Characterization of Key Odorants in Moroccan Argan Oil by Aroma Extract Dilution Analysis
Abstract
The aroma-active compounds of Moroccan argan oil are sensorily and instrumentally analyzed via gas chromatography-mass spectrometry-olfactometry (GC-MS-O). The purge and trap extraction (PTE) method is used for the extraction of volatile components. A total of 35 aroma compounds are determined including mostly alcohols and pyrazines, and some carboxylic acids, pyrroles, furans, lactones, volatile phenols, an aldehyde, and a ketone. An aroma extract dilution analysis of the aromatic fraction of argan oil isolated by the PTE method reveals 19 key odorants with flavor dilution (FD) factors ranging from 4 to 512, among which nonanal, 2,5-dimethyl-3-ethylpyrazine and 2,3-diethyl-5-methylpyrazine show the highest FD factors of 512. As for the principal scents perceived by all panelists, characteristic odor notes in argan oil are found to be roasty, nutty, fatty, earthy, and cheesy.
Practical Applications: Nut oil is one of the most widely consumed oils in many countries. The outcomes of this investigation provide valuable information for elucidation of the key odorants and aroma composition of the well-known and expensive Moroccan argan oil. Aroma is a crucial quality parameter of a foodstuff which directly influences customer preferences. Therefore, determining the key odorants of argan oil's aromatic extract isolated by purge and trap methodology is of major importance for the argan oil sector. The purge and trap extraction set for separation followed by analysis and characterization of those compounds via gas chromatography–mass spectrometry-olfactometry (GC-MS-O) is an effective practical application tool for aroma description in valuable and expensive oil samples. It is observed that the characteristic odor notes of argan oil are related to the few most powerful aroma-active compounds using the aroma extract dilution analysis technique.
The aroma-active compounds of Moroccan argan oil are sensorily and instrumentally analyzed via gas chromatography-mass spectrometry-olfactometry (GC-MS-O). The purge and trap extraction (PTE) method is used for the extraction of volatile components. A total of 19 aroma compounds are detected as aroma active by AEDA method among 35 volatiles. Characteristic odor notes in argan oil are found to be roasty, nutty, fatty, earthy, and cheesy.
1 Introduction
Argan oil, which is extracted from roasted argan (Argania spinosa L.) seeds/fruit kernels grown endemically in Morocco, is used as a cosmetic, pharmaceutical, and edible product.1 This oil contains high amounts of unsaturated (up to 80%) and saturated (up to 20%) fatty acids. In addition to minor components, argan oil includes polyphenols, tocopherols, sterols, squalene, triterpene alcohols, and volatile compounds. In fact, this oil is known for its interesting nutritional value due to a high percentage of unsaturated fatty acids and is appreciated for its particular taste and aroma. Edible argan oil is produced from roasted kernels. The oil preparative process includes fruit picking, sun-drying, peeling, kernel collection, and roasting and oil extraction.1 Therefore, the roasting process of argan seeds can dramatically affect the formation of some specific aroma compounds, particularly key odorants. Moreover, this oil is now referred as one of the world's most expensive oils.2 It not only reduces plasma cholesterol and LDL levels, but also prevents high blood pressure.3 In addition to the health benefits, it is also reported to be highly valuable in culinary with the use as a seasoning or cooking oil due to its rich flavor.4
Oil processing influences its color, flavor composition, quality, and stability. A large number of chemical compounds with different classes such as aldehydes, hydrocarbons, ketones, and furans participate in the final aroma of argan oil. Most of these compounds are produced by oxidation of fatty acid after enzymatic reactions occurring in the presence of oxygen.5 Harhar et al.6 identified the volatile compounds in fresh and dried pulp fruits of Argania spinosa L. Hydrodistillation, steam distillation, and microwave-assisted methods were used to extract the volatile oils. Oxygenated terpene derivatives are found as the main constituents in the essential oil of the argan fruit pulp. Camphor was defined as the major component in all oil samples. Recently, Gracka et al.7 determined the volatiles and aroma active compounds of raw and roasted argan oils by using the HS-SPME and SAFE methods, respectively. Additionally, El-Monfalouti et al.5 determined the volatile compounds formed during argan kernel roasting by using the solid phase micro-extraction (SPME) technique. It was reported that the roasting process played a vital role to enhance the aroma of the final product because of the formation of Strecker degradation, lipid peroxidation, and Maillard reaction.4
