The oxidative stability of microalgae oil (Schizochytrium aggregatum) and its antioxidant activity after simulated gastrointestinal digestion: Relationship with constituents
Abstract
The objective of this study was to examine the chemical composition and oxidative stability of microalgae oil, also to explore in vitro bioaccessibility and antioxidant activity of microalgae oil after simulated gastrointestinal digestion. In total, more than 50 fatty acids were identified by GC–MS analysis, with both palmitic acid (38.3%) and DHA (34.5%) being identified as major fatty acids. The contents of total phenolics and flavonoids in the various solvent extracts were measured spectrometrically, and their amounts were 39.33 ± 0.34 µg gallic acid/g and 16.08 ± 4.3 µg rutin/g, respectively. HPLC analysis showed that the contents of β-carotene, α-tocopherols, β- and γ-tocopherols (not separated) and δ-tocopherols were 136 µg/100 g, 164.4 µg/g, 317.3 µg/g, and 43.2 µg/g, respectively. Concerning sterols, cholesterol was the principal sterol at 4210.5 mg/kg and the other six main sterols were campesterol (121.4 mg/kg), 24-methylene cholesterol (192.8 mg/kg), 24-methyl-colest-7-en-3β-ol (144.6 mg/kg), ergosterol (144.8 mg/kg), stigmasterol (260.1 mg/kg) and Δ7,24-stigmastadienol (150.5 mg/kg), respectively. The overall chemical properties of the tested oils indicated that microalgae oil had a great oil quality. A Schaal oven test was used to evaluate the oxidative stability of microalgae oil. Furthermore, in vitro simulated gastrointestinal digestion was performed, and the antioxidant ability of digestion oil was determined by using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging assay, a 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical cation decolourisation activity assay, a reducing power assay, a β-carotene bleaching assay and an oxygen radical absorbance capacity (ORAC) antioxidant assay. The results showed that following simulated gastrointestinal digestion, microalgae oil displayed a good in vitro bioaccessibility and moderate antioxidant capacity. Thus, the antioxidant activity of the microalgae oil was mainly contributed by its abundant antioxidant constituents.
Practical applications: Schizochytrium aggregatum oil is a good source of DHA and of effective, bioaccessible antioxidants.
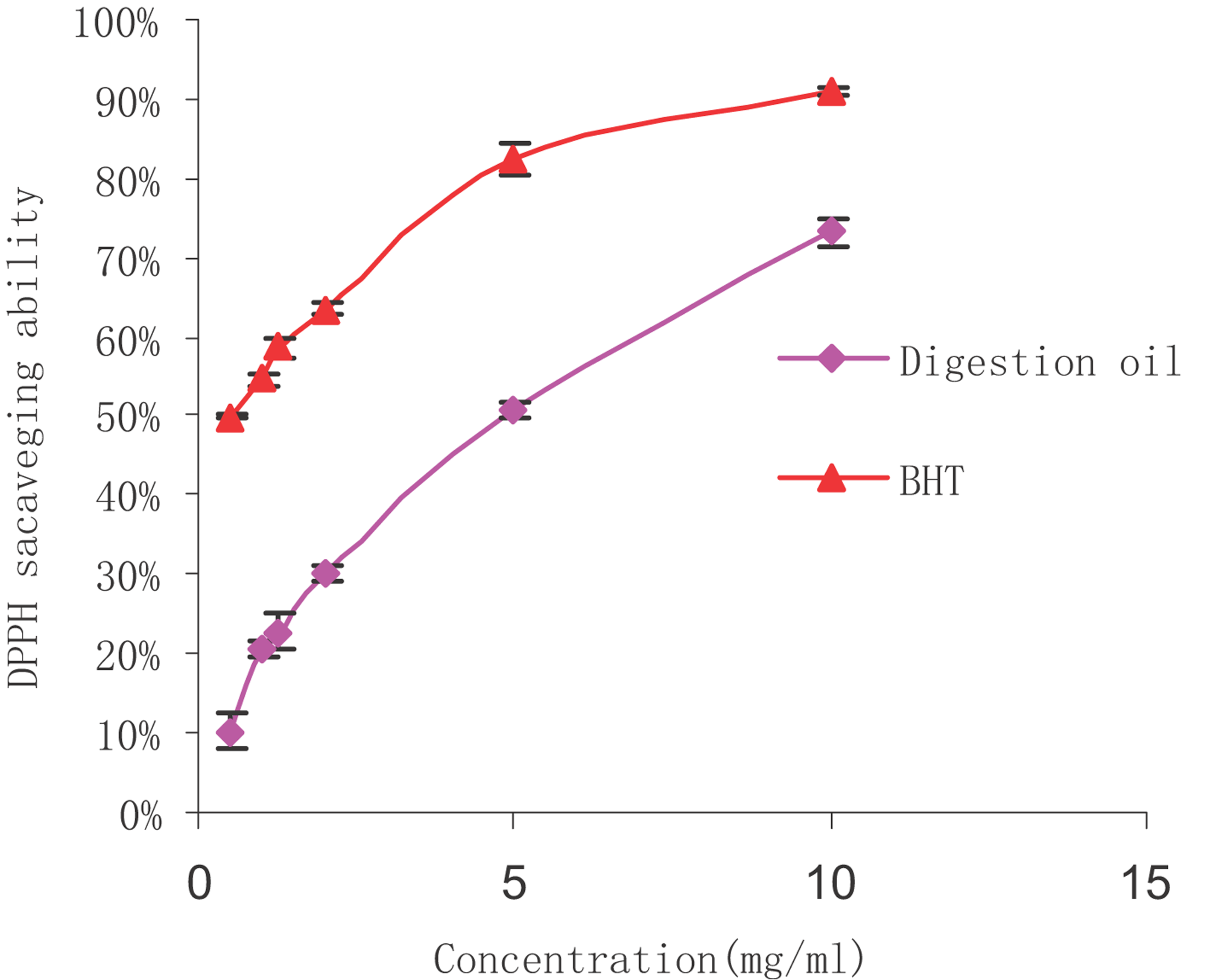
The DPPH radical is rather stable, and our study used a relatively fast method for measuring both the hydrophilic and lipophilic substances during in vitro antioxidant activity. The change of violet colour to yellow during the DPPH assay is thought to be due to the attribution of hydrogen atoms by antioxidant substances. The extent of decolourisation is a significant indicator of the sample's antioxidant ability. As shown in figure that the scavenging abilities of all the samples were well correlated with increasing concentrations, and the scavenging activity of BHT was higher than that of microalgae oil. At concentrations ranging from 0.5 to 10 mg/mL, the DPPH radical scavenging ability of digestion oil was determined at 6.8–64.9%. Lower IC50 values indicated a higher radical scavenging activity. The IC50 value of microalgae oil in our DPPH radical scavenging study was 5.76 mg/mL, and solutions of BHT had an IC50 value of 0.56 mg/mL. Although microalgae oil was shown to be weaker than BHT, it demonstrated a fairly good scavenging ability. The DPPH radical scavenging abilities of digestion oil and BHT.
