Combination of Hydrophilic or Lipophilic Natural Compounds to Improve the Oxidative Stability of Flaxseed Oil
Abstract
Oxidation of bulk oils involves radical molecules, transitions metals, and association colloids, among other factors. Combining mechanistically different antioxidants is one possible strategy to improve oxidative stability of polyunsaturated fatty acids rich oils. However, antioxidant polarity may play a critical role in the oxidative protection efficacy. Thus, the objective of this study is to formulate a hydrophilic mixture (HM) containing sinapic, ascorbic, and citric acid; and a lipophilic mixture (LM) composed of α-tocopherol, ascorbyl palmitate, and citric acid; and apply these mixtures in crude, and stripped flaxseed oils. The oxidative stability of crude oil samples containing HM and LM is comparable to that of artificial compounds mixture (ethylenediamine tetraacetic acid [EDTA] and terc-butyl hydroquinone [TBHQ]). HM is more effective than LM in crude oil, but not in stripped oil, suggesting a strong influence of minor components on antioxidant activity of natural compounds.
Practical Applications: The increasing demand for foods containing polyunsaturated fatty acids (PUFA) brings the challenge of their chemical stabilization. Here, a strategic mixture of hydrophilic natural antioxidants is effective to stabilize flaxseed oil, with effectiveness comparable to that of artificial antioxidants. Therefore, this HM constitutes a feasible and efficient option to be applied by food producers, especially in formulations that present a health claim.
1 Introduction
Oxidation is the major cause of deterioration of highly unsaturated oils, limiting, for example, the utilization of flaxseed oil.1, 2 Secondary oxidation products are produced from decomposition of hydroperoxides, causing nutrient loss, off-flavors, and color alteration.3 Furthermore, potentially cytotoxic compounds, such as 4-hydroxy-trans-2-nonenal (HNE), 4-hydroxy-trans-2-hexenal (HHE), acrolein, and malondialdehyde (MDA) can also be formed. These compounds are involved in the progression of many chronic diseases and are applied as biomarkers in clinical studies.3-5
Lipid oxidation is affected by several factors, such as oxygen, temperature, light, presence of minor components, and degree of fatty acids unsaturation.3, 6-9 Oils with a high proportion of PUFA, containing 1,4–pentadiene system in their structure, tend to show much lower oxidative stability than those containing a lower proportion of PUFA.9, 10
One strategy to control oxidation is the use of antioxidants.11 According to the “Polar Paradox Theory,” polar antioxidants tend to be more active in bulk oils while nonpolar antioxidants tend to be more active in oil-in-water emulsions.12, 13 For this reason, in order to provide maximum oxidative stability, antioxidant compounds should preferentially concentrate in the place where oxidation occurs in a higher rate, that is, at the interface of association colloids.14, 15 Therefore, the antioxidant polarity is an important factor to consider when selecting antioxidants to be applied in highly unsaturated lipid systems.12, 13, 16
Synthetic antioxidants are widely used in the food industry, such as EDTA and TBHQ.11 However, due to their controversial effects on human health, there is an increasing demand for natural antioxidants, for example, vitamins, phenolic compounds, carotenoids, among others.17 Given the limited number of approved antioxidants for food applications, several strategies have been attempted to improve antioxidant performance. One possible strategy is to combine substances able to exert antioxidant effect by different mechanisms, such as radical scavengers, metal chelators, and molecules regenerator.18 In this context, radical scavengers, such as tocopherol and phenolic compounds, could be mixed to compounds able to regenerate oxidized molecules, for example, ascorbic acid and metal chelators, for instance citric acid.19-21
In a previous study carried out by our group, Espinosa et al.21 showed that among 11 natural phenolic compounds found in red propolis, sinapic acid presented the highest antioxidant activity in emulsions containing oil rich in PUFA. Therefore, considering the importance of mixing natural compounds with different antioxidant mechanisms and the influence of antioxidant polarity on its effectiveness, the objective of this study was to evaluate the effect of a hydrophilic or a lipophilic antioxidants mixture on the oxidative stability of crude and stripped flaxseed oil.