Organoleptic properties are principle determining agents for oil consumer preferences. Among these features, aroma is a prevailing factor which has a significant role in the quality of oils. In order to clarify unexplored effects of particular aroma compounds on the general odor, the gas chromatography-olfactometry (GC-O) method was stressed as a more effective research on food aroma science. Therefore, the GC-MS-O method is a useful one applied in aroma studies to directly characterize the key odorants in food stuffs.8 This technique can be used to detect odor-active compounds present in very small amounts with a concentration above the threshold. The relative aroma intensity of compounds could be evaluated using a technique called aroma extract dilution analysis (AEDA), including GC-MS-O assessment of dilution series of aroma extract.9 Although there is a considerable amount of extant literature on the active compounds of olive oils, there are only a limited numbers of studies available on applying the GC-O technique to decode the potent aroma compounds of seed oils.4, 10, 11
Data reported in the available literature regarding the aroma and aroma-active profiles of argan oils is still very scarce. Hence, the aim of current investigation was primarily focused on determining the aroma profile of argan oil by applying the PTE system and, secondarily, on the characterization of the potent odorants by applying AEDA.
2 Experimental Section
2.1 Reagents and Samples
Water used during the analysis was subjected to a cleansing process by a Millipore-Q system (Millipore Corp., Saint-Quentin, France). Chemicals such as anhydrous sodium sulfate, dichloromethane, and internal standard for aroma analysis (4-nonanol) were obtained from Merck (Darmstadt, Germany). Standards for the aroma compounds were purchased from Sigma–Aldrich (Steinheim, Germany).
Argan fruits were collected from the Taroudant region in Morocco at the end of July, 2017. Fruits were air-dried for 2 weeks in order to discard the peel and pulp and to easily obtain the argan nuts. By hitting the argan nuts with a stone, almonds or kernels were separated from the argan shell. To prepeare the edible argan oil, kernels were gently roasted at 100 °C in clay plates for 30 min.12 The oils of roasted (alimentary use) kernels were extracted by using a cold press oil machine (Kaltpressung OI Maschine DYM 500). After pressing, the oils were filtered and kept in dark glass bottles and purged with nitrogen. To keep oxidation as low as possible, the oil samples were stored in a refrigerator (4 °C) until use. All the analyses under study were performed in triplicate.
2.2 Extraction of Volatile Compounds
The aromatic extracts of argan oil were obtained via the purge and trap extraction methodology. Special cartridges, prepared with an adsorbent, namely Lichrolut EN (200 mg, Merck), were used to trap the volatiles. This material was already declared as a suitable material for the retention of volatiles in an earlier study.13 A flow-meter, connected to a separator system to control carrier gas flow, splits the flow into diverse channels to purge samples simultaneously. Two needles were placed onto the vial providing the connections of the cartridge and nitrogen gas source by a septum to permit the purging and trapping of the volatiles. Shortly thereafter, a 20 mL vial was prepared by removing the oxygen present in the head space using nitrogen gas. Next, an 8 mL of sample was placed into the vial. The samples were pre-incubated at 60 °C for 10 min. Last, the extraction was performed for 1–1/2 h with a nitrogen flow rate equal to 500 mL min−1. Eventually, removal of the aroma compounds trapped inside of the cartridge was conducted delicately with a transition by dichloromethane (12 mL). Dehydration of the content of dichloromethane and volatiles was done with a sodium sulfate filtration, and then the isolated aromatic extract was decreased to 5 mL by using a Kuderna Danish concentrator (Sigma–Aldrich, St. Louis, USA). Eventually, the volume of aromatic extract diminished up to 200 µL with nitrogen flow. Aromatic extracts were kept at −20 °C until analysis.
