Chemoenzymatically synthesized policosanyl phenolates as autoxidation inhibitors
Abstract
In this study, ten policosanyl phenolates were synthesized via a two-step chemoenzymatic route. The intermediate vinyl esters were first chemically produced and subsequently esterified with policosanols catalyzed by Novozyme 435 (Candida antarctica lipase B). The conversion yields of the policosanyl phenolates were in the range of 4.20–37.14%. Their antioxidant activities were evaluated. Results showed that, compared with the corresponding phenolic acids, all synthesized policosanyl phenolates had better activities for peroxide value, but worse for 2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) assay; policosanyl p-coumarate and policosanyl 5-phenylvalerate showed a moderate and durable inhibition effect on lipid oxidation both in a linoleic acid system and a cooked pork system.
Practical applications: It is important to develop antioxidants from natural sources. The lipophilization of phenolic acids with fatty alcohols can improve the hydrophobicity and change the antioxidant capacity of phenols. This study focuses on policosanol, which is a food-grade mixture of long-chain aliphatic primary alcohols, having development and application potential as a cost-effective food additive. According to our results, policosanyl phenolates, which are a new range of amphiphilic antioxidant molecules, have the potential for use as alternative food antioxidants in the food industry.
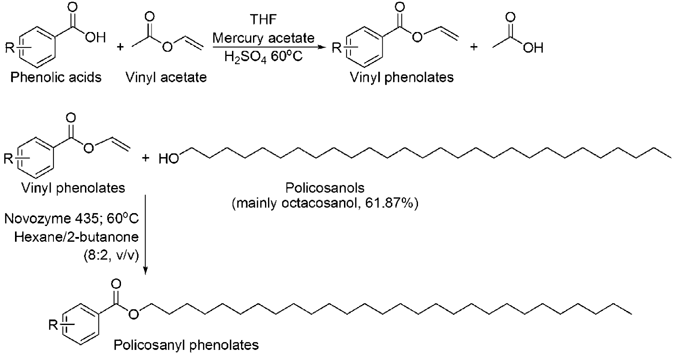
The policosanyl phenolates were synthesized via a two-step route as shown in the figure. The intermediate vinyl esters were first chemically produced and subsequently esterified with policosanols catalyzed by Novozyme 435 (Candida antarctica lipase B).
Abbreviations
-
- ABTS
-
- 2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)
-
- BHT
-
- butylated hydroxytoluene
-
- FeCl2
-
- iron (II) chloride
-
- FeCl3
-
- iron (III) chloride
-
- FTC
-
- ferric thiocyanate
-
- 1H NMR
-
- proton nuclear magnetic resonance
-
- IC50
-
- 50% inhibitory concentration
-
- IO
-
- inhibition of oxidation
-
- MDA
-
- malonaldehyde
-
- p-AnV
-
- p-anisidine value
-
- PL
-
- policosanol
-
- PV
-
- peroxide value
-
- TL
-
- trolox
-
- TBA
-
- thiobarbituric acid
-
- TBARS
-
- thiobarbituric acid reactive substances
-
- TCA
-
- trichloroacetic acid
-
- TLC
-
- thin layer chromatography
-
- UV
-
- ultraviolet
1 Introduction
Dietary lipids play an important role in food nutrition and flavor. The oxidation of lipids in food is a key cause of food quality deterioration during storage at refrigerated or frozen temperatures. The autoxidation of unsaturated fatty acids, a form of lipid oxidation, is a free-radical process that takes place between molecular oxygen and unsaturated fatty acids via a chain reaction involving initiation, propagation, and termination 1, 2. Preventing this is a challenge for manufacturers and food scientists. For many lipid-containing foods, special measures are taken to reduce or prevent oxidation, such as removing available oxygen, using gas-barrier packaging materials, and adding antioxidants 3, 4.
Many studies have been carried out to develop antioxidants from natural sources because of the concerns about the safety of synthetic antioxidants 5, 6. Phenolic acids and their derivatives have attracted significant attention as natural antioxidants, and for many years have been added to food to prevent oxidation processes 7. To enhance their hydrophobicity and improve their antioxidant properties, the esterification of phenolic acids with fatty alcohols has been carried out 8; however, the esterification of phenolic acids with long-chain aliphatic alcohols (carbon number over 22) has not yet been achieved.
