Chemical Characterization of Virgin Almond and Hazelnut Oils and Their By-Products
Abstract
Various cultivars of almonds (“Ferragnes,” “Guara,” “Largueta,” and “Marcona”) and hazelnuts (“Negret,” “Pauetet,” and “Tonda”), particularly their virgin oils and by-products, are evaluated in this study. The almond and hazelnut virgin oils present high contents of oleic acid (59–73% and 76–80%, respectively) and α-tocopherol (420–542 and 310–378 mg kg–1, respectively), as compared with other virgin vegetable oils. Aldehydes are the major contributors to their aromatic profile (54–74% almond oil and 30–40% hazelnut oil of total content), especially, benzaldehyde in almond oils (1.35–7.52 mg kg–1), and hexanal in hazelnut oils (0.99–1.27 mg kg–1). Statistical differences exist between the virgin almond and hazelnut oils and their varieties, for most of the chemical compounds studied. While all the nut varieties are high in polar phenolic compounds, “Ferragnes” almonds (1262 mg kg–1) and “Negret” hazelnuts (1720 mg kg–1) stand out. Accordingly, high antioxidant activity is also observed. Finally, the residual cakes may be considered a good source of polar phenolic compounds (823–2064 mg kg–1 almond cakes, 2261–4179 mg kg–1 hazelnut cakes), possessing high antioxidant capacity with potential applications of these by-products as functional ingredients in food and non-food formulations.
Practical Applications: Virgin nut oils are gaining consumers’ preference due to their unique organoleptic attributes and potential health effects. It is therefore very relevant to establish their specific chemical composition, directly related to their properties, and that are greatly affected by the cultivar.
1 Introduction
Nuts have been the focus of intense research1 mainly due to their potential in improving the blood lipid profile and thereby reducing the risk of coronary heart diseases.2 This beneficial effect may be attributed to the high amounts of unsaturated fatty acids, but also the dietary fiber and phytosterols in nuts that inhibit the absorption of cholesterol in the digestive tract.3
Moreover, other minor compounds found in nuts possess antioxidant activity—mainly tocopherols and phenolics4—both in vivo, reducing the oxidative damage of cellular biomolecules, such as lipids, proteins, and nucleic acids and being protective against certain types of cancer,5 and in vitro, improving the oil stability6, 7 by scavenging free radicals. It is also recognized that the appropriate intake of nuts does not increase body weight but, instead, provides satiety,8 and increases the basal metabolism.9
The Prevention with Mediterranean Diet (PREDIMED) study and other clinical trials have confirmed that the intake of nuts can lower the incidence of hypertension, diabetes, cancer, and other inflammatory conditions.10-12 Based on such evidence, in 2003, the USA Food and Drug Administration (FDA) approved a specific health claim stating that the consumption of nuts (42 g d–1, 1.5 ounces d–1), may reduce the risk of heart disease.
Almonds (Prunus dulcis L.) and hazelnuts (Corylus avellana L.) were first cultivated in the Anatolian peninsula (present-day Turkey). From there, they spread to Ancient Greece and, later, to Italy during the Roman Empire, extending throughout Europe in the Middle Ages. At the end of the 16th century, Spain introduced them to America.13
In Spain, the cultivation area for almonds and hazelnuts was over 633 500 and 13 000 ha, in 2017, with a production of around 255 000 and 10 500 tons in-shell, respectively.14 Andalucía (30%) was the greatest producer of almonds, and Cataluña (90%) for hazelnut production, respectively.14
The production and consumption of virgin oils date from 8000 years ago, and the development of a solvent extraction technology with increased performance occurred more than one century ago.15 However, it has not been until the last couple of decades that a renewed interest in consuming less processed foods appeared, demanding the production of virgin oils.15 Nowadays, a wide variety of different virgin seed and nut oils, such as those derived from almonds, pistachios, hazelnuts, pumpkin seeds, and sunflower seeds, are used in gastronomy and cosmetic formulations.15, 16
Almond and hazelnut oils are rich in unsaturated fats, principally oleic (50–81% and 76–87%, respectively) and linoleic acids (6–37% and 7–16%, respectively), and the ratio of these two fatty acids (oleic/linoleic) is considered an important criterion for establishing the quality and stability of the oil.3
Another current research focus is the valorization of food processing by-products.17 In this sense, the pressing of nut oils produces a residual, partially defatted cake, which retains nutrients and bioactive compounds. Thus, this by-product represents a natural source of phytochemicals and antioxidants for dietary supplements, as well as ingredients for functional foods.
The current study is focused on the chemical characterization of almonds and hazelnuts, particularly their virgin oils and by-products, obtained by screw pressing. Differences in the major and minor components among various varieties were highlighted since these differences may be relevant to their nutritional and health-promoting effects and affect their organoleptic properties.
2 Experimental Section
2.1 Almond and Hazelnut Samples
Nuts from four almond varieties (“Ferragnes,” “Guara,” “Largueta,” and “Marcona”) and three hazelnut cultivars (“Negret,” “Pauetet,” and “Tonda”) were supplied by the companies Almendras de la Mancha (Albacete, Spain) and “Coselva” (Tarragona, Spain), respectively. “Negret” and “Pauetet” hazelnuts were provided by the Protected Designation of Origin “Avellana de Reus” (Tarragona, Spain).
2.2 Virgin Almond Oils and Virgin Hazelnut Oils
Oils were obtained using a screw-press (Komet screw oil expeller CA59G-CA563, IBG Monforts Oekotec GmbH & Co. KG, Mönchengladbach, Germany) equipped with a nozzle of 6 mm in diameter, and operating at a screw speed of 50 rpm (for almonds) and 30 rpm (for hazelnuts). The press was run for several minutes (10–15 min) to increase the barrel temperature (by the electrical resistance ring) to the required for processing the nuts (about 50 °C). The extracted crude oil was then centrifuged (5000 rpm) to get rid of the remaining plant material. Obtained oil samples were kept in amber bottles, with limited headspace, to protect them from light and oxygen. To protect from oxidative degradation, residual cakes were stored in vacuum-packed pouches. All samples were stored at 4 °C in darkness until analyzed.