2.3 GC-MS-Olfactometry Analysis
A combined GC (Agilent 6890), FID (flame ionization detector), mass selective detector (Agilent 5973-MSD) and a sniffing (Gerstel ODP-2, Maryland, USA) system was used to perform aroma and aroma-active analysis.
2.4 AEDA Analysis
To perform a characterization on the potent aroma compounds of argan oil, the AEDA methodology was employed.16 This analysis was performed by diluting the aroma extracts with dichloromethane step by step in the rates of 1:1, 1:2, 1:4, 1:8, 1:16,…,1:512 and so forth. Three experienced panelists smelled the aromatic extracts using an olfactometric port in a GC-MS-O device.11 The dilutions and sniffing procedure continued until no odor was smelled. During this procedure, each odor perceived from the olfactometric port was represented as flavor dilution (FD) factor comprising 4, 8, 12…, 512, and so forth. The ratio mentioned above meant that the greater the FD factor of a key aroma compound, the more effective it was on the aroma profile.17, 18
2.5 Representativeness Test of the Aromatic Extract/Sensory Descriptive Analysis
2.5.1 Panel, Preparation and Presentation of the Samples
In the sensory analysis, a panel composed of 10 assessors (five females and five males between the ages of 23 and 45) was selected from the Food Engineering Department of Cukurova University in Adana, Turkey. Each panelist was trained to distinguish scents precisely.
In the current study, a cardboard sniffing strip (Granger-Veyron, Lyas, France) was applied to determine the representativeness of the aroma extracts. The extant literature shows that the cardboard strips are suitable for the representativeness test of some oils and spices.19, 20 For the representativeness tests, a 10 mL of argan oil aroma extract was poured in a labeled 25 mL brown flask as a reference. The aromatic extracts of argan oil were adsorbed by a cardboard strip to perform a comparison for representativeness test. Further information on this part of the work was mentioned in our earlier study.8
2.5.2 Intensity and Similarity Tests
In the sensory analysis, similarity and intensity tests are quite important to determine the closeness of argan oil and its aromatic extracts. The smell and the retentions of the aroma were memorized by panelists simultaneously with a reference sample to determine the similarity and intensity of their scents. A 100 mm of grade-less scale was used for each test. The distance marked of the sample on the scale was measured by reading it as the centimeters from the left part.
2.6 Descriptive Analysis
Nine descriptors composed of phenol, roasty, fatty, nutty, alcohol, cheesy, green, buttery, and earthy that provides their decisive aroma were determined by the expert panelists. Aroma profile analyses were performed by orthonasally scoring descriptors given above on a 100 mm scale anchored on both sides for the intensity of attributes by “none” and “very strong.”
2.7 Statistical Analysis
Analysis of the variance test (t-test) was carried out on data obtained from the sensorial scores of reference (original) sample and its aromatic extracts using the software package SPSS, version 22.0 (Chicago, IL, USA).