Policosanols (PLs), which refer to a mixture of long-chain (C24–C34) aliphatic primary alcohols, are natural supplements derived from sugar cane, wheat (germ, bran, shoots, and flour), and beeswax 9, 10. Recently, there has been significant interest in PLs because of their perceived benefits in treating diabetes, hypercholesterolemia, platelet aggregation, and endothelial cell damage 11-14. Currently, a number of dietary supplements containing PLs are commercially available in the US 9. Recently, a few studies have focused on the esterification of PLs to increase their bioavailability 15, 16. Such food-grade mixtures would be cost effective for the food industry and thus have significant development and application potential as food additives. For this reason, the esterification of policosanyl phenolates is attempted in this study.
In the food industry, the lipase-catalyzed esterification of PLs would offer several advantages, such as milder reaction conditions, selective specificity, minimization of side reactions, fewer intermediary and purification steps, and a more environmentally friendly process 17, 18. However, many research papers have indicated that the conversion of these esters in lipase-catalyzed esterification is significantly affected by the functional groups present in phenolic acids (such as the carboxylic acid, substituents on the aromatic ring, and the double bond in the side alkyl chain), as well as by the carbon length of the fatty alcohols 19, 20.
Hence, in this study, a two-step chemoenzymatic route to synthesize policosanyl phenolates was investigated, in which the intermediate vinyl phenolate was first chemically produced to provide easy access to the active site of lipase, and this was subsequently esterified, using commercially available lipases to catalyze the reaction. The free radical scavenging capacities and inhibition activities of lipid oxidation were also assessed.
2 Materials and methods
2.1 Chemicals
Lipase from Candida rugosa (1179 U/mg), Amano lipase PS-IM (30 U/mg), lipase from Pseudomonas cepacia (37.8 U/mg), and Novozyme 435 (Candida antarctica lipase B, 5 U/mg) were purchased from Sigma-Aldrich Korea (Seoul, Korea). One unit is defined as the microequivalents of fatty acid will be hydrolyzed from a triglyceride in 1 hr at pH 7.2 at 37°C.
The PLs used in this study were obtained from the Riotto Biological Technology Co., Ltd. (Xi'an, Shanxi province, China). They contained 61.87% octacosanol (C28), 20.04% triacontanol (C30), 12.13% hexacosanol (C26), and 5.99% other minor policosanols. Cinnamic acid, m-coumaric acid, p-coumaric acid, ferulic acid, 3–4-dimethoxycinnamic acid, sinapic acid, phloretic acid, hydrocinnamic acid, 4-phenylbutyric acid, and 5-phenylvaleric acid were purchased from Sigma-Aldrich Korea (Seoul, Korea). Iron (II) chloride (FeCl2) and iron (III) chloride (FeCl3) were purchased from Shinyo Pure Chemicals Co., LTD. (Minoo Osaka, Japan). Glacial acetic acid was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Sulfuric acid was purchased from Junsei Chemical Co. LTD. (Tokyo, Japan). Hydrogen chloride was purchased from Daejung Chemicals & Metals Co., LTD. (Siheung, Korea). All other chemicals and solvents, unless otherwise specified, were guaranteed reagent grade and purchased from Sigma-Aldrich Korea (Seoul, Korea). Ground pork was purchased from a local supermarket.
2.2 Chemical synthesis of vinyl phenolates
Vinyl phenolates were chemically synthesized through vinyl interchange reactions of phenolic acids and vinyl acetate 21. Briefly, vinyl acetate (10 mL), mercury acetate (400 µL, 396 mg/mL), and tetrahydrofuran (3 mL) were mixed with 0.5 g of the corresponding phenolic acid in a 25 mL vial, which was sealed with a rubber stopper. The mixture was stirred with a magnetic stirrer (200 rpm) under nitrogen for 3 min. Next, sulfuric acid (2 µL), which served as an activator, was added to the mixture. The reaction mixture was placed in a water bath at 60°C, with stirring (200 rpm) using a magnetic stirrer. The reaction was stopped after 12 h by neutralizing the mixture with sodium acetate. The solvent was removed from the reaction mixture by evaporation. And the products were separated and purified using silica-gel column chromatography, eluted with a gradient solvent consisting of hexane and ethyl acetate (from 32:1 to 4:1, v/v). Above reaction and column chromatography were monitored by TLC (TLC, silica-gel-precoated, flexible TLC sheets, 4 cm × 10 cm, Merck KGaA, Darmstadt, Germany) using hexane and ethyl acetate (4:1, v/v) as elution solvent. Products were visualized under ultraviolet (UV) light at 254 nm using TLC Visualizer (CAMAG Scientific Inc., Wilmington, NC). The percentage yield is the ratio of the amount of product actually obtained to the maximum amount of product possible.