Additionally, three commercial virgin almond oils (VAOs) and five commercial virgin hazelnut oils (VHOs), acquired from “Oleonucis” (Cuenca, Spain), “Azada” (Tarragona, Spain), “La Tourangelle” (Loire Valley, France), “Cauvin” (Saint-Gille, France), and “Clovis” (Reims, France), were studied.
2.3 Moisture and Oil Contents
The water content of almonds and hazelnuts and their residual cakes were determined by oven-drying at 100 °C until constant weight.18 The oil content was determined by Soxhlet extraction, according to the Association of Official Analytical Chemists.19
2.4 Fatty Acid Composition
The fatty acid methyl esters (FAME) were determined using an Agilent Technologies gas chromatograph (HP 6890; Santa Clara, CA) with a flame ionization detector, after derivatization by shaking of a solution of oil in heptane (0.5 g in 3 mL) with 0.5 mL of 2 n methanolic-KOH, according to the European Regulation EC 2568/91 and subsequent amendments, corresponding to the American Oil Chemists’ Society (AOCS) method Ce 1j-07-91.20 A fused silica capillary column (60 m length × 0.25 mm i.d.) coated with SP-2380 phase (0.2 µm thickness; Supelco, Madrid, Spain) was used. Helium (flow rate of 1 mL min–1) was employed as carrier gas. Temperatures of 220, 185, and 250 °C for the injector, oven, and detector were used, respectively, employing an injection volume of was 1 µL. Content of individual fatty acid were reported as total amount a percentage.
2.5 Tocopherols
Tocopherols were analyzed, as defined in the AOCS method Ce 8–89.21 A total of 0.4 g of ground almond/hazelnut kernels or residual cake in n-hexane (twice: 6 + 4 mL) was mixed for 2 min by vortex and 5 min by ultrasound and finally centrifugated at 2000 × g for 10 min. Prior to analysis the combined extracts (supernatants) were filtered. Overall 0.1 g of nut oil in 10 mL n-hexane was analyzed using a high-performance liquid chromatograph (Agilent Technologies 1100 series) employing a silica gel LiChrosorb Si-60 column (particle size 5 µm, 250 mm × 4.6 mm id; Sugerlabor, Madrid, Spain), n-hexane/2-propanol (98.5:1.5; 1 mL/min) as the eluent, and a fluorescence detector (Waters 470, Milford, MA; 290 and 330 nm excitation and emission wavelengths, respectively).
2.6 Phenolic Compounds
The total polar phenolic (TPP) content was determined, as reported by Vázquez et al. and Gutfinger.22, 23 The solid residue obtained from the n-hexane extraction (Section 2.5) was extracted twice in MeOH:H2O:HCOOH (80:20:0.1; 10 + 10 mL), mixing 2 min by vortex and 5 min by ultrasound and finally centrifugated at 2000 × g for 10 min. The combined extracts (supernatants) were filtered before analysis.
5 g of nut oil was dissolved in 10 mL n-hexane and 10 mL of MeOH:H2O:HCCOH (80:20:0.1). The solution was vortexed for 2 min, ultrasonicated for 5 min and then centrifuged at 2000 × g for 10 min. The polar fraction was finally separated and filtered. Polar extracts aliquots (100−500 µL) were moved to a 10 mL volumetric flask adding the Folin–Ciocalteu reagent (0.5 mL) and water (up to 8.5 mL). A total of 1.5 mL of saturated NaCO3 solution (20%, w/v) was added to the reaction solution 3 min later. The absorbance at 725 nm of the mixture was read using a UV–vis spectrophotometer (Agilent Technologies 8453) and a gallic acid calibration curve.
2.7 Antioxidant Activity
Antioxidant capacity was measured by the radical scavenging effect of the methanolic-extract against the synthetic radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH), as reported previously.24, 25 An aliquot of the polar extract (100 µL; same as for TPP, Section 2.6), was dissolved to a methanolic-DPPH solution (2.9 mL, 6 × 10−5 m) and kept in dark for 30 min. The decrease in absorbance was determined at 515 nm (Agilent 8453 spectrophotometer) using a Trolox calibration curve for quantification.
The oxygen radical absorbance capacity (ORAC) was assessed26 by pre-incubation of 20 µL of polar extract and 120 µL fluorescein (70 nm) at 37 °C for 15 min. Sixty microliters of 24 mm 2,2’-azobis(2-amidinopropane) dihydrochloride was then added and the solution was incubated at 37 °C measuring its kinetics every minute for 80 min. The assay was performed in Nunclon black 96-well flat-bottom plates (Sigma–Aldrich, Madrid, Spain), employing a plate reader (528 and 485 nm emission and excitation, respectively) and a Trolox calibration curve.
2.8 Volatile Compounds
A solid-phase microextraction (SPME) prior to a GC–MS analysis was used to determine the volatile compounds in the VAO and VHO samples, via adaptation of a literature method.27 Virgin oil (1.5 g) was spiked with 4-methyl-2-pentanol (as the internal standard) to a concentration of 1.5 mg kg–1 and put in a silicone septum 10 mL vial. The SPME sampling was carried out exposing the DVB/Carboxen/PDMS fiber (50/30 µm, 2 cm long; Supelco) into the sample headspace kept 30 min at 40 °C. The fiber was then drawn back into the needle and desorbed in the injection port (5 min) of a gas chromatograph (Agilent Technologies 6850) equipped with a mass detector (Agilent Technologies 5975C). Compounds were isolated using a DB-Wax column (30 m × 0.25 mm × 0.25 µm; Agilent Technologies) employing: an injection port temperature of 260 °C; 0.8 mL min–1 of helium flow; and an oven temperature ramp of 35 °C for 10 min, 3 °C min–1 to 160 °C and then 15 °C min–1 to 200 °C (kept for 5 min). For the identification of the volatile compounds, the NIST MS library, the Kovats retention indices, and the mass spectra of standard substances (Sigma–Aldrich) were used.