2 Experimental Section
2.1 Material and Chemicals
Cold pressed flaxseed oil was purchased from Pazze Indústria de Alimentos Ltda (Panambi/Rio Grande do Sul, Brazil), stored in the dark at room temperature (20 °C). Ascorbyl palmitate, ascorbic acid, α-tocopherol, citric acid, sinapic acid, silicic acid, and activated charcoal were purchased from Sigma-Aldrich (Sigma Chemical, St. Louis, MO, USA). All reagents were HPLC grade.
2.2 Study Design
To compose the LM, α-tocopherol was used as radical scavenger and ascorbyl palmitate as α-tocopherol regenerator. To formulate the HM, sinapic acid was applied as radical scavenger while ascorbic acid was selected as sinapic acid regenerator. In both mixtures, citric acid was used as metal chelator. The proportion of compounds in each mixture was defined according to previous studies.12, 22-29 Bulk oils with high proportion of PUFA naturally contain minor compounds with pro or antioxidant activity. For this reason, the antioxidant effectiveness of the mixtures was evaluated in crude flaxseed oil and in flaxseed oil stripped from its minor compounds. Table 1 shows the proportion of each compound applied to formulate LM and HM. It was included a sample composed only of bulk oil without addition of antioxidants (CONT), as well as a sample containing a mixture of TBHQ and EDTA, which are approved artificial antioxidants (ART). All combinations presented in Table 1 were initially dissolved in ethanol (1 mL) and added to 10 mL of crude or stripped flaxseed oil in 20 mL -amber vials. Afterward, ethanol was evaporated under nitrogen and opened vials were stored at 40 °C for 15 days.30 After this period, chemical markers of lipid oxidation (peroxide value [PV], malondyaldehyde [MDA], and volatile compounds), fatty acids, sinapic acid, and α-tocopherol contents were evaluated.
2.3 Methods
2.3.1 Flaxseed Oil Stripping
Flaxseed oil had its minor compounds stripped according to the methodology proposed by Khan and Shahidi,6 and modified by Waraho et al.31 using an open-column chromatographic system (29.5 mm internal diameter, 350 mm in length; Wilmad Labglass, New Jersey, USA) connected to a vacuum system. For the stripping process, 30 g of flaxseed oil was dissolved in 60 mL of hexane, introduced into the column and eluted for 30 min, followed by the addition of 100 mL (three times) and 70 mL of hexane. Eluates were collected in glass flasks immersed in an ice bath. Finally, hexane was vaccum-evaporated (Heildolph Rotavac Valve Tec., Germany) for 30 min at 30 °C. To remove traces of hexane, samples were flushed with nitrogen. Stripped oils were protected from light during all the procedure and stored at −80 °C until use.
2.3.2 Determination of PV
PV was determined according to Shanta and Decker.32 The absorbance of samples was measured at 510 nm using a UV–vis mini 1240 spectrophotometer. A standard curve was prepared with known concentrations of cumene hydroperoxide (CHP) (0.03–0.93 mg CHP; R2 = 0.9978) and samples were analyzed in quintuplicate. Results were expressed as meq O2/L oil.
2.3.3 Determination of MDA Content
MDA concentration was determined by High-Performance Liquid Chromatography (HPLC) (model 1200, Agilent Technologies Inc., Palo Ato, USA) following the protocol of Hong et al.33 Samples were analyzed utilizing a reverse-phase C18 column (250 mm × 4.6 mm; 5 μm; Phenomenex, Torrance, California, USA) and quantified by fluorescence detection at an excitation wavelength of 515 nm and emission of 553 nm. The HPLC pump delivered an isocratic mobile phase composed of 60% phosphate buffer (50 mmol, pH 7.4) and 40% methanol at a flow rate of 1.0 mL min−1. A standard curve was prepared using 1,1,3,3-tetraetoxipropano (TEP) (24.3–185.9 μg mL−1 TEP; R2 = 0.996), analysis were carried out in quintuplicate and results expressed as mg MDA/L oil.