2.3 Synthesis of policosanyl cinnamate for enzyme screening
Esterifications were performed following Torres et al. 22 with slight modification. Briefly, the esterification reactions were carried out in 10 mL glass vials at 60°C under stirring using magnetic stirrers (200 rpm) in an incubator. A binary mixture of hexane and 2-butanone (3 mL, 8:2, v/v) was used as the solvent. The reaction mixtures were prepared using 17.4 mg (0.1 mmol) of vinyl cinnamate, 40 mg (0.1 mmol) of PLs, 300 mg of molecular sieves, and 300 U of different lipases. The four lipases tested for screening are listed under Chemicals section. The reactions were monitored using TLC with an eluent mixture of hexane and ethyl acetate (4:1, v/v), and were visualized under UV light at 254 nm using TLC Visualizer (CAMAG Scientific Inc.). After 4 days, the reactions were stopped and the mixtures were filtered to remove the enzymes. The components of each reaction mixture were analyzed and compared through TLC Rf and area using TLC Visualizer and VideoScan (CAMAG Scientific Inc.).
2.4 Enzymatic synthesis of policosanyl phenolates
0.1 mmol of the corresponding vinyl phenolates were placed into 10 mL glass vials together with 40 mg (0.1 mmol) of PLs, 300 mg of molecular sieves, 300 U (60 mg) of Novozyme 435, and 3 mL of solvent (hexane/2-butanone, 8:2, v/v). The reaction mixtures were incubated under stirring at 60°C. The reactions were monitored using TLC, with an eluent mixture of hexane and ethyl acetate (4:1; v/v), and were visualized under UV light at 254 nm. After 4 days, the reactions were stopped and the mixtures were filtered to remove the enzyme. The solvents were removed from the reaction mixtures by evaporation. The products were separated and purified using silica-gel column chromatography, eluted with a gradient solvent consisting of hexane and ethyl acetate (from 32:1 to 4:1, v/v). The percentage yield is the ratio of the amount of product actually obtained to the maximum amount of product possible.
2.5 Proton nuclear magnetic resonace (1H NMR) and fourier transform infrared (FT-IR) analysis
The structure of above purified vinyl phenolates and policosanyl phenolates were fast confirmed by 1H NMR and FT-IR. Particularly, 1H NMR spectra were recorded at 400 MHz in CDCl3, using a Fourier-transform-NMR spectrometer (Bruker Korea, Seongnam, Korea), with tetramethylsilane as the internal standard. Signal processing and interpretation were performed using the Bruker DPX 400 MHz (9.4T) package. Chemical shifts were expressed as δ values. The FTIR spectra were recorded using a JASCO FT/IR-4100 spectrophotometer (Easton, MD). Attenuated total reflectance was used for the FTIR measurements in the frequency range of 4000–500 cm−1.
2.6 ABTS assay
The antioxidant capacities of policosanyl phenolates and their corresponding phenolic acid were screened by ABTS assay. Assays were performed using the method reported by Re et al. 23 with slight modifications. ABTS diammonium salt (2 mM) and potassium persulfate (3.5 mM) were dissolved in distilled water, and kept in the dark at room temperature for 24 h before use. Ten microliter of samples (at a different concentration in ethyl acetate) were reacted with 290 µL of ABTS solution for 10 min in a 96-well plate (Corning Inc., Corning, NY). The absorbance at 750 nm was determined using an EL-800 Universal microplate reader (Bio-Tek Instrument Inc., Winooski, VT). BHT and TL were used as positive controls. The percentage inhibition was calculated in the following way: % inhibition = [(Acontrol–Ablank)-(Asample–Ablank)]/(Acontrol–Ablank) × 100. Where Acontrol is the absorbance of ABTS solution (290 µL) with ethyl acetate (10 µL), Ablank is the absorbance of distilled water (290 µL) with ethyl acetate (10 µL), Asample is the absorbance of test compounds. The results were expressed as IC50 of ABTS radical scavenging, where IC50 means the concentration of antioxidant necessary to decrease the initial concentration of ABTS radical by 50%.