2.9 Pigments and Color
Chlorophyll and carotenoid compounds were determined at 670 and 472 nm, respectively, in cyclohexane, using specific extinction values, as described by Minguez-Mosquera et al.28 A Konica Minolta CR-400 colorimeter (Tokyo, Japan) was employed to measure the color (CIELAB coordinates: L*, lightness; a*, redness; and b*, yellowness) of ground almonds and hazelnuts, their residual cakes, and virgin nut oils. The C illuminant was used, and the instrument was precalibrated against a standard tile.
2.10 Reference Materials, Reagents, and Solvents
FAME mixture (C14–C22, certified reference material), tocopherols mixture, α-tocopherol (from vegetable oil), γ-tocopherol (>96%, HPLC), Folin–Ciocalteu reagent, gallic acid, Trolox, DPPH, and 4-methyl-2-pentanol were acquired from Sigma–Aldrich (Steinheim, Germany). All solvents used were supplied by Merck (Darmstadt, Germany) and of analytical, HPLC, or spectroscopic grade.
2.11 Statistical Analysis
Analytical determinations and experiments were performed at least in duplicate. Analysis of variance (ANOVA) and principal components analysis (PCA) were carried out employing the statistical software XLSTAT 19.5 (Addinsoft, Paris, France). One-way ANOVA was performed using Duncan's test. Means were considered statistically different at p < 0.05. PCA was employed to infer the effect of nut cultivar on the chemical composition of the virgin oils. A Pearson correlation matrix of n-type standardized data was used without applying rotation; commercial nut oils characteristics were treated as supplementary data.
3 Results and Discussion
3.1 Major Composition of Nuts, Their Virgin Oils, and By-Products
The studied almond varieties possessed an oil content ranging from 45.8% (“Largueta”) to 57.9% (“Ferragnes”; Figure 1), showing significant differences between the varieties studied. In comparison, hazelnuts showed a significantly higher oil content (>62%), with a maximum of 69.9% (“Negret”). These data concurred with those found in the literature.29 For the residual cakes left after the mechanical extraction of the virgin oil, the fat contents of 7.9–13.8% (almond cakes) and 10.7–11.4% (hazelnut cakes) were relatively low (Figure 1).

Oil and moisture contents (%) in studied almond and hazelnut cultivars and their residual cakes. A, almond; H, hazelnut; FE, Ferragnes; GU, Guara; LA, Largueta; MA, Marcona; NE, Negret; PA, Pauetet; TO Tonda. Different letters show statistical differences between varieties of the same kind of nut or cake (almond or hazelnut).
The moisture contents of the nuts were in the ranges of 3.5–6.2% (almonds) and 2.7–4.1% (hazelnuts; Figure 1), which were slightly lower than those obtained in the corresponding by-products (≈6% for almond cakes, ≈5% for hazelnut cakes) since the separation of the oil concentrates the other components in the residual cake. A higher water content (>8%) could reduce the shelf life and quality of the nuts, due to fungal proliferations29 and lead to contamination by aflatoxin-producing fungi. However, the total aflatoxin contents in the almonds and hazelnuts were far below the limit established by Spanish law (<10 µg kg–1; Spanish RD 475/1988).
From the fatty acid profiles of the obtained VAOs and VHOs (Figure 2), both oils were high in oleic acid, which varied from 59% (“Largueta”) to 73% (“Ferragnes”) among the almond cultivars compared with the narrower range of 76% (“Tonda”) to 80% (“Negret”) for the hazelnut cultivars. These high percentages of oleic acid are close to that found in olive oil30 and other virgin nut oils, such as pistachio (55–74%),31 but greater than those detected in other virgin nut oils, like walnut, in which linoleic acid is the main fatty acid (59.6–62.4%).32
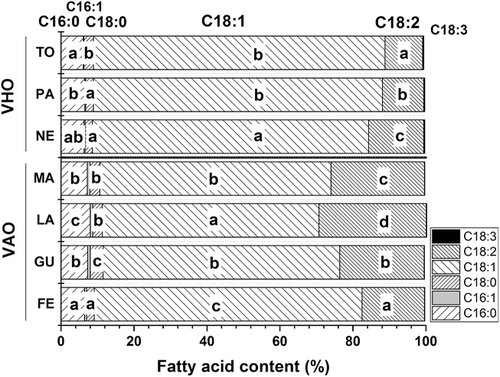
The essential fatty acid linoleic acid (n-6), was the second most abundant fatty acid in both nut oils (17–29% in almond oil; 10–15% in hazelnut oil; p = 0.011), with the greatest proportions found in “Largueta” and “Negret” hazelnut varieties. Based on these values, the intake of a tablespoon (14 g) of VAO or VHO would provide about 25% and 15% of the recommended dietary allowance (RDA) of 11 and 14 g linoleic acid d–1 for female and male adults, respectively (Food and Nutrition Board, US National Academy of Sciences, 2016). Hence, the oils could be nutritionally labeled as a “good source” (>10% RDA) or having a “high content” (>20% RDA) of this nutrient.
Other published data reported similar oleic acid values, detecting about 65% in almonds and 80% in hazelnuts, and linoleic acid at ≈25% in almonds and 10% in hazelnuts.29, 33
The examined commercial oils (three almond and five hazelnut) had no cultivar information on their labels. It was determined that the commercial almond oils contained 67.8–68.7% oleic acid and 20.1–22.8% linoleic acid, values that were closest to the “Guara” oil studied. The commercial hazelnut oils analyzed, possessed 75.5–81.1% oleic acid and 9.1–12.9% linoleic acid.