Abbreviations
-
- AAPH
-
- 2,2′-azobis(2-amidinopropane)dihydrochloride
-
- ABTS
-
- 3-ethylbenzothiazoline-6-sulfonic acid
-
- DPPH
-
- 2,2-diphenyl-1-picrylhydrazyl
-
- MDA
-
- malondialdehyde
-
- ORAC
-
- oxygen radical absorbance capacity
1 Introduction
The exogenous substances and endogenous metabolites in the body may produce ROS, which are generated endogenously through different enzymes such as NADPH oxidase, xanthine oxidase (XO) and cytochrome P450 as well as through the mitochondrial respiratory chain 1. ROS are capable of oxidising various biomolecules, which can result in DNA damages, carcinogenesis, cell death and tissue damage. In healthy bodies, ROS production is constantly balanced by enzymatic and non-enzymatic antioxidant defenses. Oxidative stress can lead to a physiological imbalance of pro-oxidants over antioxidants, resulting in possible damage to the organism 2. ROS including hydroxyl radicals, superoxide anions, hydrogen peroxide and non-radical compounds are formed by different mechanisms. These types of ROS are closely related with many chronic and degenerative diseases such as atherosclerosis, cancer, ageing, cardiovascular illnesses and other inflammatory diseases 3, 4. Antioxidants are known to effectively eliminate free radicals and to have some preventive and therapeutic effects on a number of disorders and diseases 5, 6.
Schizochytrium sp., a heterotrophic algae, contains large amounts of DHA. DHA was widely applied as a nutritional ingredient in foods and it was mainly extracted from fatty fish such as tuna, mackerel, herring and salmon. However, algae are the primary producers of DHA in the ecosystem, whilst fatty fish are short of the necessary enzymatic activities to produce this fatty acid. Also, the accumulation of DHA in fatty fish is mainly attributed to the consumption of algae. DHA-rich lipids drawn from algae have considerable advantages over those drawn from the conventional fish oil source. DHA from algae has no seasonal variation, is free from any fishy smell, does not cause marine pollution and has lower processing costs. DHA from algae also has fewer of the toxic persistent organic pollutants that accumulate in fish 7, 8. Researchers have revealed that in addition to DHA's significant role in basic brain and visual functions 9, 10, there is a positive correlation between the appropriate intake of DHA and a range of health benefits. These benefits include the suppression of vascular proliferation or inflammation 11, 12, strong cardioprotective effects 13, 14, protective and suppressive effects on colon cancer 15, 16, reductions in various diseases including diabetes 17 and Parkinson's disease 18 and an anti-ageing function 19.
In researching the effects of food ingredients, in vitro methods for simulating the gastrointestinal digestive tract are now widely used, as these methods are simple, rapid, safe and do not involve the ethical restrictions required for in vivo methods 20. Indeed, whereas in vitro methods have limitations such as constituting only a static model of digestion, the more dynamic measurement of bioavailability with in vitro models can be well correlated with outcomes obtained from animal models and human studies 21. In vitro methods have been extensively used for bioaccessibility assessment and the determination of changes in dietary components during the digestive tract. Such methods have been used to test the processing of foods such as polyphenols, anthocyanins and flavanones 22-24. However, relatively few studies have been done to evaluate the bioaccessibility and antioxidant ability of microalgae oil after the digestion process.
In this study, the constituents of microalgae oil (refined) were systematically analysed, and an in vitro model of the gastrointestinal tract was used to simulate the chemical and biological gastrointestinal conditions. The aim of this investigation was to explore the constituents and oxidative stability of microalgae oil and to evaluate the bioaccessibility and antioxidant ability of digested samples through tests involving radical scavenging methods.