2.7 Determination of peroxide value (PV)
Linoleic acid, which is a free fatty acid model system, was employed for assessing the inhibition effect of samples and the reference antioxidant compounds (BHT, TL) on PV. The method was performed following Sakanaka et al. 24 with slight modifications. One milliliter of each sample (at a different concentration in ethyl acetate) and nine milliliter of linoleic acid solutions (2.5% in ethanol) was mixed in test tubes (15 mL volume). The tubes were sealed tightly with caps and kept at 60°C in the dark. The same reaction without samples was used as the control. The fatty acid samples were taken on 7 days for measurement of PV using ferric thiocyanate (FTC) method.
PVs were determined as described by Lips et al. 25 with slight modifications. Briefly, the free fatty acid sample (0.5 mL) was mixed with 0.02 M FeCl2 solution (5 µL, in 10% HCl), and kept for 3 min, and then 30% (w/v) ammonium thiocyanate (5 µL) was added. The absorption of the resulting colored system was measured at 500 nm using a spectrophotometer (SECOMAM, Ales Cedex, France). The same reaction without fatty acid samples was used as blank. The PVs of the lipid samples were calculated from a Fe3+ standard calibration curve using the following equation: PV = [(Asample–Ablank) × m]/(55.8 × 2 × w). Where Asample, Ablank, w, and m are the sample absorbance, blank absorbance, sample weight, and calibration curve slope respectively. The inhibition oxidation (IO) indexes were calculated from the PVs using the following equation: % IO = 100–(PVsample/PVcontrol) × 100, where PVsample and PVcontrol are the PVs, of the samples and control, respectively. The results were expressed as IC50 of PV inhibition, where IC50 means the concentration of antioxidant necessary to reduce by 50% the oxidation.
2.8 Determination of total oxidation (TOTOX) value in linoleic acid model system
The linoleic acid model system was employed on TOTOX. Briefly, one milliliter of sample (1 mM in ethyl acetate) and nine milliliter of linoleic acid solutions (2.5% in ethanol) were mixed in test tubes (15 mL volume). The tubes were sealed tightly with caps and kept at 60°C in the dark. The same reaction without samples was used as the blank. The fatty acid samples were taken on 0, 1, 3, 5, 7, and 14 days for measurement of PV and p-AnV.
PVs were determined using FTC method s as above described. The results were expressed in meq/kg linoleic acid.
Standard measurements of p-AnV were performed according to the IUPAC method 2.504 26. The absorption was measured at 350 nm using a spectrophotometer (SECOMAM).
The so called TOTOX value was calculated using p-anisidine value (p-AnV) in conjunction with PV in the industry 27. It is calculated as followed: TOTOX value = 2 × PV–p-AnV.
2.9 Antioxidant activity in cooked ground pork model system
The cooked ground pork model system was used for assessing the antioxidant activity 28. Briefly, fresh ground pork and distilled water mixture (1 g/ml) was treated with samples or reference compound (BHT) at a level of 100 µM. A blank without any antioxidant was prepared. The mixtures were homogenized and then cooked at 80°C in a water bath for 15 min. The cooked samples were kept at 4°C for 14 days and were taken on 0, 4, 7, and 14 days for measurement of thiobarbituric acid reactive substances (TBARS) values.
The TBARS value is an indicator of secondary products (mainly is MDA) in lipid oxidation. In this assay, the MDA is reacted with TBA to form a pink MDA–TBA complex, which is measured spectrophotometrically at its absorption maximum of 532 nm 29. An aliquot (5 mL) of lipid sample was mixed with TCA (2.5 mL; 10% in deionized water), followed by addition of TBA (2.5 mL; 0.02 M in deionized water). The mixture was heated at 80°C in a water bath for 15 min, and then was centrifuged at 10,000 rpm for 10 min. The upper absorbance was read at 532 nm using a spectrophotometer (SECOMAM). The TBARS values were calculated from an MDA standard calibration curve. The results were expressed in µmol of MDA/kg of meat.