3.2 Tocopherols, Polar Phenolic Compounds, and Antioxidant Properties
All the VAOs and VHOs were rich in α-tocopherol, displaying statistically different mean values of 480 mg kg–1 for VAO and 350 mg kg–1 for VHO (Table 1a,b), in agreement with Robbins et al. and Bacchetta et al.33, 34 Relatively lower amounts have been recorded in other virgin nut oils, including pistachio (6–30 mg kg–1),31 walnut (≈25 mg kg–1)32 and macadamia (22–30 mg kg–1),35 as well as seed oils, such as soybean and linseed (80 mg kg–1)36 and olive (150 mg kg–1).37 “Marcona” (420 mg kg–1) and “Largueta” (542 mg kg–1) almonds, and “Pauetet” (310 mg kg–1) and “Tonda” (378 mg kg–1) hazelnuts defined the minimum and maximum of the ranges. Moreover, both nut oils possessed a small amount of γ-tocopherol (2–5% of the total), as well as 3–5 mg kg–1 of α-tocotrienol (data not shown), depending on the variety.
| Almond | Ferragnes | Guara | Largueta | Marcona | Commercial oils (range) | |
|---|---|---|---|---|---|---|
| α-T* | Nut | 312 ± 21b | 298 ± 5b | 374 ± 33c | 237 ± 6a | – |
| γ-T* | 14.0 ± 1.5c | 3.6 ± 0.1b | 13.0 ± 0.3c | 1.7 ± 0.2a | – | |
| α-T* | Oil | 475 ± 5b | 484 ± 34b | 542 ± 2c | 420 ± 3a | 407–592 |
| γ-T* | 26.3 ± 2.6b | 10.8 ± 1.0a | 27.9 ± 0.9b | 6.7 ± 0.4a | 12–133 | |
| α-T* | Cake | 91 ± 11b | 94 ± 7b | 75 ± 11b | 38 ± 2a | – |
| γ-T* | 4.1 ± 0.9c | 2.0 ± 0.4b | 1.6 ± 0.0b | 0.2 ± 0.0a | – | |
| Total polar phenols** | Nut | 1 262 ± 50c | 1 171 ± 31c | 1 093 ± 56b | 709 ± 46a | – |
| Oil | 5.8 ± 1.8a | 5.6 ± 1.5b | 3.4 ± 0.3a | 5.7 ± 1.2a | 26.9–31.5 | |
| Cake | 2 064 ± 217d | 1 696 ± 586c | 1 252 ± 157b | 823 ± 97a | – | |
| DPPH*** | Nut | 3.2 ± 0.6b | 2.2 ± 0.8a | 2.3 ± 0.2ab | 1.8 ± 0.6a | – |
| Oil | 0.02 ± 0.01a | 0.03 ± 0.1a | 0.05 ± 0.02b | 0.06 ± 0.02b | 0.04–0.16 | |
| Cake | 7.5 ± 0.4d | 6.0 ± 1.2c | 4.0 ± 0.3b | 2.1 ± 0.5a | – | |
| ORAC**** | Nut | 79.7 ± 4.9b | 70.3 ± 7.8b | 72.0 ± 9.7b | 39.2 ± 5.1a | – |
| Oil | 0.53 ± 0.08a | 0.80 ± 0.04b | 0.48 ± 0.03a | 1.30 ± 0.03c | 1.24–1.72 | |
| Cake | 197.4 ± 20.9d | 152.2 ± 15.3c | 102.2 ± 9.4b | 57.6 ± 8.9a | – | |
| Pigments | Chlor. | 8.5 ± 1.3a | 18.0 ± 1.7c | 10.6 ± 0.0ab | 12.1 ± 0.5b | 2.3–8.2 |
| Carot. | 5.3 ± 0.6a | 8.8 ± 0.7b | 5.8 ± 0.0a | 5.7 ± 0.5a | 1.8–5.1 |
- Values in the same row with different lower-case letters are significantly different at p < 0.05 by Duncan test. *Quantified using α-tocopherol as standard. **Quantified by Folin using gallic acid as standard. ***Quantified by radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH) using Trolox as standard. ****Quantified by oxygen radical absorbance capacity (ORAC) using Trolox as standard. Chlor., chlorophylls; carot., carotenoids; α-T, α-tocopherol; γ-T, γ-tocoferol.
| Hazelnut | Negret | Pauetet | Tonda | Commercial oils (range) | |
|---|---|---|---|---|---|
| α-T* | Nut | 194 ± 4a | 200 ± 5a | 235 ± 3b | – |
| γ-T* | 2.0 ± 0.1b | 0.8 ± 0.1a | 1.0 ± 0.1a | ||
| α-T* | Oil | 321 ± 4a | 310 ± 7a | 378 ± 17b | 86–423 |
| γ-T* | 5.5 ± 0.4b | 6.0 ± 0.0b | 3.2 ± 0.2a | 2.7–81.3 | |
| α-T* | Cake | 41 ± 2 | 42 ± 0 | 49 ± 5 | – |
| γ-T* | 0.9 ± 0.1a | 1.2 ± 0.3a | 1.8 ± 0.5b | ||
| Total polar phenols** | Nut | 1 720 ± 53c | 1 324 ± 33b | 1 086 ± 38a | – |
| Oil | 12.1 ± 0.9a | 10.4 ± 0.4b | 11.3 ± 1.3ab | 12.2–47.9 | |
| Cake | 4 179 ± 176c | 3 439 ± 89b | 2 261 ± 44a | – | |
| DPPH*** | Nut | 5.8 ± 0.3c | 4.0 ± 1.1b | 2.9 ± 0.2a | – |
| Oil | 0.05 ± 0.00a | 0.05 ± 0.02ab | 0.09 ± 0.04b | 0.02–0.10 | |
| Cake | 13.9 ± 1.3c | 10.3 ± 0.9b | 8.1 ± 1.0a | – | |
| ORAC**** | Nut | 77.1 ± 19.2b | 58.6 ± 10.2a | 56.5 ± 4.3a | – |
| Oil | 1.81 ± 0.05b | 1.17 ± 0.03a | 2.17 ± 0.32c | 1.06–4.61 | |
| Cake | 179.7 ± 15.8c | 158.7 ± 38.6b | 122.5 ± 13.9a | – | |
| Pigments | Chlor. | 15.6 ± 0.5c | 13.7 ± 6.5b | 10.9 ± 0.8a | 5.3–8.6 |
| Carot. | 7.5 ± 0.1 | 6.6 ± 1.4 | 7.9 ± 0.4 | 2.8–5.1 |
- Values in the same row with different lower-case letters are significantly different at p < 0.05 by Duncan test. *Quantified using α-tocopherol as standard. **Quantified by Folin using gallic acid as standard. ***Quantified by radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH) using Trolox as standard. ****Quantified by oxygen radical absorbance capacity (ORAC) using Trolox as standard. Chlor., chlorophylls; carot., carotenoids; α-T, α-tocopherol; γ-T, γ-tocoferol.