2 Chemicals and methods
2.1 Chemicals
Microalgae oil was provided by Runke Bioengineering Co. Ltd. (Shantou, China). Sodium BF3/methanol (14% w/w) reagent, Folin–Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid (Vc), butylated hydroxytoluene and 2,2′-azobis(2-amidinopropane)dihydrochloride (AAPH) were all purchased from Sigma–Aldrich Co. LLC. (St. Louis, MO, USA). Lecithin, 3-ethylbenzothiazoline-6-sulfonic acid (ABTS), β-carotene and α-tocopherol were bought from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan). Rutin, fluorescein and thiobarbituric acid (TBA) were supplied by Guangzhou Qiyun Bioengineering Co. Ltd. (Guangzhou, China). Taurodeoxycholate (NaTDC) was purchased from Tianjin Heowns Biochemical Technology Co., Ltd. (Tianjin, China). Neocuproine was bought from Chengdu Geleixiya Chemical Technology Co., Ltd. (Chengdu, China) and methyl-β-cyclodextrin was provided by Aladdin Industrial Corporation (Shanghai, China). All other solvents and chemicals were of at least analytical grade.
2.2 Identification and quantification of fatty acids by GC–MS
The fatty acid composition of microalgae oil was analysed using a modification of the method developed by Li et al. 25. In summary, 0.2 g of microalgae oil was first saponified in 5 mL of methanol NaOH solution for 10 min at 60°C and then esterified at 60°C for 30 min in BF3/methanol (14% w/w) reagent. One millilitre of n-hexane and 1 mL of distilled water were added to the mixture, then the upper n-hexane layer was drawn out for filtration to a volume of 1.5 mL. The sample was then analysed by a GC–MS (Shimadzu QP-2010, Japan) equipped with a DB-5ms capillary column (30 m × 0.25 mm). Helium was used as the carrier gas, with a flow rate of 0.5 mL/min and a 20:1 split ratio. The temperature was set to rise from 35 to 230°C at 5°C/min and the injector temperature was 250°C. The injection volume was 1 µL. Fatty acids were determined by comparing their retention times and were quantified by the area normalisation method.
2.3 Extraction and determination of total phenolic content and flavonoid content
The phenolic fractions and flavonoid parts were extracted based on the modified method developed by Servili et al. 26. Five groups of samples of oil (2 g each) were obtained and added with 3 mL of different solvents, respectively. The five solvents added were (a) 3 mL of methanol, (b) 3 mL of ethanol, (c) 2 mL of methanol mixed with 1 mL water, (d) 2 mL of ethanol together with 1 mL of water and (e) 3 mL of water. These solutions were marked as methods a–e, respectively. Then the solutions were shaken vigorously in a vortex apparatus for 5 min and centrifuged at 10 000 rpm for 5 min to obtain the solvent layers for each sample. The extractions were done in triplicate.
The total phenolic contents were determined according to the Ping's method 27. In each case, 1 mL of extraction was added to 2 mL of Folin–Ciocalteu reagent and mixed with 2 mL of 20% Na2CO3. The compound was then shaken thoroughly and left to react in darkness for 1 h. The absorbance was determined at 760 nm. A calibration curve of gallic acid was plotted, and the total phenolics were expressed as gallic acid equivalents (µg/g).
The total flavonoid content was measured using the method proposed by Dobravalskyte et al. 28 with a tiny modification. One millilitre of the extraction was added to 1 mL of aluminium chloride (3%) and then left at RT for 40 min. The absorbance was determined at 415 nm. The calibration curve of rutin was obtained, and the flavonoid content was expressed as a rutin equivalent.
2.4 Determination of β-carotene
β-Carotene was determined with the modified method of GB/T 5009.83-2003 29. In summary, 10 g of the sample was added to a flask and mixed with petroleum ether/acetone (8:2 v/v) to extract several times until the upper layer was colourless. After rotary evaporation under vacuum pressure, the sample was dissolved in a small amount of petroleum ether and purified with an alumina column chromatography petroleum in which ether/acetone (95:5 v/v) acted as the eluent. Then the sample was made up to a constant volume of 10 mL for filtration, and 20 µL of the sample was injected into a Spherisorb C18 (4.6 mm × 150 mm). Measurement was done at a wavelength of 448 nm, with methanol/acetonitrile (9:1) treated as a mobile phase at a flow rate of 1.2 mL/min. The standard curve of β-carotene was obtained, and the β-carotene content of the sample was obtained from the calibration curve.
2.5 Determination of tocopherols
Tocopherols were analysed by HPLC according to the standard method of GB/T 5009.82-2003 30. First, 2 g of sample was added to a flask mixed with 30 mL of ethanol, 5 mL of ascorbic acid (10%), 2 mL of benzopyrene solution (10 µg/mL) and 10 mL of KOH (50%). The flask was placed in a boiling water bath circumfluence to saponify for 30 min. Then the mixture was extracted, washed with water and concentrated in a rotary evaporator. The concentrated liquor was dissolved in 2 mL of ethanol and centrifuged at 5000 rpm. Twenty microlitres of the upper solution was subjected to the HPLC system (Agilent 1100, Japan) with an Ultrasphere ODS column (25 cm × 4.6 mm × 5 µm). Detection was performed by the fluorescence detector set at the 298 nm (excitation) and 325 nm (emission) wavelengths, and the mobile phase was methanol/water (98:2) at a rate of 1.7 mL/min. Benzopyrene was compared as an internal standard substance and the standard curves of α-, β-, γ-, and δ-tocopherols were obtained. The tocopherols contents were quantified by an internal standard method.