3 Results and Discussions
3.1 Sensory Analysis
3.1.1 Odor Sensory Profiles
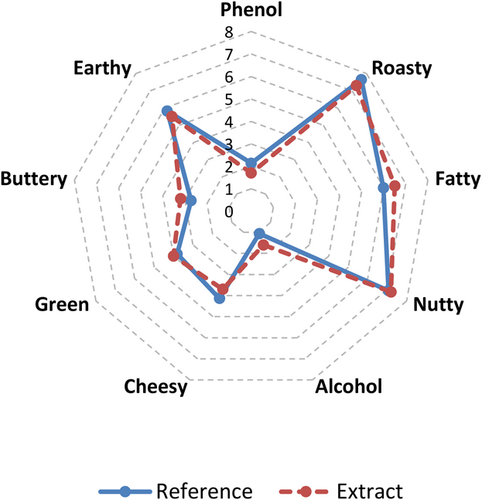
The aromatic extracts obtained from the argan oil were compared with the original argan oil sample (reference sample) for odor by 10 panelists. The principle intensity ratings for the original (reference) sample and their extracts were drawn on a spider graph employing nine descriptors (Figure 1). Odor descriptors of the original (reference) sample and their extracts were depicted as phenol, roasty, fatty, nutty, alcohol, cheesy, green, buttery, and earthy (Figure 1). To the best of our knowledge, this is the first detailed report about the sensory analysis of argan oil. Among the descriptors, roasty, nutty, fatty, and earthy notes had the highest scores, while phenol, alcohol, green, buttery, and cheesy exhibited the lowest scores. Figure 1 demonstrates it is clear that the organoleptic attributes of aromatic extracts were congruently evaluated by the panelists. In addition, no statistical differences were detected between the origin (reference) sample and the aromatic extracts based on the nine descriptors. The results showed that the most dominant flavor was roasty, nutty, and earthy, which is known to be formed mainly by pyrazines and their derivatives. In this manner, it can be proven that the presence of pyrazines played a vital role in the unique flavor of argan oils. In addition, fatty acid derived from products via thermal degradation provided fatty and cheesy notes to the argan oil.
3.1.2 Similarity and Intensity Evaluation of Aromatic Extract
The main purpose of the similarity and intensity evaluation tests was to compare the representativeness of the odor of the aromatic extract with that of the reference sample. The outcome of the similarity and intensity values of extract was satisfactory. The similarity scores of the obtained aromatic extract using the PTE technique on smelling strips was detected to be 71 mm on a 100-mm unstructured scale. The obtained average similarity scores in the same extract was established as 76 mm. According to comparison with the vast literature review, the similarity score of the aromatic extract was found to be at reasonable levels. For instance, in an earlier study Amanpour et al.8 measured the similarity scores of extracts isolated by four different methodologies from the Iranian saffron samples as 71.8, 61.1, 55.4, and 37.8 mm on a 100-mm unstructured scale. In another study, the similarity score of the aromatic extract obtained from the cherry tomato was detected as 70.4 mm by Selli et al.21
3.2 Aroma Compounds of Argan Oil
In the present study, Table 1 shows that the aroma compounds of the Moroccan argan oil were identified, quantified, and enumerated by means of detected aroma groups and their linear retention indexes. A total of 35 volatile compounds were identified in the oil samples. Most of volatiles detected in this study were consistent with those of previously published studies conducting with thermal processing of seed oils such as argan, sesame oil, and so forth.22 In the case of argan oil, pyrazines and pyrroles play crucial role by giving unique taste and aroma to this product. It is mentioned in the extant literature that the distinctive nutty scent of argan oil mainly occurs due to pyrazines formed from the Maillard type non-enzymatic reactions between reducing sugar and free amino acid during the roasting process.23 According to the results of our study, the main aroma groups of argan oil were alcohols, pyrazines, pyrroles and organic acids, which constituted of 20.94, 27.56, 22.21, and 17.48%, respectively. These compounds represented over 88% of the total volatiles in argan oil. According to the results of the quantification analysis performed by FID, the total aroma concentration of argan oil was found to be 15 868 µg kg−1. Apart from those groups, furans, lactones, volatile phenols, a ketone, and an aldehyde were other volatile groups detected in this work.