Given these amounts, the intake of a tablespoon (14 g) of VAO or VHO will provide more than 40% and 30% of the RDA of 15 mg vitamin E d–1 for adults; Food and Nutrition Board, US National Academy of Sciences, 2016), allowing the nutritional labeling of these oils as providing a “high content” (>20% RDA) of this nutrient.
Tocopherols are apolar compounds that separate into the oily phase during the extraction, explaining the low residual concentrations of these compounds in the almond (α-tocopherol, 38–94 mg kg–1) and hazelnut cakes (α-tocopherol, 41–49 mg kg–1). Indeed, a moderate relationship existed between the oil and tocopherol contents in the by-products. For example, “Ferragnes” almond cake had 11% fat and 91 mg kg–1 α-tocopherol, and “Largueta” almond cake contained 8% fat and 75 mg kg–1 α-tocopherol.
For most nuts, the polar phenolic compounds tend to lie in the range 1100–2900 mg kg–1,38 except pistachios (7500 mg kg–1)31 and walnuts (12 500 mg kg–1).32 “Marcona” (709 mg kg–1 as gallic acid) and “Ferragnes” (1262 mg kg–1) possessed the least and highest amounts, respectively (Table 1a), among the studied almond cultivars. Significantly more quantities occurred in the hazelnuts (p = 0.002), with the maximum detected in “Negret” hazelnuts (1720 mg kg–1; Table 1b). Similar values have been reported for roasted almonds (1140 mg kg–1 as gallic acid) and hazelnuts (1110 mg kg–1),39 although higher amounts (2400 mg kg–1 for almonds and 2900 mg kg–1 for hazelnuts),40 have also been published, possibly affected by the variety being investigated.
The residual cakes could be expected to possess an added value for applications in food formulations since they are a good source of phenolic compounds that concentrate in the by-product due to their polar properties. Consistent with the trend observed in the nuts, “Ferragnes” almond cake (2064 mg kg–1 as gallic acid; Table 1a) and “Negret” hazelnut cake (4179 mg kg–1 as gallic acid; Table 1b) showed the highest TPP concentrations.
Small quantities of phenolic compounds existed in the VAOs (3.4–5.8 mg kg–1, Table 1a) and VHOs (10.4–12.1 mg kg–1, Table 1b), which was expected, given their polar characteristics, although the VHO oils had markedly more than the VAOs (p = 0.001). These results were consistent with those described by Górmas et al.41 (6.0 mg kg–1 VAO and 19.9 mg kg–1 VHO) but less than that specified in almond oils extracted with solvent (38 mg kg–1).42 Phenolic contents similar to those indicated in the studied VAOs and VHOs are found in other virgin seed oils, such as sunflower, rapeseed, and pumpkin (10–30 mg kg–1 as caffeic acid).43
There were significant differences in the DPPH and ORAC antioxidant activities of the polar phenolics in the methanolic extracts between the almond and hazelnut varieties. As expected, considering the greater quantities of polar phenolics, the hazelnut varieties also had higher DPPH radical scavenging properties than the almond ones. For the DPPH assay, a minimum of 1.8 mmol kg–1 and a maximum of 3.2 mmol kg–1 (“Ferragnes”) was measured in almond nuts (Table 1a). Overall, however, “Negret” hazelnuts demonstrated the greatest DPPH antioxidant capacity (5.8 mmol kg–1; statically higher than the other varieties), confirming the literature data.39
ORAC values of 39.2 mmol kg–1 (“Marcona”; significantly lower than the other varieties; Table 1a) to 79.7 mmol kg–1 (“Ferragnes”) for almonds, and 56.5 mmol kg–1 (“Tonda”) to 77.1 mmol kg–1 (“Negret”; significantly higher than the other varieties; Table 1b) for hazelnuts were observed. In comparison, several other nuts possess a lower ORAC antioxidant activity (cashew: 20 mmol kg–1; macadamias: 17 mmol kg–1; pine nut: 7 mmol kg–1),38 except pistachios (80 mmol kg–1)31 and walnuts (130 mmol kg–1).32
It was not surprising that the residual cakes revealed a more potent antioxidant activity when compared with their corresponding nuts, given the phenolic fraction is concentrated in the by-product, as already discussed above. By-products derived from “Ferragnes” almonds (7.5 mmol kg–1 DPPH; 197.4 mmol kg–1 ORAC; Table 1a) and “Negret” hazelnuts (13.9 mmol kg–1 DPPH; 179.2 mmol kg–1 for ORAC; Table 1b) showed the highest antioxidant activity.