2.6 Identification and quantification of sterols
The oil was pretreated following a procedure described by ISO 12228: 1999 31. The sample was then analysed by a GC–MS equipped with a DB1701ms capillary column (30 m × 0.25 mm × 0.25 µm). The column temperature was held at 120°C for 2 min, and then programmed to 270°C at 20°C/min, held for 25 min. The detector temperature was set at 300°C. Helium was used as the carrier gas at a flow rate of 1.2 mL/min. An internal standard, cholestanol, was used for sterols quantification. Sterols were determined by comparing their retention times and were quantified by the internal standard method. The sample was analysed in triplicate.
2.7 Measurements of chemical properties
International Organization for Standardization (ISO) methods were used for the determination of peroxide value, acid value, water content and unsaponifiable matter of the oil according to ISO 3960: 2005 32, ISO 660: 2005 33, ISO 665: 2000 34 and ISO 3569: 2000 35, respectively. The TBA value was determined according to the method of Wang et al. 36.
2.8 Storage oxidative stability test
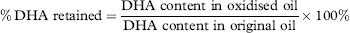
2.9 In vitro simulated gastrointestinal digestion
In vitro digestion experiments were done as described by Sek et al. 37. The standard digestion buffer contained 50 mM of tris-maleate (pH 7.5), 150 mM of NaCl and 5 mM of CaCl2 · 2H2O. NaTDC was chosen as a source of bile salt, and lecithin (LC) was used as a model phospholipid to prepare phospholipid/bile salt mixed micelles. The ratio of bile salt to LC used was 4:1, which was consistent with the ratio in human bile 38, 39. The LC/NaTDC micellar solutions were obtained by dissolving the lecithin in chloroform and evaporating the chloroform under a vacuum at 45°C for 5 min, generating a thin film on the bottom of the flask. Then, NaTDC was added and dissolved with the digestion buffer. The mixture was equilibrated for 12 h to form clear, pale yellow liquor.
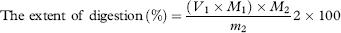
2.10 Antioxidant activity
Different methodological approaches result in scattered outcomes, which can be conflicting and hardly comparable. Many antioxidant methods are available, and because their results depend on different mechanisms, they strictly rely on different oxidant/antioxidant models applied and different lipophilic/hydrophilic balances involved 40. As different antioxidant assays are based on different mechanisms, a single assay can only produce a relative result. Therefore, this study has applied five complementary test systems to confirm the antioxidant activity of the sample.
2.10.1 DPPH radical-scavenging assay
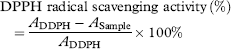
2.10.2 ABTS radical cation decolourisation activity
The radical scavenging ability was measured using a modified ABTS radical decolourisation assay 27, 42. The ABTS solution was made by mixing 5 mL of 7 mmol ABTS and 88 µL of 140 mmol potassium persulphate solution. This solution was then left in the dark at normal temperature for 16 h. A 5 mL sample of this reagent was placed in a glass petri plate and frozen at −20°C. Then the frozen sample was lyophilised in a vacuum freeze-drier (ALPHA 1-4, German Christ). Finally, FD-ABTS radical powder was dissolved in the solvent (methanol:chloroform (1:1 v/v) to produce 0.700 ± 0.020 at 752 nm. Oil was dissolved in a methanol:chloroform solution (1:1 v/v) for dilution to definite concentrations. A 2 mL FD-ABTS solution was added to 200 µL of diluted oil solution. The absorbance was determined at 752 nm after 10 min at RT against the blank. A calibration curve of α-tocopherol was plotted, and the outcomes were expressed as α-tocopherol equivalent antioxidant ability values (µmol α-tocopherol/g).
2.10.3 Reducing power assay
The reducing power of digestion on the oil was measured by using a modified version of the method developed by Özyürek et al. 43. To summarise the process, various concentrations of oil (ranging from 0.5 to 10 mg/mL) were dissolved in acetone–water (9:1 v/v) solutions that contained 2% methyl-β-cyclodextrin (M-β-CD) as a solubiliser. Then, solutions of 1 mL CuCl2 (1.0 × 10−2 M), 1 mL neocuproine (Nc) solution (7.5 × 10−3 M) and 1 mL of NH4Ac solution (1 M of ammonium acetate buffer) at pH 7.0 were mixed together with 1.2 mL of the samples. The absorbance was determined at 450 nm after 30 min at RT. BHT was compared as the control. The degree of decrease in absorbance indicated the degree of reducing power.
2.10.4 β-Carotene bleaching assay
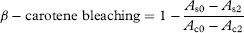
2.10.5 ORAC antioxidant assay
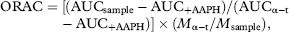
3 Results and discussion
3.1 Identification of microalgae oil fatty acids
Table 1 displays the major fatty acid contents of the microalgae oil. The oil was mainly composed of palmitic acid (C16:0, 38.3%), DHA (C22:6, 34.5%), tetradecanoic acid (C14:0, 4.6%), eicosatetraenoic acid (C20:4, 4.3%), stearic acid (C18:0, 2.4%) and pentadecanoic acid (C15:0, 1.9%). These six major fatty acids of the oil comprised more than 80% of the total fatty acids. These findings were in agreement with studies carried out by other investigators who found that the two major fatty acids in the oil were DHA and palmitic acid, and the content of DHA ranged from 30 to 45%, depending on the extraction methods used 47, 48. Moreover, it was found that microalgae oil from Scenedesmus sp. mainly contained C18:1 (49.6%) and Nannochloropsis sp. oil mainly contained C16:1 (32.9%), whilst both of them had a low polyunsaturated content and DHA was not detected, which differ with our oil 49. Whilst the oil produced from the Dinoflagellate contained DHA as much as 44.98%, which is higher than ours 49.