| No. | LRIa) | LRIb) | Aroma compounds | Concentrationc) | Identificationd) |
|---|---|---|---|---|---|
| Alcohols | |||||
| C1 | 102126 | 1009 | 2-Methyl-2-butanol | 276 ± 23.7 | LRI,MS,Std |
| C2 | 117719 | 1182 | 3-Penten-2-ol | 187 ± 5.9 | LRI,MS,Std |
| C3 | 121827 | 1216 | Isoamyl alcohol | 1612 ± 11.4 | LRI,MS,Std |
| C4 | 125328 | 1230 | Pentanol | 326 ± 15.7 | LRI,MS,Std |
| C5 | 124529 | 1254 | 2-Hexanol | 222 ± 12.1 | LRI,MS,Std |
| C6 | 155630 | 1545 | 2,3-Butanediol | 195 ± 6.1 | LRI,MS,Std |
| C7 | 157831 | 1578 | 1,3-Butanediol | 249 ± 14.8 | LRI,MS |
| C8 | 160332 | 1591 | 1,2-Propanediol | 125 ± 0.9 | LRI,MS,Std |
| C9 | 187928 | 1881 | Benzyl alcohol | 75 ± 1.3 | LRI,MS,Std |
| C10 | 193728 | 1916 | Phenylethyl alcohol | 55 ± 2.0 | LRI,MS,Std |
| Total | 3322 ± 94 | ||||
| – | |||||
| Pyrazines | |||||
| C11 | 127228 | 1263 | Methyl pyrazine | 1099 ± 13.1 | LRI,MS,Std |
| C12 | 132928 | 1297 | 2,5-Dimethylpyrazine | 1181 ± 40.1 | LRI,MS,Std |
| C13 | 135233 | 1325 | 2,6-Dimethylpyrazine | 393 ± 10.7 | LRI,MS,Std |
| C14 | 134328 | 1343 | Ethyl pyrazine | 164 ± 9.4 | LRI,MS |
| C15 | 140133 | 1367 | 2-Ethyl-6-methylpyrazine | 177 ± 9.3 | LRI,MS,Std |
| C16 | 139835 | 1386 | 2-Ethyl-5-methylpyrazine | 257 ± 14.9 | LRI,MS,Std |
| C17 | 142633 | 1411 | 2,3,5-Trimethylpyrazine | 431 ± 21.9 | LRI,MS,Std |
| C18 | 144734 | 1459 | 2,5-Dimethyl-3-ethyl pyrazine | 409 ± 17.1 | LRI,MS,Std |
| C19 | 148835 | 1470 | 2,3-Diethyl-5-methyl pyrazine | 262 ± 3.3 | LRI,MS,Std |
| Total | 4373 ± 140 | ||||
| – | |||||
| Acids | |||||
| C20 | 145036 | 1450 | Acetic acid | 1432 ± 24.3 | LRI,MS,Std |
| C21 | 154436 | 1538 | Propanoic acid | 324 ± 1.4 | LRI,MS,Std |
| C22 | 155435 | 1562 | Isobutanoic acid | 291 ± 2.2 | LRI,MS,Std |
| C23 | 163336 | 1628 | Butanoic acid | 218 ± 3.1 | LRI,MS,Std |
| C24 | 172237 | 1671 | 2-Methylbutanoic acid | 341 ± 13.3 | LRI,MS |
| C25 | 186137 | 1854 | Hexanoic acid | 168 ± 2.0 | LRI,MS,Std |
| Total | 2774 ± 46 | ||||
| – | |||||
| Furans | |||||
| C26 | 167038 | 1651 | Furfuryl alcohol | 439 ± 1.9 | LRI,MS,Std |
| C27 | 172934 | 1753 | 5-Methylfurfuryl alcohol | 294 ± 19.6 | LRI,MS,Std |
| Total | 733 ± 21.5 | ||||
| – | |||||
| Pyrroles | |||||
| C28 | 113939 | 1153 | 1-Methyl-1H-pyrrole | 3363 ± 14.7 | LRI,MS |
| C29 | 205740 | 2010 | 2-Formylpyrrole | 162 ± 1.5 | LRI,MS,Std |
| Total | 3525 ± 16.2 | ||||
| – | |||||
| Lactones | |||||
| C30 | 158941 | 1600 | γ-Valerolactone | 190 ± 0.6 | LRI,MS,Std |
| C31 | 164036 | 1614 | γ-Butyrolactone | 399 ± 3.7 | LRI,MS,Std |
| Total | 589 ± 4.37 | ||||
| – | |||||
| Phenols | |||||
| C32 | 199035 | 1989 | Phenol | 46 ± 0.3 | LRI,MS,Std |
| C33 | 208336 | 2090 | p-Cresol | 110 ± 3.5 | LRI,MS,Std |
| Total | 156 ± 3.83 | ||||
| – | |||||
| Ketone | |||||
| C34 | 129342 | 1287 | 2-Octanone | 190 ± 5.7 | LRI,MS,Std |
| – | |||||
| Aldehyde | |||||
| C35 | 139031 | 1395 | Nonanal | 206 ± 4.5 | LRI,MS,Std |
| – | |||||
| General Total | 15868 ± 341 | ||||
- a)LRI retention indices obtained from literature.17, 24-40 b)LRI retention indices on DB-WAX column. c)Concentration mean values based on three repetitions as μg kg−1. d)Identification methods of identification; LRI (linear retention index), Std (chemical standard); when only MS or LRI is available for the identification of compounds, it must be considered as an attempt of identification.