On the contrary, in virgin nut oils, the polar antioxidant activity was low, according to their small TPP content (Table 1a,b), but similar to other tree nut oils.44
For the commercial oils, the almond oils had similar α-tocopherol contents to each other (407–592 mg kg–1; Table 1a), and a low proportion of γ-tocopherol (maximum of 133 mg kg–1), whereas, a much wider range of α-tocopherol (86–423 mg kg–1; Table 1b) was observed in the commercial hazelnut oils. For the TPP content of the commercial oils, once again, a much greater maximum content (31.5 mg kg–1 for almond and 47.9 mg kg–1 for hazelnut oils) was measured relative to the studied VAOs and VHOs, probably because of the different extraction conditions employed.
3.3 Volatile Compounds
Table 2a,b summarizes the volatiles of the studied VAOs and VHOs, respectively, observing significant differences for each of the volatile compound families between the two types of nut oils. It is well known that the volatiles are related to sensory notes, such as fruit/banana (hexanol), oily/green–sweet (hexanal), fruity (pentanol),45 and bitter almonds (benzaldehyde).46
| Compounds* | Ferragnes | Guara | Largueta | Marcona | Commercial oils (range) |
|---|---|---|---|---|---|
| Acetic acid | 0.24 ± 0.01b | 0.22 ± 0.05b | 0.12 ± 0.00a | 0.19 ± 0.03ab | 1.09–20.15 |
| Hexanoic acid | 0.12 ± 0.01b | 0.11 ± 0.01b | 0.00 ± 0.00a | 0.17 ± 0.01c | 0.25–1.66 |
| Σ Acids | 0.35 ± 0.01b | 0.34 ± 0.04b | 0.12 ± 0.00a | 0.36 ± 0.02b | 1.47 ̶ 21.81 |
| Benzyl alcohol | 0.14 ± 0.01a | 0.37 ± 0.04c | 0.34 ± 0.01c | 0.27 ± 0.01b | 0.00–0.59 |
| 2,3-Butadienol | NDa | 0.14 ± 0.02b | NDa | NDa | ND |
| Heptanol | NDa | NDa | NDa | 0.12 ± 0.01b | ND |
| Hexanol | 0.13 ± 0.01a | 0.27 ± 0.02a | 0.54 ± 0.02b | 1.89 ± 0.17c | 0.17–0.71 |
| 2-Methyl-1-butanol | 0.43 ± 0.01b | NDa | 0.58 ± 0.01c | 0.59 ± 0.02c | 0.00–2.92 |
| Pentanol | NDa | 0.65 ± 0.02d | 0.14 ± 0.00b | 0.50 ± 0.05c | 0.00–0.38 |
| Σ Alcohols | 0.71 ± 0.02a | 1.43 ± 0.05b | 1.60 ± 0.02c | 3.37 ± 0.24d | 0.17–4.16 |
| Benzaldehyde | 1.35 ± 0.22a | 7.30 ± 0.59b | 7.14 ± 0.48b | 6.28 ± 0.23b | 0.00–6.37 |
| Hexanal | NDa | 0.23 ± 0.05b | 0.28 ± 0.02b | 1.22 ± 0.15c | 0.75–5.79 |
| Σ Aldehydes | 1.35 ± 0.21a | 7.52 ± 0.64b | 7.42 ± 0.46b | 7.51 ± 0.38b | 3.06–7.11 |
| (2-Methylbutylidene)-cyclopentane | NDa | 0.25 ± 0.01c | 0.19 ± 0.01b | 0.14 ± 0.03b | ND |
| 3-Ethyl-2,5-Dimethyl-1,3-Hexadiene | NDa | 0.13 ± 0.02c | 0.10 ± 0.01b | NDa | ND |
| Σ Hydrocarbons | 0.00 ± 0.00a | 0.38 ± 0.03d | 0.29 ± 0.01c | 0.13 ± 0.03b | ND |
| 4-Hydroxy-4-methyl-2-pentanone | NDa | 0.43 ± 0.09c | 0.18 ± 0.03ab | 0.34 ± 0.13bc | 0.00–0.33 |
| Σ Ketones | 0.00 ± 0.0a | 0.43 ± 0.09d | 0.18 ± 0.03b | 0.34 ± 0.13c | 0.00–0.33 |
| Longifolene | 0.11 ± 0.02 | 0.14 ± 0.0 | 0.15 ± 0.01 | 0.15 ± 0.00 | 0.00–0.21 |
| α-Terpineol | NDa | NDa | 0.23 ± 0.04b | 0.20 ± 0.02b | ND |
| Σ Terpenes | 0.11 ± 0.02 | 0.14 ± 0.0 | 0.38 ± 0.05 | 0.35 ± 0.02 | 0.00–0.21 |
| Total | 2.52 ± 0.21a | 10.23 ± 0.60b | 9.98 ± 0.45b | 12.05 ± 0.79c | 9.55–27.78 |
- Values in the same row with different lower-case letters are significantly different at p < 0.05 by Duncan test. *Quantified using 4-methyl-2-pentanol as internal standard. Com, commercial.