2.3.4 Fatty Acids Composition
Oil samples (10 mg) were transferred to screw-caped tubes containing 1 mg of tricosanoic acid methyl ester (C23:0) as internal standard, 50 µL of 0.5% BHT ethanol solution, and 1 mL of 0.5 M methanolic NaOH, according to Shirai et al.34 Samples were placed in a boiling water bath for 5 min, then cooled at room temperature. After that, 2 mL of BF3 were added to the samples and boiled in water bath for 5 min. Samples were cooled at room temperature and 1 mL of isooctane was added, followed by vigorous homogenization. Then, 5 mL of a saturated NaCl solution was added and samples homogenized gently. The organic phase was extracted and dried. The samples were re-suspended in 500 μL of isooctane, transferred to vials and analyzed in triplicate. Fatty acids quantification was carried out using a gas chromatography equipped with a G3243A MS detector (Agilent 7890A GC System; Agilent Technologies Inc.). A fused silica capillary column (J&W DB-23 Agilent 122–236; 60 m × 250 mm inner diameter) was used to separate sample compounds. Injection volume was 1 µL. High-purity Helium was used as the carrier gas at a flow rate of 1.3 mL min−1 with a split injection of 50:1. The oven temperature was programed from 80 °C to 175 °C at a rate of 5 °C min−1, followed by another gradient of 3 °C min−1 to 230 °C, which was maintained for 5 min. The GC inlet and transfer line temperatures were 250 and 280 °C, respectively. GC-MS was performed using 70 eV EI in scan acquisition and recorded as TIC. All mass spectra were acquired over an m/z range of 40–500. Fatty acids in samples were tentatively identified by NIST mass library (National Institute of Standards and Technology [NIST]; Gaithersburg, MD, USA) and by comparing the retention times with those of four purified standard mixtures of fatty acid methyl esters (4-7801; 47085-U; 49453-U and 47885-U; Sigma Chemical Co.). Results were expressed as % of fatty acids or mg g−1 oil.
2.3.5 Determination of Tocopherol and Sinapic Acid Content
Tocopherol and sinapic acid concentration were performed using HPLC (model 1200, Agilent Technologies Inc.). Compounds separation was carried on a reverse-phase C18 column (Agilent, Santa Clara, California, USA, 150 × 4.6 mm2; 5 µm). Tocopherol content in oils was determined using a modified method from Gliszczyńska-Świgło & Sikorska.35 Oils (100 μL) were diluted in 1 mL isopropanol and injected into HPLC, using an isocratic mobile phase consisting of 50% acetonitrile and 50% methanol, at a flow rate of 1 mL min−1 and at 30 °C. The eluate was detected using a fluorescent detector set at a wavelength of 295 nm (excitation) and 325 nm (emission). A calibration curve was prepared using standards (α-tocopherol: 0–1046.4 ppm, R2 = 0.9974 and γ-tocopherol: 0–4317 ppm, R2 = 0.9996) and analysis were carried out in triplicate. Results were expressed as ppm. To determinate sinapic acid concentration in oils supplemented with the HM, 100 μL oil was diluted in 1 mL of isopropanol, in duplicate and injected in the HPLC using a gradient elution adapted from da Silveira et al.36 consisting of acetonitrile (A), and an aqueous formic acid solution 0.1% (B). (A) increased from 8% to 92% in 11 min. The flow rate was of 1 mL min−1 and the column temperature was maintained at 30 °C. The eluate was detected using a diode array detector set at a wavelength of 325 nm. Identification of sinapic acid was carried out by comparing the retention time and UV–vis spectra of peaks in samples to those of genuine standard, and quantification was performed using a standard curve (97–1147.56 ppm, R2 = 0.9931). Results were expressed as ppm.
2.3.6 Determination of Volatile Compounds
The volatile content in oil samples was determined according to Gómez-Cortés et al.37 with some modifications. Oil samples (10 mL) were sealed in 20 mL glass vials (10 mL of headspace). A Combi PAL autosampler was used for automated SPME analysis. The vials were agitated (400 rpm) for 15 min at 50 °C. A 50/30 µm Stableflex 23Ga fiber (DivynilBenzene/Carboxen/Polydimethylsiloxane; DVB/CAR/PMDS – 57298-U; Supelco; Bellefonte, PA, USA) was inserted into the headspace and the vial was agitated for 60 min at 50 °C. The fiber was injected into the GC-MS (Agilent 7890A GC System, Agilent Technologies Inc.), at 250 °C for 60 min, using a splitless mode. During the GC analysis, the fiber thermally cleaned at 250 °C for 60 min. A capillary column (19091S-433UI HP-5 ms Ultra Inert, 30 m, 0.25 mm, 0.25 μm, 5%-Phenyl-methylpolysiloxane, Agilent Technologies) was used. Ultra-pure Helium was the carrier gas at a constant flow of 1 mL min−1. The oven temperature started at 40 °C 5 min−1, increased to 100 °C at 4 °C min−1, to 220 °C at 17 °C min−1 and held at 220 °C for 10 min. Volatile compounds identification was carried out using a G3243A MS detector (Agilent 7890 A GC System, Agilent technologies Inc.). The ion source and quadrupole temperatures were 230 and 150 °C, respectively. All mass spectra were acquired in the electron-impact mode with an ionization voltage of 70 eV, adopting a mass range of m/z 35–350. Scan monitoring was used as the data acquisition mode. Compounds were tentatively identified (>70% of match) by using NIST mass library, and analysis were performed in duplicate, except for CONT that was just analyzed one time. Results were expressed as area ratio of the sample to internal standard (mbik).