2.10 Statistical analysis
All experiments were repeated three times and the results are given as the mean ± SD. All data were analyzed using ONOVA. Significant differences were assessed with a Duncan test (p < 0.05). The statistical analyses were carried out using IBM SPSS version 19.0 software (IBM, NY).
3 Results and discussion
3.1 Chemical synthesis of vinyl phenolates
Our first experiments involved the direct esterification of phenolic acids with PLs catalyzed by lipases, but the conversions were rather low (the data have not been presented). Many researches indicated that the conversion of those esters was significantly affected by the functional groups (carboxylic acid, substituents on aromatic ring, and double bond in side alkyl chain) of phenolic acids and the carbon length of fatty alcohols in lipase-catalyzed esterification 30. Lipase-catalyzed acylation takes place through the formation of an acyl-enzyme intermediate; the nature of reactants has a significant effect on the reactivity 31. A better solution is offered by the introduction of electron-withdrawing substituents. According to Shahidi and Chigorimbo-Murefu 32, 33, the use of vinyl esters increases the reaction rate several-fold, because of facile access of vinyl esters to the active site of lipase and formation of the intermediate adduct, which favors alcoholysis with policosanols. As Fig. 1 showed, in our approach to the synthesis of policosanyl phenolates, the initial step was therefore the production of activated phenolic acid acyl donors, i.e., vinyl phenolates, via a chemical vinyl exchange reaction.
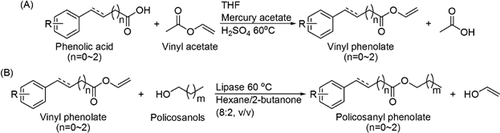
Ten vinyl phenolates were successfully synthesized via chemical vinylation, and purified by flash column chromatography. As Table 1 showed, the Rf values of vinyl phenolates were higher than those of the corresponding phenolic acids, because the synthesized vinyl phenolates had higher hydrophobicities. Their structures were fast confirmed by 1H NMR spectroscopy and compared with those of the corresponding phenolic acids. Three new peaks around δ 7.39 ppm, 4.96 ppm, and 4.62 ppm appeared in the vinyl phenolates spectra, which indicated that the vinyl phenolates were successfully synthesized by vinyl exchange reactions. The yields of synthesized vinyl phenolates were quite different which ranged from 14.59 to 61.26%. The low yields were the results of side reactions and some loss of product during silica-gel column isolation; the differences among the phenolate yields may be caused by different structural fragments in the reaction materials.
 |
||||||||||||||
| No. | Name | 1H NMR (400 MHz, CDCl3, δ) | Yield (%) | Rf (TLC) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic group | Vinyl group | Poliosanyl groups | ||||||||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1' | 2' | 1” | Alkyl chains | ||||
| – | Policosanols | – | – | – | – | – | – | – | – | – | 3.64 | 0.90–1.70 | – | – |
| 1a | Cinnamic acid | 7.48 | 7.35 | 7.30 | 7.35 | 7.48 | 7.72 | 6.39 | – | – | – | – | – | 0.20 |
| 1v | Vinyl cinnamate | 7.30 | 7.20 | 7.20 | 7.20 | 7.30 | 7.93 | 6.54 | 7.39 | 4.62(a); 4.96(b) | – | – | 58.18 | 0.80 |
| 1b | Policosanyl cinnamate | 7.51 | 7.39 | 7.36 | 7.39 | 7.51 | 7.66 | 6.43 | – | – | 4.19 | 0.91–1.70 | 11.28 | 0.90 |