| Compounds* | Negret | Pauetet | Tonda | Commercial oils (range) |
|---|---|---|---|---|
| Acetic acid | 0.77 ± 0.02a | 0.94 ± 0.01b | 0.88 ± 0.03b | 0.85–16.42 |
| Hexanoic acid | 0.27 ± 0.02b | 0.19 ± 0.02a | 0.24 ± 0.01ab | 0.00–1.72 |
| Σ Acids | 1.04 ± 0.00a | 1.13 ± 0.01b | 1.12 ± 0.04ab | 1.44–18.14 |
| 2,3-Butadienol | NDa | 0.14 ± 0.01b | NDa | ND |
| 2,3-Dimethyl-1-butanol | NDa | 0.16 ± 0.02b | 0.15 ± 0.00b | 0.00–0.81 |
| Heptanol | NDa | 0.09 ± 0.01b | 0.11 ± 0.00b | ND |
| Hexanol | NDa | 0.14 ± 0.00b | 0.23 ± 0.03c | 0.00–0.42 |
| 2-Methyl-1-butanol | 0.13 ± 0.00b | NDa | NDa | 0.00–1.19 |
| Pentanol | 0.29 ± 0.00b | 0.31 ± 0.01c | 0.20 ± 0.00a | 0.00–0.16 |
| 2-Pentanol | 0.12 ± 0.00a | 0.20 ± 0.01b | 0.14 ± 0.00a | 0.00–0.37 |
| Σ Alcohols | 0.54 ± 0.01a | 1.04 ± 0.07c | 0.83 ± 0.02b | 0.00–2.43 |
| Benzaldehyde | NDa | 0.09 ± 0.01b | NDa | 0.00–0.30 |
| Furfurale | NDa | 0.09 ± 0.01b | NDa | 0.28–10.50 |
| Heptanal | 0.14 ± 0.03b | NDa | NDa | 0.00–0.49 |
| Hexanal | 1.21 ± 0.01b | 0.99 ± 0.05a | 1.27 ± 0.06b | 0.68–3.47 |
| Σ Aldehydes | 1.35 ± 0.02b | 1.16 ± 0.04a | 1.27 ± 0.06ab | 1.86–14.47 |
| 4-Hydroxy-4-methyl-2-pentanone | 0.30 ± 0.02 | 0.43 ± 0.09 | 0.37 ± 0.11 | 0.24–2.25 |
| Σ Ketones | 0.30 ± 0.02 | 0.43 ± 0.09 | 0.37 ± 0.11 | 0.24–2.25 |
| Longifolene | 0.12 ± 0.03ab | NDa | 0.19 ± 0.06b | 0.00–0.25 |
| Σ Terpenes | 0.12 ± 0.03ab | NDa | 0.19 ± 0.06b | 0.00–0.25 |
| Total | 3.36 ± 0.01 | 3.76 ± 0.08 | 3.78 ± 0.13 | 5.56–33.19 |
- Values in the same row with different lower-case letters are significantly different at p < 0.05 by Duncan test. *Quantified using 4-methyl-2-pentanol as internal standard. Com, commercial.
For the VAOs, the most important family found in all the varieties studied was that of aldehydes (1.35–7.52 mg kg–1; Table 2a). “Ferragnes” had the lowest amount (1.35 mg kg–1) while the other three oils had a much higher content, with little difference between each other. Benzaldehyde, which provides the characteristic bitter almonds aroma,47 was the main aldehyde (52–74% of total), followed by hexanal (0–10%), in agreement with earlier authors.48, 49
Alcohols were the second major family, accounting for 14% (“Ferragnes”) to 30% (“Marcona”) of the total volatiles. Hexanol was the main contributor and was most abundant in “Marcona” (1.89 mg kg–1). The acids, hydrocarbons, ketones, and terpenes showed close concentrations to each other, indicating about 0.30 mg kg–1 for each family.
For the VHOs, their volatile content was much lower than that measured in the VAOs, and their profile (Table 2a) featured aldehydes (31–40%), acids (30–31%), and alcohols (16–28%). Hexanal was the main aldehyde, ranging in concentration from 0.99 mg kg–1 (“Pauetet”) to 1.27 mg kg–1 (“Negret”), consistent with previous work.50 It is important to remark that hexanal is not only a marker of secondary oxidation but an intrinsic characteristic of certain virgin oils aroma.45 “Negret” was the only studied variety that presented heptanal (0.14 mg kg–1) in its volatile profile, and “Pauetet” was the one variety to show both furfural (0.09 mg kg–1) and benzaldehyde (0.09 mg kg–1).
As verified in Table 2b, “Pauetet” VHO possessed the highest concentration of alcohols (1.04 mg kg–1), followed by “Tonda” (0.83 mg kg–1) and “Negret” (0.54 mg kg–1), and all three were statistically different from each other (p = 0.003). Pentanol, related to fruity aroma,51 was the major contributor to this family.
Table 2a,b highlights the broad concentration ranges of the volatiles from the different families present in the commercial almond and hazelnut oils, as exemplified by the acids (1.47–21.81 mg kg–1 almonds and 1.44–18.14 mg kg–1 hazelnuts), aldehydes (3.06–7.11 mg kg–1 almonds and 1.86–14.47 mg kg–1 hazelnuts), and alcohols (0.17–4.16 mg kg–1 almonds and 0.00–2.43 mg kg–1 hazelnuts). The high concentration of acids (18.14 mg kg–1), as well as the presence of filbertone (5-methyl-(E)-2-hepten-4-one), pyrazines, and furans (data not shown), is probably related to the extraction conditions employed in the commercial oils (mainly high temperatures) and the use of roasted nuts,52, 53 which contributes to the typical nutty–roasty aroma.54
Volatile alcohols and acids are constantly found in the headspace of different nuts oils (e.g., ref. [55]). They are generally formed by the oxidation action of enzymes or microorganisms during storage. However, in the current study (similarly to others) the experimental oils were freshly prepared by screw pressing (working temperature of about 50 °C) and stored in dark at 4 °C. Therefore, the oxidation process may also have occurred during the storage of the nuts. An important increase in acids and alcohols is observed at higher extraction temperature (>100 °C) or employing roasted nuts (e.g., ref. [53]), as indeed observed in the commercial oils studied (Table 2a,b), as mentioned above.
3.4 Pigments and Color
Chlorophylls (8.5–18.0 mg kg–1) and carotenoids (5.3–8.8 mg kg–1) were present in the VAOs, especially “Guara” (Table 1a), and the ranges were not dissimilar to those revealed in the VHOs, which varied from 10.9 mg kg–1 (“Tonda”) up to 15.6 mg kg–1 (“Negret”) and from 6.6 mg kg–1 (“Pauetet”) to 7.9 mg kg–1 (“Tonda”), respectively.