| Fatty acids | Commercial name | Content (%) |
|---|---|---|
| C14:0 | Tetradecanoic acid | 4.6 ± 0.02 |
| C15:0 | Pentadecanoic acid | 1.9 ± 0.01 |
| C16:0 | Palmitic acid | 38.3 ± 0.09 |
| C16:1 | hexadecenoic acid | 0.9 ± 0.02 |
| C16:2 | Hexadecadienoic acid | 0.8 ± 0.03 |
| C16:3 | hexadecatrienoic acid | 0.8 ± 0.02 |
| C17:0 | Heptadecanoic acid | 0.9 ± 0.04 |
| C18:0 | Stearic acid | 2.4 ± 0.02 |
| C18:1 | Octadecenoic acid | 3.9 ± 0.01 |
| C18:2 | Octadecenedioic acid | 0.9 ± 0.03 |
| C18:3 | Octatecatrienoic acid | 0.8 ± 0.02 |
| C20:0 | Arachidic acid | 0.7 ± 0.02 |
| C20:4 | Eicosatetraenoic acid | 4.3 ± 0.01 |
| C22:0 | Behenic acid | 0.8 ± 0.02 |
| C22:6 | DHA | 34.5 ± 0.08 |
| SFA | Saturated fatty acid | 49.6 ± 0.22 |
| MUFA | Monounsaturated fatty acid | 4.8 ± 0.03 |
| PUFA | Polyunsaturated fatty acids | 42.1 ± 0.18 |
3.2 Extraction and determination of total phenolic content and flavonoid content
As it is well established, phenolic acids, flavonoids and tannins are associated with the in vitro antioxidant ability of plants and food extracts, mainly due to their redox properties that make them act as hydrogen donors, reducing agents, hydroxyl radical quenchers, singlet oxygen quenchers and metal chelators 50. In addition, it has also been testified that in vivo (except may be in the digestive tract), polyphenols do not act directly as quencher of ROS but rather through the modulation of the expression or the activity and pro- and antioxidant enzymes 51.
The total phenolic content is listed in Table 2, as derived using a gallic acid standard curve based on y = 0.0052x + 0.003 (R2 = 0.9999). Li et al. 's 52 research showed that the phenolic content of the hexane fraction, the ethyl acetate fraction and the water fractions of Schizochytrium sp. were approximately 13.61 ± 0.01 mg GAE/g, 0.96 ± 0.01 mg GAE/g and 1.37 ± 0.03 mg GAE/g, respectively. These results showed a much higher phenolic content than ours. This difference in results may be due to the oil refining process, especially in the hydrated degumming process, leading to the loss of hydrosoluble phenolics. However, our results were higher than those obtained by Gong et al. 53. This difference in results may be due to different means used for extracting phenolics. In addition, the total phenolic content of the different fractions of 23 microalgae was evaluated in Li et al.'s 52 study and the phenolic content of hexane fractions varied from 2.12 to 39.87 mg GAE/g due to different species of microalgaes.
| Method | The total phenolics (µg GAE/g) |
|---|---|
| a | 34.93 ± 0.82 |
| b | 39.33 ± 0.34 |
| c | 22.16 ± 1.20 |
| d | 21.1 ± 0.91 |
| e | 15.54 ± 0.82 |
- a–e represent different extraction methods.
The total flavonoid content was obtained using the linear curve y = 0.0038x + 0.0609 (R2 = 0.9996) with a rutin equivalent, which when applied to method e gave a result of 16.08 ± 4.3 µg/g. The other four methods (a–d) did not produce good results, as they yielded turbid phenomena following the mixture of reagents. This outcome may be due to aluminium chloride's low solubility in methanol or ethanol.
It has been well attested that phenolics and flavonoids have great antioxidant ability 51. Although the content of these compounds in microalgae oil is not high, they do make certain contributions to the total antioxidant capacity of the oil.
3.3 Determination of β-carotene and tocopherols
The compound β-carotene has been proven to be one of the most important carotenoids in the human diet, and it plays a role in scavenging singlet molecular oxygen and peroxyl radicals as antioxidants 54, 55. The current tests indicated that β-carotene was the major carotenoid in microalgae oil, with a content of 136 µg/100 g. This result was consistent with Gong et al.'s study 53, which showed that DHA oil extracted from Schizochytrium aggregatum had abundant carotene.
It is also well established that natural oils in general, particularly microalgae oils, contain high amounts of vitamin E. The vitamin E content measured in our assays is shown in Table 2. The contents of α-tocopherols, β- and, γ-tocopherols (not separated) and δ-tocopherols were 164, 317 and 43.2 µg/g, respectively. Higher amounts of tocopherols were detected by Zhang 56, who found that the α-tocopherol content in microalgae oil was 234 µg/g, the β- and γ-tocopherols (not separated) were 676 µg/g, and the δ-tocopherol content was 109 µg/g. These results were nearly double those found in our study (Table 3).
| Tocopherols | Content (µg/g) |
|---|---|
| α-Tocopherols | 164.4 ± 8.1 |
| β- and γ-tocopherols | 317.3 ± 11.2 |
| δ-Tocopherols | 43.2 ± 4.3 |
3.4 Identification and quantification of sterols
Sterols are important bioactive compounds of oils, which are known due to their good physiological function and great antioxidation activity. Sterol composition for microalgae oil in Table 4 is presented. The oil contained a high amount of sterols at 5224.6 mg/kg. We identified seven sterols and cholesterol was the principal sterol in all the sterols and constituted 4210.5 mg/kg. The other six main sterols were campesterol (121.4 mg/kg), 24-methylene cholesterol (192.8 mg/kg), 24-methyl-colest-7-en-3β-ol (144.6 mg/kg), ergosterol (144.8 mg/kg), stigmasterol (260.1 mg/kg) and Δ7,24-stigmastadienol (150.5 mg/kg), respectively. To the best of our knowledge, there are no published reports describing sterols in microalgae oil, eliminating possibility to do comparison.