| 2a | m-Coumaric acid | 6.75 | – | 6.75 | 7.04 | 6.75 | 7.62 | 6.41 | – | – | – | – | – | 0.09 |
| 2v | Vinyl m-coumarate | 6.93 | – | 6.78 | 7.24 | 7.02 | 7.62 | 6.38 | 7.28 | 4.58(a); 4.90(b) | – | – | 22.66 | 0.34 |
| 2b | Policosanyl m-coumarate | 6.99 | 4.91(OH) | 6.84 | 7.26 | 7.09 | 7.60 | 6.40 | – | – | 4.19 | 0.89–1.70 | 5.59 | 0.50 |
| 3a | P-coumaric acid | 7.20 | 6.70 | – | 6.70 | 7.20 | 7.70 | 6.51 | – | – | – | – | – | 0.06 |
| 3v | Vinyl p-coumarate | 7.45 | 6.88 | – | 6.88 | 7.45 | 7.76 | 6.33 | 7.45 | 4.64(a); 5.03(b) | – | – | 14.59 | 0.32 |
| 3b | Policosanyl p-coumarate | 7.37 | 6.78 | – | 6.78 | 7.37 | 7.56 | 6.21 | – | – | 4.10 | 0.81–1.62 | 4.2 | 0.46 |
| 4a | Ferulic acid | 6.60 | 3.52 (-CH3) | 5.50 (OH) | 6.60 | 6.60 | 7.55 | 6.37 | – | – | – | – | – | 0.05 |
| 4v | Vinyl ferulate | 6.97 | 3.89 (-CH3) | 5.85 (OH) | 6.87 | 7.07 | 7.66 | 6.28 | 7.32 | 4.54(a); 4.89(b) | – | – | 55.37 | 0.25 |
| 4b | Policosanyl ferulate | 7.04 | 3.94 (-CH3) | 5.85 (OH) | 6.91 | 7.10 | 7.61 | 6.30 | – | – | 4.19 | 0.90–1.70 | 6.57 | 0.41 |
| 5a | 3,4-Dimethoxycinnamic acid | 6.72 | 3.78 (-CH3) | 3.78 (-CH3) | 6.72 | 6.72 | 7.58 | 6.48 | – | – | – | – | – | 0.03 |
| 5v | Vinyl 3,4-dimethoxycinnamate | 7.02 | 3.88 (-CH3) | 3.88 (-CH3) | 6.82 | 7.11 | 7.73 | 6.32 | 7.37 | 4.66(a); 4.95(b) | – | – | 31.75 | 0.29 |
| 5b | Policosanyl 3,4-dimethoxycinnamate | 6.87 | 3.92 (-CH3) | 3.92 (-CH3) | 7.06 | 7.12 | 7.63 | 6.31 | – | – | 4.20 | 0.89–1.69 | 7.68 | 0.42 |
| 6a | Sinapic acid | 6.21 | 3.78 (-CH3) | – | 3.78 (-CH3) | 6.21 | 7.61 | 6.43 | – | – | – | – | – | 0.02 |
| 6v | Vinyl sinapate | 6.89 | 3.84 (-CH3) | – | 3.84 (-CH3) | 6.89 | 7.67 | 6.36 | 7.38 | 4.59(a); 4.93(b) | – | – | 24.63 | 0.13 |
| 6b | Policosanyl sinapate | 6.79 | 3.92 (-CH3) | 5.73 (OH) | 3.92 (-CH3) | 6.79 | 7.58 | 6.30 | – | – | 4.19 | 0.89–1.70 | 4.97 | 0.23 |
| 7a | Phloretic acid | 7.11 | 6.71 | - | 6.71 | 7.11 | 2.86 | 2.61 | – | – | – | – | – | 0.19 |
| 7v | Vinyl phloretate | 6.80 | 5.88 | - | 5.88 | 6.80 | 2.95 | 2.71 | 7.28 | 4.60(a); 4.86(b) | – | – | 16.24 | 0.27 |
| 7b | Policosanyl phloretate | 7.09 | 6.78 | 4.89 (OH) | 6.78 | 7.09 | 2.88 | 2.59 | – | – | 4.06 | 0.90–1.58 | 17.92 | 0.43 |
| 8a | Hydrocinnamic acid | 7.06 | 7.16 | 7.06 | 7.16 | 7.06 | 2.76 | 2.51 | – | – | – | – | – | 0.30 |
| 8v | Vinyl hydrocinnamate | 7.19–7.38 | 2.99 | 2.73 | 7.38 | 4.56 (a); 4.84 (b) | – | – | 20.51 | 0.71 | ||||
| 8b | Policosanyl hydrocinnamate | 7.09–7.24 | 2.88 | 2.56 | – | – | 4.00 | 0.83–1.50 | 27.12 | 0.92 | ||||
 |
|||||||||||||||
| No. | Name | 1H NMR (400 MHz, CDCl3, δ) | Yield (%) | Rf (TLC) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic group | Vinyl group | Poliosanyl groups | |||||||||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 1' | 2' | 1” | Alkyl chains | ||||
| 9a | 4-phenylbutyric acid | 7.10 | 7.19 | 7.10 | 7.19 | 7.10 | 2.57 | 1.90 | 2.25 | – | – | – | – | – | 0.37 |
| 9v | Vinyl 4-phenylburate | 7.21 | 7.32 | 7.21 | 7.32 | 7.21 | 2.69 | 2.01 | 2.43 | 7.32 | 4.61(a); 4.89(b) | – | – | 36.72 | 0.78 |
| 9b | Policosanyl 4-phenylburate | 7.18 | 7.26 | 7.18 | 7.26 | 7.18 | 2.55 | 1.99 | 2.26 | – | – | 4.07 | 0.90–1.63 | 31.39 | 0.87 |