2.4 Statistical Analysis
Results were expressed as mean ± standard error of mean (SEM). Sinapic acid and α-tocopherol contents in the oils (crude and stripped) were compared by Mann–Whitney, while the four treatments were compared by one way ANOVA followed by Tukey test, using software Statistica v. 13.4 (TIBCO Software Inc, Round Rode, Texas, USA). An alpha value of 5% was adopted to reject the null hypothesis.
3 Results
Table 2 exhibits the fatty acid composition, PV, and γ-tocopherol concentration of crude and stripped flaxseed oils. No difference was observed in the fatty acids proportion and PV was in accordance with legislation, <15 meq O2 kg−1 oil, 26 confirming that the stripping procedure was adequately performed. In addition, the absence of γ-tocopherol in stripped oil suggested that minor components were removed by the column. Oxidative markers measured in the samples treated with ART, HM, and LM are shown in Figure 1. All treatments showed reduction of PV compared to CONT, indicating that all antioxidants mixtures were effective to protect both crude and stripped oils from oxidation. Figure 1a shows PV for crude flaxseed oil. Among the mixtures, LM samples showed the highest PV (p < 0.001), while there was no difference between ART and HM (p = 0.991). This result suggests that HM was as effective as ART and promoted a better antioxidant protection than LM. In contrast, for stripped oils (Figure 1b), no difference was found in PV among the mixtures.
| Fatty acids [%] | Crude | Stripped |
|---|---|---|
| 16:0 | 11.58 ± 1.12 | 11.12 ± 0,60 |
| 16:1 n7 | 1.01 ± 0.00 | 0.18 ± 0.05 |
| 18:0 | 8.79 ± 0.36 | 9.20 ± 0,73 |
| 18:1 n9 c | 22.48 ± 0.89 | 26.38 ± 0.46 |
| 18:2 n6 c (LNA) | 14.51 ± 0.29 | 16.04 ±0,34 |
| 18:3 n6 (GLA) | 0.42 ± 0.00 | - |
| 18:3 n3 (ALA) | 41.86 ± 2.85 | 36.75 ± 1.09 |
| 20:0 | 0.24 ± 0.00 | 0.26 ± 0.2 |
| 22:0 | 0.10 ± 0.00 | 0.18 ± 0.02 |
| SFA | 20.60 ± 1.71 | 20.77 ± 1.35 |
| MUFA | 22.99 ± 1.39 | 26.56 ± 0.50 |
| PUFA | 56.58 ± 2.93 | 52.79 ± 1.22 |
| PV (meq O2/L) | 1.38 ± 0.24 | 3.85 ± 0.27 |
| γ-Tocopherol (ppm) | 288.73 ± 10.72 | – |
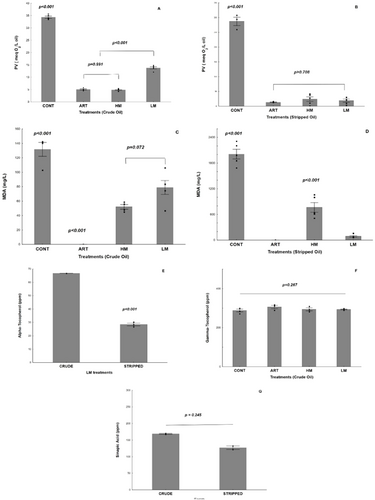
MDA concentration in crude and stripped oils is shown in Figure 1c and d, respectively. MDA was not detected in crude flaxseed oil treated with ART (Figure 1c), while HM and LM did not differ from one another. However, there was a trend of lower MDA content in HM samples than in LM samples (p = 0.072), showing that the HM could also be more effective at reducing MDA formation in crude oil (Figure 1c). Contrarily, in stripped samples (Figure 1d), the MDA content was higher in HM than in LM (p < 0.001), suggesting an influence of minor compounds on the formation of secondary products of lipid oxidation.