The color (data not shown) of almond flour (3.9–9.8 a*; 16.9–20.5 b*), depended on the variety and was comparable to the hazelnut flours (5.3–6.5 a*; 16.7–17.9 b*). These color values were close to those measured in the residual cakes after pressing (as already noted by Karaman et al.).56 Both VAO (–3.5 a*, 16.0 b*) and VHO (–2.0 a*, 40.0 b*) were pale yellow, consistent with an earlier report.41
3.5 PCA of VAO and VHO Chemical Characteristics
Figure 3 provides the PCA biplot, allowing visual observation of the differences between the virgin oils processed from different almond and hazelnut varieties, as well as the commercial samples studied. The composition of the fatty acids, tocopherols, and volatiles of the VAOs (four experimental and three commercial samples) and VHOs (three experimental and five commercial samples), explained 84.54% of the data variance (principal component 1 [F1], 63.72%; principal component 2 [F2], 20.82%).
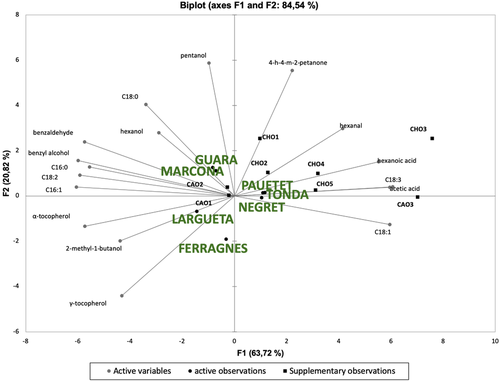
F1 was most positively related to acetic acid (0.970), hexanoic acid (0.883), and hexanal (0.663), among the volatile compounds, and oleic acid (C18:1, 0.952) and linolenic acid (C18:3, 0.952) fatty acids. Conversely, F1 was most negatively associated with the benzyl alcohol (–0.959) and benzaldehyde (–0.920) volatiles, palmitoleic (C16:1, –0.969), linoleic (C18:2, –0.948) and palmitic (C16:0, -0.889) fatty acids, and α- (–0.918) and γ-tocopherol (–0.690).
F2 was positively related to pentanol (0.939), 4-hydroxy-4-methyl-2-pentanone (0.887), and stearic acid (C18:0, 0.647), and negatively correlated with γ-tocopherol (–0.705) and 2-methyl-1-butanol (–0.317), leading to the separation of “Guara” and “Marcona” (positive y-axis) from “Largueta” and “Ferragnes” (negative y-axis) almond varieties. In contrast, the three hazelnut cultivars were situated very close together and near their commercial oils (positive y-axis).
In this sense, the PCA distinctly separated the almond from the hazelnut oils studied, except commercial oil CAO3 (Figure 3), mainly based on their different fatty acid profile (a higher content in linoleic acid (C18:2) in almonds rather than linolenic (C18:3) and oleic (C18:1) in hazelnut oils), as well as tocopherols and benzaldehyde contents. As concerned the commercial oils studied, those from almonds appeared to be closest to the cold-press virgin oils of the same nut prepared in this study, in particular the “Marcona” and “Largueta” cultivars (except commercial oil CAO3, as already mentioned above); similarly, the commercial hazelnut oils are closest to the experimental ones obtained in this study, in particular “Pauetet” and “Tonda” cultivars (except commercial oil CHO3).
4 Conclusions
Statistical differences were found between VAOs and VHOs studied, and their varieties, for most of the chemical compounds studied, except chlorophylls and carotenoids. However, a higher number of samples from different geographical and pedoclimatic origins are needed to generalize these findings. VAO and VHO are rich—as compared with other vegetable oils, such as walnut, sunflower, pumpkin or flaxseed—in oleic (59–73% and 76–80%, respectively) and linoleic acids (17–29% and 10–15%, respectively) and α-tocopherol (420–542 mg kg–1 and 310–378 mg kg–1, respectively). These compounds are of nutritional and health interest and therefore confer added values to these oils, as well as a higher oxidative stability as compared to linolenic-rich vegetable oils. The volatiles are mainly composed by aldehydes (54–74% almonds, 30–40% hazelnuts), with benzaldehyde as the principal compound in almond oils (1.35–7.52 mg kg–1), and hexanal, the main aldehyde in hazelnut oils (0.99–1.27 mg kg–1). A sensory evaluation would contribute to define the characteristics and preferences of the attributes perceived by consumers. Among the cultivars, “Ferragnes” VAO could be highlighted for its oleic acid content (73.4%), “Largueta” for α-tocopherol (542 mg kg–1), and “Marcona” for volatiles (12.1 mg kg–1). Unlike the VAOS, the three VHOs analyzed were highly similar to each other, although “Tonda” had the highest levels of oleic acid (79.9%) and α-tocopherol (378 mg kg–1). Varietal or origins effects on composition and properties are of great commercial interest as product differentiation and for their relationship to global quality and added value of the virgin nut oils. All the nuts displayed a high content of polar phenolic compounds, of great relevance for their potential health effects, emphasizing “Ferragnes” almonds (1262 mg kg–1) and “Negret” hazelnuts (1720 mg kg–1). Consequently, high antioxidant activity was also observed (averages of 2.4 mmol kg–1 DPPH and 65.3 mmol kg–1 ORAC in almonds, and 4.2 mmol kg–1 DPPH and 64.1 mmol kg–1 ORAC in hazelnuts). Finally, the residual cakes may be considered a good source of polar phenolic compounds (823–2064 mg kg–1 almond cakes, 2261–4179 mg kg–1 hazelnut cakes), possessing strong antioxidant capacity, with potential applications of this by-product as a functional ingredient in food and non-food formulations.
Abbreviations
-
- VAO
-
- virgin almond oil
-
- VHO
-
- virgin hazelnut oil
-
- TPP
-
- total polar phenolics
Acknowledgements
This research project was supported by the Junta de Comunidades de Castilla-La Mancha, and the European Regional Development Fund (FEDER; ref. POII-2014-003-P).
Conflict of Interest
The authors declare no conflict of interest.