Among all the aroma groups, alcohols were the most dominant ones in terms of number of identified compounds. A total of 10 different volatile alcohols were identified and quantified. Isoamyl alcohol (1612 µg kg−1) represented the highest concentration. Similarly, compared to some vegetable and kernel oils, this alcohol also existed in argan oil.23, 24 In addition, 2-methyl-2-butanol, 3-penten-2-ol, pentanol, 2-hexanol, 2,3-butanediol, 1,3-butanediol, 1,2-propanediol, benzyl alcohol, and phenylethyl alcohol were other volatile alcohols found in argan oil. Total concentration of alcohols was found to be 9202 µg kg−1.
Pyrazines, known as typical nutty and burnt notes contributors, were the second major aroma group which comprised 19.97% of overall aroma compounds concentration in argan oil. Among the pyrazines, 2,5-dimethylpyrazine and methyl pyrazine were the two dominant aroma compounds found in argan oil (1181 and 1099 µg kg−1, respectively). Both of these compounds were also found in other nut oils, especially those treated with roasting during their production. Due to the increasing temperature during the pre-roasting or roasting processes of argan kernels, their concentrations drastically increased and provided its unique burnt, nutty flavor.6 Moreover, as the human nose is very sensitive to pyrazine compounds, they play an important role in consumer preferences.
Another important and thermal relevant aroma group found in argan oil was pyrroles, which impart burnt and earthy characteristic odors in several kernel oils. Pyrroles identified in the present study were also previously determined in the extant literature, especially in sesame oil. According to the results, 1-methyl-1H-pyrrole and 2-formylpyrrole were found in argan oil with a concentration of 3363 and 162 µg kg−1.
Similarly, compared to other seed oils, fatty acid derived volatile organic acids were also present in argan oil due to oxygen reactions with unsaturated fatty acids which comprise hydroperoxides, and then revealed several volatile and non-volatile secondary compounds. Higher temperatures drastically contribute to the formation of volatile compound through lipid oxidation. The extant literature mentions that acid compounds were one of the important aroma groups.5 In the present study, acetic, propanoic, isobutanoic, butanoic, 2-methylbutanoic and hexanoic acids were the identified organic acids in argan oil. In particular, acetic acid was found in higher concentration.
Furans are frequently found in most seed oils. These compounds occur in case of either lipid peroxidation or degradation of carbohydrates. Among furans, furfuryl alcohol and 5-methylfurfuryl alcohol were identified in argan oil samples with the concentration of 439 and 294 µg kg−1. Those volatiles are important compounds, formed via degradation of carbohydrates since they possess flavor properties and mainly occur incrementally with the increase of heat during roasting process.5
Gamma lactones such as γ-valerolactone and γ-butyrolactone found in this work were also mentioned in an earlier study as a thermal process derived from volatiles.25 Similarly, in the case of argan oil, occurrence of these compounds was probably formed via the roasting process of argan kernels.