Flaxseed oil contains γ-tocopherol in its composition and just traces of α-tocopherol. Thus, the concentration of α-tocopherol was only determined in the oils added of LM (Figure 1e). Compared to the initial added amount of α-tocopherol in LM (500 ppm), it was found a higher reduction in stripped oils (94.31%) than in crude oils (86.64%). As the stripping procedure removes all tocopherol isomers, γ-tocopherol concentration was only determined in crude oil (Figure 1f), with no change observed among the treatments (p = 0.267), suggesting that, in our model, γ-tocopherol did not exert any antioxidant action in crude oil stored at 40 °C/15 days. This assumption was confirmed by comparing the γ-tocopherol concentration in crude oils before (292.69 ± 7.04 ppm) and after oxidation (293.79 ± 2.88 ppm) taking into account the mean value of the four treatments (p = 0.673). Figure 1g shows the consumption of sinapic acid in crude and stripped oils added of HM. Although no difference in the sinapic acid content (p = 0.245) was observed between crude and stripped oils (167.28 ± 3.40 ppm and 139.81 ± 22.27 ppm, respectively), almost half of the initial added concentration (500 ppm) was consumed during oil oxidation. There was not observed sinapic acid peak at 325 nm on crude oil sample, confirming no interference of minor compounds on sinapic acid quantification.
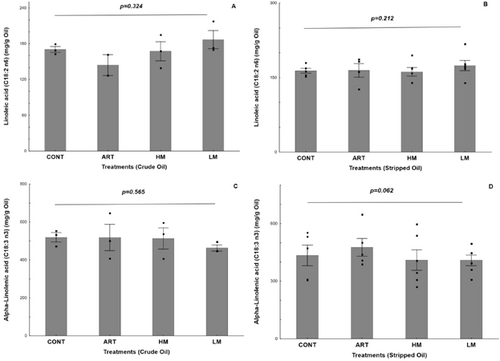
Linoleic (LNA) and α-linolenic (ALA) acids represent the major PUFA in flaxseed oil and their contents are shown in Figure 2. In general, fatty acids concentrations are not used as oxidation markers because a significant increase in primary and secondary oxidation products can be observed without any significant change in fatty acids content. This statement was confirmed in our study with regard to LNA and ALA measured in crude and stripped oils (Figure 2a–d).
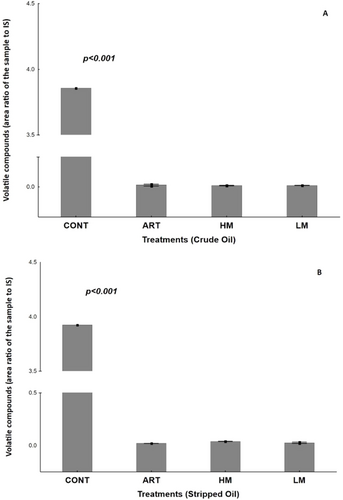
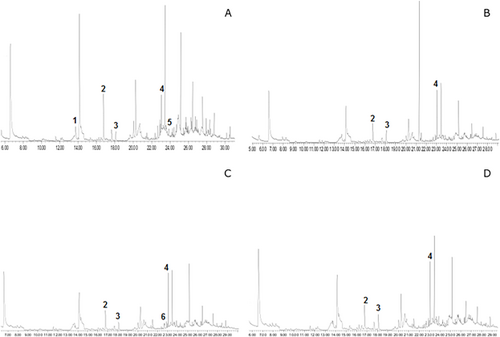
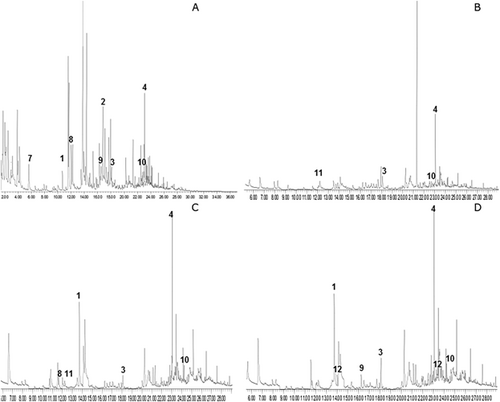
Volatile compounds were also measured as secondary oxidation products. All treatments reduced total volatile compounds (Figure 3) when compared with CONT, here adopted as a reference value, for both crude (Figure 3a) and stripped samples (Figure 3b). Although no differences were found in the total volatile compounds content among LM, HM and ART samples, the volatile profile changed. Table 3 presents the major volatiles identified in the samples, being 3.5-octadien-2-one, 2.4-heptadienal, nonanal, and tridecane found both in crude and stripped oils. However, 2.4-heptadienal and 3.5-ocatedien-2-one were only found in CONT groups of crude and stripped samples respectively. Figures 4 and 5 show a typical chromatogram obtained for each sample. Major peaks in crude (Figure 4a–d) and stripped oils (Figure 5a–d) were highlighted.