| Sterols | Content (mg/kg) |
|---|---|
| Cholesterol | 4210.5 ± 29.1 |
| Campesterol | 121.4 ± 6.3 |
| 24-Methylene cholesterol | 192.8 ± 6.8 |
| 24-Methyl-colest-7-en-3β-ol | 144.6 ± 3.2 |
| Ergosterol | 144.8 ± 5.3 |
| Stigmasterol | 260.1 ± 9.5 |
| Δ7,24-Stigmastadienol | 150.5 ± 4.1 |
| Total | 5224.6 ± 59.3 |
3.5 Measurements of chemical properties
The chemical properties of the microalgae oil were evaluated as Table 5. The oil had an acid value of 0.12 mg KOH/g, a peroxide value of 0.98 meq/kg, a TBA value of 0.07 mg/kg and a water content of 0.07%, which were all rather low and reached the national standards of edible oil. In addition, it was also found that the unsaponifiable matter was 0.95%, which may mainly involved tocopherols, sterols and other minor antioxidant substances, which not only enrich the oil but also enhance the oxidative stability of the oil.
| Parameters | Results |
|---|---|
| Peroxide value (meq/kg) | 0.98 ± 0.01 |
| Acid value (mg KOH/g) | 0.12 ± 0.02 |
| Water content (%) | 0.07 ± 0.01 |
| Unsaponifiable matter (%) | 0.95 ± 0.02 |
| TBA value (mg/kg) | 0.07 ± 0.01 |
3.6 Storage oxidative stability test
A POV evaluation was done to monitor the oxidative stability during Schaal oven heating. This index is a rather sensitive indicator of oxidation levels for lipids in which peroxides or hydroperoxides are the primary products formed. Increases in heating time brought great changes in the POV, and the values were positively and strongly correlated with the exposition time. These results confirmed the oxidative effects of Schaal oven heating. The POV of microalgae oil at 60 ± 1°C for 12 days are shown in Fig. 1a. The POV of the sample exhibited an increasing trend over the 12 days. It can be seen from the figure that the POV increased slowly in the first 6 days and then ascended drastically in the following days until the final value at 12 days was 29.462. This phenomenon can be ascribed to the existence of antioxidants such as VE and phenolics in the oil. In the incipient stage, antioxidants protected the oil from oxidisation. Then, with the reduction of antioxidant substances, the oil started to deteriorate and the POV value increased quickly.
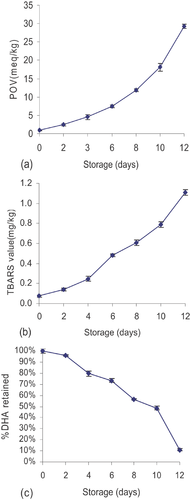
TBARS is used as an indicator for determining secondary products of oxidation. TBA reacts with MDA and forms a red compound that can be determined at 532 nm. The outcomes of our TBA assays are shown in Fig. 1b. The TBARS levels increased as the days went on, and the trend of the curve was similar to that of the POV. The TBARS values varied from 0.075 meq/kg on Day 1, to 0.48 meq/kg on Day 6, to a value of 1.108 meq/kg on Day 12. The antioxidants in the oil showed a rather strong protective effect for reducing the secondary products of oxidation.
The DHA content showed a similar trend to that of the POV and TBA values. The oxidative stability decreased during oven heating, as heat caused a degradation of polyunsaturated fatty acid, an accelerated oxidation reaction and an increase in the formation of main and secondary substances of oxidation. Hence, the antioxidant constituents in the oil demonstrated a protecting effect in the initial phase.
3.7 In vitro simulated gastrointestinal digestion
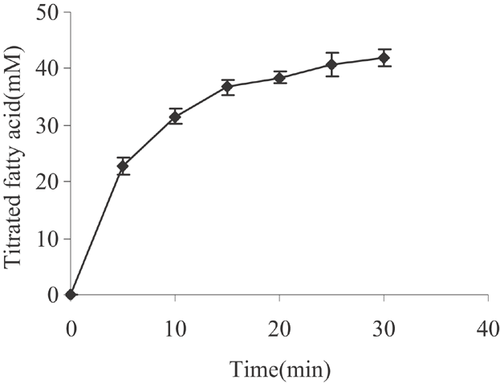
In vitro digestion models are increasingly being used to assess the performance of lipid under conditions that imitate lipid digestion in the small intestine. An in vitro simulation of human digestion and absorption was used to test microalgae oil and the released fatty acid was measured by pH-stat titration. Figure 2 reveals the change in fatty acid produced during simulated gastrointestinal digestion process and titratable fatty acid corresponds to fatty acid released from the triglycerides in response to digestion by enzymes in the pancreatin extract. Before adding the enzyme (time 0), a crude emulsion of bile salt and mixed micelles was clearly visible, appearing as small oil droplets, which were clear but slightly yellow. Once the enzyme was added, the solution became white and dense, as lipolysis proceeded and presumably contributed to the formation of calcium fatty acid soap 57. During oil lipolysis, there was a continuous decrease in triglycerides, a transient accumulation in diglyceride and a gradual increase in monoglyceride. In vivo hydrolysis of oil is a dynamic process, the amount of free fatty acid released increased rapidly in the initial period (0–15 min), which may be due to enough of the pancreatin extract, then the rate of free fatty acid release ascended more slowly (15–30 min). By the end of the digestion phase (30 min), 41.9 ± 0.4 mM fatty acid was titrated, and the extent of digestion at 30 min reached 79.4%, which revealed that microalgae oil has an excellent in vitro bioaccessibility.