3.3 GC-MS-O Results
The potent aroma compounds of the Moroccan argan oil were characterized by AEDA using the GC-MS-O technique to determine the FD factors. The results of the olfactometric analysis are summarized in Table 2.
| No. | Aroma-active compounds | LRIa) | Odor descriptionb) | FD factorc) | OT (ppm)d) | OAVe) | References |
|---|---|---|---|---|---|---|---|
| 1 | Isoamyl alcohol | 1216 | Alcohol | 64 | 100 | 16.12 | 43 |
| 2 | Pentanol | 1230 | Green | 16 | 1.502 | 2.17 | 44 |
| 3 | 2-Octanone | 1287 | Overripe fruit | 32 | 50 | 3.81 | 45 |
| 4 | 2-Hexanol | 1254 | Green, fatty | 128 | 15.2 | 14.63 | 46 |
| 5 | 2,5-Dimethylpyrazine | 1297 | Roasty | 256 | 80 | 14.77 | 47 |
| 6 | 2,6-Dimethylpyrazine | 1325 | Roasty | 16 | 400 | ≤1 | |
| 7 | 2-Ethyl-5-methylpyrazine | 1386 | Nutty | 256 | 16 | 16.06 | 47 |
| 8 | Nonanal | 1395 | Fatty | 512 | 1 | 206 | 46 |
| 9 | 2,5-Dimethyl-3-ethyl pyrazine | 1459 | Nutty | 512 | 8.6 | 47.56 | 45 |
| 10 | 2,3-Diethyl-5-methyl pyrazine | 1470 | Earthy | 512 | nd | – | |
| 11 | Unknown 1 | 1483 | Roasty | 16 | |||
| 12 | Propanoic acid | 1538 | Fatty | 32 | 100 | 3.23 | 48 |
| 13 | Isobutanoic acid | 1562 | Cheesy | 256 | 1 0 | 30.00 | 48 |
| 14 | Butanoic acid | 1628 | Buttery | 4 | 204 | 1.07 | 46 |
| 15 | Unknown 2 | 1664 | Floral | 16 | |||
| 16 | 2-Methylbutanoic acid | 1671 | Buttery | 4 | 180 | 1.89 | 46 |
| 17 | Unknown 3 | 1706 | Wet | 32 | |||
| 18 | Hexanoic acid | 1854 | Cheesy | 8 | 93 | 1.80 | 49 |
| 19 | Unknown 4 | 1896 | Spicy | 64 | |||
| 20 | p-Cresol | 2090 | Phenol | 16 | 55 | 2.00 | 50 |
- a) LRI retention indices on DB-WAX column. b)Odor description as perceived by trained panelists during olfactometry. c)FD factor is the highest dilution of the extract at which an odorant is determined by AEDA. d)Odor threshold values of aroma-active compounds. e)The OAV obtained by dividing concentration of the compounds by their threshold.
The odor variability of argan oil was affected by the kernel roasting and oil extraction process. Based on the AEDA, a total of 20 odor-active compounds characterized the argan oil including alcohols (3), pyrazines (5), organic acids (5), a ketone, an aldehyde and a phenol with FD factors in the range of 4 and 512 FD. In addition, Table 2 shows four unknown compounds perceived at the sniffing port of chromatography, but not determined by the GC-MS.