| OIL | Treatment | 2.4-Heptadienal | 3.5 Octadien-2-one | Nonanal | Tridecane | Octadecanoic acid | Nonanoic acid | 2-Octenal | Tetradecane | 2.4-Hexadienal |
|---|---|---|---|---|---|---|---|---|---|---|
| Crude | ||||||||||
| CONT | 0.611 | 1.864 | 0.381 | 0.596 | 0.402 | – | – | – | – | |
| ART | ND | 0.006 ± 0.002 | 0.003 ± 0.001 | 0.006 ± 0.002 | – | – | – | – | – | |
| HM | ND | 0.004 ± 0.000 | 0.002 ± 0.000 | 0.002 ± 0.002 | – | 0.003 ± 0.000 | – | – | – | |
| LM | ND | 0.005 ± 0.001 | 0.002 ± 0.000 | 0.004 ± 0.000 | – | – | – | – | – | |
| Stripped | CONT | 0.209 | 0.928 | 0.281 | 0.443 | 0.606 | 0.693 | 0.613 | 0.147 | – |
| ART | 0.002 | – | 0.006 ± 0.000 | 0.013 ± 0.001 | – | – | – | – | – | |
| HM | 0.015 ± 0.000 | – | 0.002 ± 0.000 | 0.016 ± 0.000 | – | 0.001 ± 0.000 | – | – | 0.001 ± 0.000 | |
| LM | 0.013 ± 0.004 | – | 0.004 ± 0,001 | 0.009 ± 0.001 | – | – | – | – | – |
4 Discussion
Our results indicated that both LM and HM mixtures showed antioxidant activity in crude and stripped oils. However, the mixtures behaved differently according to the type of oil. HM showed better antioxidant effect in crude flaxseed oil than LM, as evidenced by the lower PV and trend of lower MDA concentration. In contrast, for stripped oil, there was no difference in PV between LM and HM, but the MDA content was lower in LM than in HM, indicating that LM were, globally, more effective at protecting stripped flaxseed oil.
Results for crude flaxseed oil agree with the “Polar Paradox Theory,” which states that polar antioxidants are more effective in bulk oils than nonpolar ones.13 Although the “Polar Paradox” has been consistently confirmed by several studies,38-40 others have reported contradicting conclusions. For example, Pereira-Caro et al.41 observed that the chain length of hydroxytyrosol alkyl esters (C0–C18) did not affect their antioxidant effectiveness in stripped sunflower oils. In another study, Mohanan et al.2 found that ascorbyl palmitate was better at protecting crude flaxseed oil from oxidation than the more hydrophilic tannic acid.
Oils are complex systems containing minor surface-active compounds, such as hydroperoxides, amphiphilic molecules (phospholipids, mono, and diacylglycerols), natural antioxidants (carotenoids, tocopherols), sterols, transition metals, and traces of water. These minor surface-active compounds have been reported to play a key role in the oxidation rate due to the formation of association colloids.7, 42 Association colloids are structures composed of an aqueous core stabilized by amphiphilic compounds, with the polar head directed to the aqueous phase and the nonpolar tails facing the oil phase.1 Studies have evidenced that oxidation is most prevalent at the water-oil interface of association colloids, where surface-active compounds accumulate, favoring, or retarding lipid oxidation.7, 39, 43 Thus, it is possible that the more polar compounds in HM partitioned into the water phase while those in LM would locate farther from oxidation sites.1, 38 This fact could explain why HM was more effective than LM in crude flaxseed oil.