 |
||||||||||||||||
| No. | Name | 1H NMR (400 Hz, CDCl3, δ) | Yield (%) | Rf (TLC) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic group | Vinyl group | Poliosanyl groups | ||||||||||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1' | 2' | 1” | Alkyl chains | ||||
| 10a | 5-Phenylvaleric acid | 7.07 | 7.16 | 7.07 | 7.16 | 7.07 | 2.55 | 1.63 | 1.57 | 2.23 | – | – | – | – | – | 0.32 |
| 10v | Vinyl 5-phenylvalerate | 7.15 | 7.28 | 7.15 | 7.28 | 7.15 | 2.63 | 1.64 | 1.64 | 2.44 | 7.28 | 4.53(a); 4.88(b) | – | – | 61.26 | 0.80 |
| 10b | Policosanyl 5-phenylvalerate | 7.19 | 7.28 | 7.19 | 7.28 | 7.19 | 2.65 | 1.71 | 1.71 | 2.36 | – | – | 4.09 | 0.91–1.65 | 37.14 | 0.84 |
3.2 Lipase-catalyzed synthesis of policosanyl phenolates
Initially, four commercially available lipases, as listed under “Chemicals”, were screened for their ability to catalyze the transesterification reaction between the PLs and vinyl cinnamate. The components of each reaction mixture were analyzed by TLC with reference compounds. The results are shown in Fig. 2. Vinyl cinnamate, methyl cinnamate, and cinnamic acid were used as standards to indicate the starting reactant and side products. A new peak appeared at an Rf value of 0.78, representing the products of policosanyl cinnamate. Novozyme 435 showed the highest conversion yield of policosanyl cinnamates. This might result in a relative deep and wide active site which can easy accept the substrates. The ideal biocatalyst should provide a faster reaction speed, higher conversion yield, less side products, and should be inexpensive. Novozyme 435 was considered the best fit to the above requirements and was thus used for the remaining reactions for the policosanyl phenolates in this study.
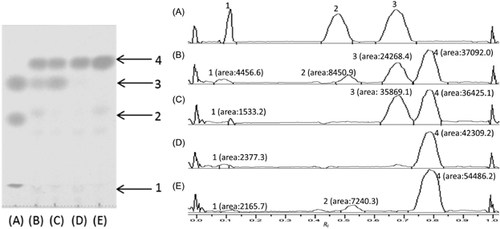
Here, ten policosanyl phenolates were successfully synthesized via vinyl phenolate intermediates, using Novozyme 435 as the catalyst. It is well established that the activity of a lipase can be greatly affected by the choice of organic solvent, and good correlations between the reaction rate and solvent polarity have been achieved 34-36. According to our previous work, a binary mixture of hexane and 2-butanone (8:2, v/v), which is an unreactive and nontoxic solvent, was used as the solvent to maintain good lipase activity and reactants solubility. Moreover, 3 Å molecular sieves were used to remove the water produced, in order to shift the reaction to the left of equation. Although we attempted to improve the lipase activity using the methods described above, the conversion yields were still low, i.e., in the range of 4.20–37.14% (Table 1); with the resulting order of yields being: 10b > 9b > 8b > 7b > 1b > 5b > 4b > 2b > 6b > 3b. The results showed that the lipase activity could be influenced by the different types and positions of functional groups of the phenolates. Specifically, the double bond in the side alkyl chain significantly inhibits lipase activity, while without the double bond, a longer alkyl side chain offers easier access to the lipase active site; similarly, substituents on the aromatic ring also hinder access to the acceptor binding site, with the hydroxyl group having a greater effect than the methoxyl group.