3.8 Antioxidant activity
3.8.1 DPPH radical-scavenging assay
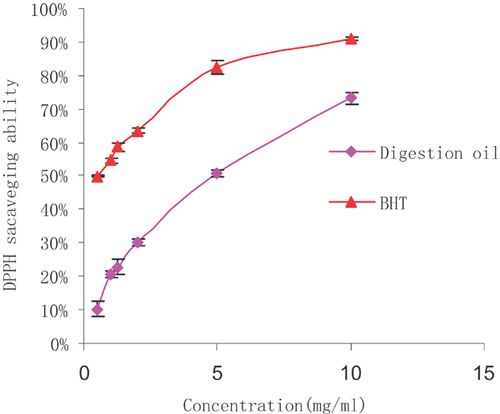
The DPPH radical is rather stable, and our study used a relatively fast method for measuring both the hydrophilic and lipophilic substances during in vitro antioxidant activity 58. The change of violet colour to yellow during the DPPH assay is thought to be due to the attribution of hydrogen atoms by antioxidant substances. The extent of decolourisation is a significant indicator of the sample's antioxidant ability. As shown in Fig. 3, the scavenging abilities of all the samples were well correlated with increasing concentrations, and the scavenging activity of BHT was higher than that of microalgae oil. At concentrations ranging from 0.5 to 10 mg/mL, the DPPH radical scavenging ability of digestion oil was determined at 6.8–64.9%. Lower IC50 values indicated a higher radical scavenging activity. The IC50 value of microalgae oil in our DPPH radical scavenging study was 5.76 mg/mL, and solutions of BHT had an IC50 value of 0.56 mg/mL. Although microalgae oil was shown to be weaker than BHT, it demonstrated a fairly good scavenging ability.
3.8.2 ABTS+ radical cation decolourisation activity
The ABTS radical cation (ABTS+) is produced by a reaction of ABTS with potassium persulphate and it is a blue-green chromogen with an absorption maximum at 752 nm 59. The loss of blue colour in ABTS+ is due to an attribution of hydrogen atoms by antioxidant substances. The outcome of our assay showed that digested microalgae oil had a certain level of ABTS radical-scavenging activity, with an α-tocopherol equivalent antioxidant capacity of 42.071 µg/mg, using the linear curve y = 0.0047x + 0.0201 (R2 = 0.9979). This phenomenon can be interpreted as a function of the large amounts of tocopherols and other minor antioxidant constituents in the oil. In other studies, Li et al. 52 found that the ABTS+ radical scavenging capacity of the hexane fraction, the ethyl acetate fraction and the water fraction of Schizochytrium sp. was (respectively) 0.12 ± 0.03, 0.16 ± 0.18 and 4.57 ± 0.11 µmol Trolox/g, which was relatively lower than our findings. This difference in results may be partly attributed to the different methods used for handing the samples. Another reason is that the modified assay used in our study overcame the drawback of low lipid solubility in ABTS, which made the ABTS radical decolourisation assay applicable to non-hydrophilic substances.
3.8.3 Reducing power assay
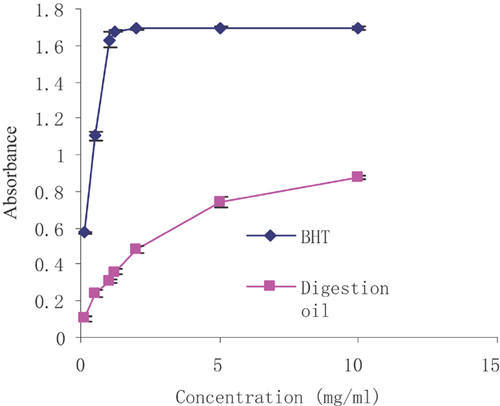
The reducing power of a substance can serve as an important indicator of its potential antioxidant ability. Antioxidants are considered to break free radical chain reactions by donating hydrogen atoms or electrons to free radicals, and this activity can be reflected in the reducing capacity 60. The reducing power of the oil and of the standard both increased with increasing concentrations. At concentrations of 0.1, 0.5, 1, 1.25, 2, 5 and 10 mg/mL, the reducing power of microalgae oil was 0.103, 0.243, 0.311, 0.359, 0.481, 0.742 and 0.874, respectively. BHT at the same concentrations had reducing power values of 0.575, 1.103, 1.629, 1.678, 1.692, 1.696 and 1.696, which were higher than those of the samples. These results indicated that the digested microalgae oil has moderate hydrogen atom or electron donating ability, which is associated with its antioxidant ability (Fig. 4).