In contrast, in stripped flaxseed oil, HM, and LM showed equivalent PV. Oils stripped from their minor compounds have their surface-active compounds partially or fully removed, drastically reducing the amount of association colloids.7, 42, 43 Thus, it is possible that factors other than polarity may have driven the antioxidant action. For example, it is possible that in the absence of association colloids, antioxidants did not partition according to their hydrophobicity, leading hydrophilic, and lipophilic antioxidants to have comparable effectiveness as radical scavengers, as reported by Laguerre et al.42 in a study with chlorogenic acid alkyl esters (C2-C16) applied to stripped corn oil.
Stripped oil samples treated with LM had lower levels of MDA than those treated with HM. It is possible that the absence or lower amount of association colloids impaired the action of hydrophilic antioxidants in inhibting hydroperoxide decomposition, suggesting that other mechanisms are involved in this reaction.38, 39 For instance, Frankel,44 reported that the hydrogen donating ability of α-tocopherol inhibits the formation of MDA precursors. It was found a higher consumption of α-tocopherol in stripped than in crude oil. Therefore, we suppose that in the absence of association colloids, the higher solubility of α-tocopherol in stripped than in crude oils could have been more significant than polarity for oil oxidative protection.
In our study, γ-tocopherol did not exert any antioxidant protection in crude flaxseed oil, as its concentration did not vary significantly between fresh and oxidized oil samples. In a previous study, Nogueira et al.45 oxidized flaxseed oil at 60 °C for 15 days and observed total depletion of γ-tocopherol. Thus, the lack of γ-tocopherol depletion can be partially attributed to the lower temperature used in this study. In addition, crude flaxseed oil presents other compounds with antioxidant activity such as β-carotene and plastochromanol-8, a γ-tocotrienol homologue. β-Carotene, that can inhibit autoxidation and shows singlet oxygen chelator properties, is more susceptible to degradation than tocopherols.46 Plastochromanol-8 has antioxidant capacity similar to that of γ-tocopherol, only differing in size chain length and lipophilicity, and could have been consumed at 40 °C before γ-tocopherol,46, 47 However, specific analysis of these two compounds must be carried out to confirm this hypothesis. Similar depletion of sinapic acid was observed in crude and stripped oils added of HM, suggesting that when considered only antioxidant activity of sinapic acid, it was not influenced by association colloids and minor compounds presence.
Volatile compounds are also secondary products formed from the decomposition of hydroperoxides. Compounds tentatively identified in crude and stripped samples mainly comprised aldehydes, ketones, and alkanes, and have been reported in other studies that analyzed vegetable oils oxidation.48, 49 In general, nonanal and tridecane showed up in all oxidized samples, independent on the applied treatment. However, it was observed that the volatile profile varied among samples. These differences suggest that the type of antioxidant (HM or LM), presence of association colloids, and oil minor compounds affect the pathways of volatile compounds formation, influencing the type and amount of volatile compounds formed from flaxseed oil oxidation. Despite of the profile differences, total volatile compounds content indicated that HM and LM had comparable effectiveness at protecting both crude and stripped oils. This study contributed to a practical application aiming to replace artificial by natural antioxidants in foods, but more analysis must be done to better clarify the chemical mechanisms involved in these results.
5 Conclusions
HM containing three natural organic acids showed better antioxidant protection of crude flaxseed oil than LM. Moreover, HM presented comparable efficacy to ART in preventing the formation of primary and secondary oxidation products. Our results also suggested that combining different mechanisms of the already approved natural compounds could be a good strategy to provide oxidative stability of oils rich in PUFA, with a high potential to replace artificial antioxidants.
Abbreviations
4-HHE, 4-hydroxy-hexanal; 4-HNE, 4-hydroxy-nonenal; ALA, α-linolenic acid; ART, artificial antioxidant mixture; CONT, sample without antioxidant; HM, hydrophilic mixture; LM, lipophilic mixture; LNA, linoleic acid; MDA, malondialdehyde; PUFA, polyunsaturated fatty acids; PV, peroxide value.
Acknowledgements
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES, and Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (grant n° 2017/ 08066–1).
Conflict of Interest
The authors declare no conflict of interest.