The only aldehyde detected in argan oil extract was nonanal which represented the strongest (FD = 512) aroma-active compound providing a fatty odor. As stated in Table 2, the OAV of this compound was calculated as the strongest smell of argan. This aldehyde was also found as the main aroma component of the pistachio oil.10
Odors detected in argan oil including the roasty, nutty, and earthy notes were associated with five pyrazine derivatives: 2,5-dimethyl-3-ethyl pyrazine and 2,3-diethyl-5-methyl pyrazine (FD = 512), 2-ethyl-5-methylpyrazine and 2,5-dimethylpyrazine (FD = 256), and 2,6-dimethylpyrazine (FD = 16). These pyrazines were detected as potent aroma-active compounds in the studied sample, representing 57% of the total pyrazine compounds identified in argan oil. Additionally, they considered aroma-active notes with roasty, nutty, and earthy as the typical odor of argan oil. The majority of the above-mentioned key pyrazines have already been detected in other edible oils.11
Acids were important as aroma-active compounds in argan oil. Isobutanoic acid (FD = 256, cheesy) was one of the main contributors of argan oil aroma, followed by propanoic acid (FD = 32, fatty odor), hexanoic acid (FD = 8, cheesy odor). This result was also supported by calculated OAVs provided in Table 2. Isobutanoic acid possessed the highest OAV, followed by propanoic acid in argan oil. In addition, 2-methylbutanoic acid and butanoic acid (FD = 4) were also aroma-active acids, which were mildly contributed to the aroma of argan oil providing a buttery odor note.
Alcohols were also important as key aroma compounds in the argan oil sample. 2-Hexanol was the main aroma-active alcohol of argan oil (FD = 128), associating with green and fatty odor notes, followed by isoamyl alcohol (FD = 64, alcohol). Pentanol (FD = 16) was another aroma-active compound known as the main contributor to the green note of argan oil aroma.
2-Octanone was the aroma-active ketone compound detected by olfactometry analysis with 32 FD, providing an overripe fruit odor. Furthermore, four unknown compounds contributed to the overall aroma of argan oil. Unknown I (LRI = 1483), a key odorant of argan oil providing a roasty attribute, was detected with the highest FD factor equal to 16. Unknown II (LRI = 1664, FD = 16) and unknown III (LRI = 1706, FD = 32) provided floral and wet odor notes which contributed to overall aroma of argan oil, while unknown IV (LRI = 1896) with a 64 FD factor provided a spicy odor note.
The fatty, roasty, nutty, earthy, and cheesy notes with the highest FD factors relatively dominated the characteristic odor of argan oils obtained from roasted argan kernels. This result was also consistent with the results of the sensory analysis.
4 Conclusions
In the current study, the objective was to determine the aroma and aroma-active compounds of the edible Moroccan argan oil. In this manner, the detailed characterization of argan oil aroma, key odorants and determination of sensory analysis were performed for the first time, particularly for aroma-active compounds. As for the sensory analysis, an aromatic extract demonstrated highly similar description scores. A total of 35 aroma compounds were identified in this oil including mainly alcohols, pyrazines, pyrroles, and organic acids.
Alcohols were determined as the most abundant chemical group by means of number of identified volatiles. Alcohols followed by pyrazines and pyrrole compounds were the main contributors to the overall scent of argan oil. Many heat-derived compounds were identified in the sample due to the roasting process applied in argan seeds before oil production.
According to the data revealed from olfactometric analysis a total of 19 odor-active compounds characterized the argan oil including alcohols (3), pyrazines (4), acids (5), phenol (1), aldehyde (1), ketone (1), and unknown compounds (4). The strongest aroma-active compound of argan oil was found to be nonanal (fatty odor note), 2,5-dimethyl-3-ethyl pyrazine (nutty odor note) and 2,3-diethyl-5-methyl pyrazine (earthy odor note). These findings were in accordance with their OAVs. In addition, 2,5-dimethylpyrazine, 2-ethyl-5-methylpyrazine, and isobutanoic acid represented the second important aroma-active compounds (FD = 256) with roasty, nutty, and cheesy notes.
Conflict of Interest
The authors declare no conflict of interest.