3.8.4 β-Carotene bleaching assay
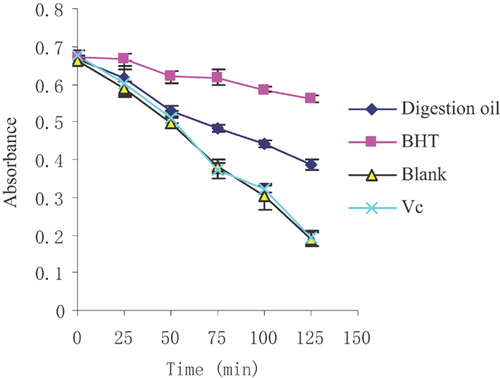
The β-carotene bleaching assay is a proton-transfer-based test in which the scavenging of peroxyl radicals produced by the oxidisation of linoleic acid is believed to be a proton-transfer-based reaction. In this reaction, the antioxidants act as a chain-breaking compound in the process of lipid peroxidation 61. In this assay, the existence of antioxidants hindered β-carotene bleaching by neutralising free radicals to decrease the extent of discolouration determined at 434 nm. As is shown in Fig. 5, both algae oil and BHT exhibited greater antioxidant activity than vitamin C, and BHT had a better protective effect on β-carotene than algae oil. The lipid peroxidation inhibition of digested oil, BHT and vitamin C were near 41, 77 and 2%, respectively. Microalgae oil showed a moderate antioxidant ability in this assay.
3.8.5 ORAC antioxidant assay
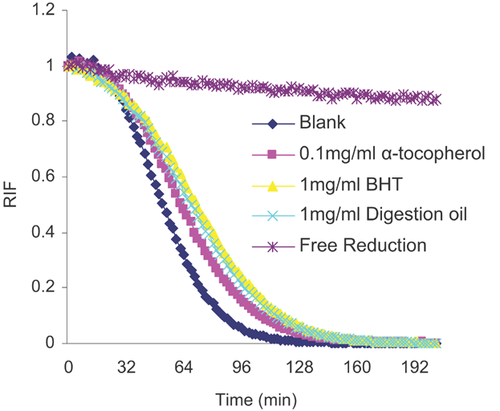
The ORAC method has been extensively applied to characterise the antioxidant ability of pure or complex compounds, and this assay has been adopted by the industry to such a degree that some manufacturers label ORAC values in their product descriptions 62. In this assay, 7% M-β-CD was used to sufficiently solubilise lipophilic substances. It can be seen from the data that the digestion oil, BHT and α-tocopherol all had a great effect on limiting the reduction of AAPH. The equivalent compound used was α-tocopherol, and the ORAC values of BHT and digestion oil were 0.137 and 0.162, respectively. The oil therefore showed good oxygen radical scavenging ability (Fig. 6).
It has been testified that microalgae oil from S. aggregatum has a great antioxidant ability in our study. Besides, extracts from other algaes also revealed antioxidant capacity in existing literature. To the best of our knowledge, red algae lipid extracts of Champia sp. showed a similar antioxidant ability with the synthetic antioxidant BHT, whilst Porphyra sp. revealed relatively poor radical scavenging activity, which was far less than BHT 63. Brown seaweed lipid extracts, E. radiata and M. pyrifera, demonstrated greater radical scavenging activity over BHT, which correlated with its compounds such as phenolics, fucoxanthin and sterols 63, 64.
Many studies have been done on the antioxidant abilities of various oils, and the main antioxidant components of these oils have been found to be VE content, phenolics, flavonoids, tocopherols, carotenoids and sterols 45, 65-67. However, only a few related studies on microalgae oil extracted from S. aggregatum have been done. In this study, it was discovered that microalgae oil contains abundant unsaturated fatty acids, especially DHA, tocopherols, phenolics, flavonoids, carotenoids and sterols. Furthermore, microalgae oil exhibited significant antioxidant capacity in tests of its DPPH radical scavenging ability and reducing power. Its performance in the β-carotene bleaching assay was great and it showed a high scavenging ability of the superoxide anion radical in ORAC test. The in vitro antioxidant activities of microalgae oil may be explained by the existence of tocopherols, phenolics, flavonoids, carotenoids and sterols, and by the outstanding free radical scavenging abilities and great antioxidant abilities of these substances 68-70. We have reports that some in vitro antioxidant capacity experiments on DHA on cells have been performed, and DHA has been proven to have radical scavenging activity and protective effects on oxidative cells 8, 71. Moreover, many in vivo epidemiological and experimental studies have proven that DHA can exert an excellent preventive effect on the production of free radicals by inhibiting NAD(P)H oxidase and well enhance the activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) of brain, liver, heart or serum, revealing a great antioxidant capacity in vivo studies 19, 71-73. Although DHA has been demonstrated to possess antioxidant properties, we remain uncertain whether DHA has revealed an antioxidant ability in our in vitro antioxidant assays. Further relative studies in this area are needed.
4 Conclusions
This study investigated the constituents, oxidation stability, in vitro bioaccessibility and antioxidant activity of microalgae oil following simulated gastrointestinal digestion. The assay results showed that microalgae oil had a good bioaccessibility and exhibited great antioxidant activity, which can be mainly ascribed to the abundant minor antioxidant constituents in the oil. The polyphenols, tocopherols and other minor components found in the oil can protect the oil against oxidation by acting as ROS quencher but further in vivo experiment are necessary to determine the ability of the oil to modulate in vivo oxidative stress. In addition, microalgae oil displayed good oxidation stability in the early phase of the Schaal oven test, which showed the protective effect of the antioxidant constituents on PUFA. On the basis of the research presented here, it can be concluded that microalgae oil is a good source of the n-3 PUFA and the natural antioxidants that are essential for body health.
This study was financially supported by China's State Oceanic Administration's Public Science and Technology Research Funds Projects for the Ocean (201005020) and a special project of the Economic Innovation Development Area (GD2012-A01).
The authors have declared no conflict of interest.