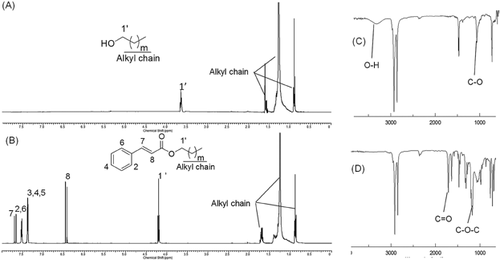
After being purified by flash column chromatography, the structures of the policosanyl phenolates were confirmed. The results are shown in Table 1 and Fig. 3. The TLC Rf values of the policosanyl phenolates are higher than those of their corresponding intermediate products and phenolic acids, indicating higher hydrophobicity. In the 1H NMR spectra, the characteristic peaks of each phenolate group were not modified, while the characteristic alkyl peaks of the policosanols were detected in the range δ 0.90–1.70 ppm, instead of the vinyl peaks. The change in the chemical shift of the α-H in the policosanyl group from δ 3.73 ppm to δ 4.19 ppm indicated binding with the phenolic group. In the IR spectra, the policosanols exhibited a strong absorption at 1061 cm−1, which was assigned to the C-O vibration via the oxygen atom of the hydroxyl group. A broad band around 3150–3500 cm−1 corresponded to the O-H vibration of the hydroxyl group of the policosanols. Policosanyl phenolates exhibited strong sharp bands at 1708 and 1180 cm−1, which indicated the functional groups CO and C-O-C, respectively.
3.3 Antioxidant capacity of policosanyl phenolates
In order to have a better understanding of the antioxidant capacity of policosanyl phenolates, they were valued together with starting materials using ABTS and FTC assay.
The ABTS system, which is commonly used for evaluating antioxidant activity, was used in this study to assess the free-radical-scavenging capacities of policosanyl phenolates. The ABTS IC50 data are shown in Fig. 4. Our results showed that 3a, 4a, and 6a had the best activities, and were better than the positive controls; however, the activities of the other phenolic acids were low. But all policosanyl phenolates showed no activity or in a low level. Phenolic compounds need hydroxyl groups to act as hydrogen donors to enable them to act as efficient free-radical scavengers; this could explain why 1a, 5a, 8a, 9a, and 10a have no activities; the activity of 2a was lower than that of 3a, because it was increased by the hydroxyl group being para position to the alkyl substituent; the double bond in the alkyl substituent would be a key factor in the activity of 7a being lower than that of 3a. Furthermore, the activities could be improved by bulky groups being ortho position to a hydroxyl group 37. However, a significant decrease in activity was observed after the phenolic acids were esterified with PLs. This could be because the long alkyl chain limited the reactivity of the hydroxyl hydrogen toward the free radical, or changed the balance of resonance delocalization. The low activities of 4b and 6b showed that the above properties were still worked 38.
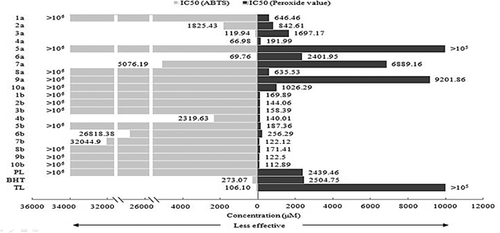
PVs, which represent the peroxide concentration, are used as indicators of lipid oxidation. In this study, PVs were measured using the FTC method; the IC50 results are shown in Fig. 4. Except 5a, 7a, and 9a, all other compounds showed inhibition effect on PV compared with BHT. Moreover, dramatic increases of PV inhibition activity were obtained after esterification of phenolic acids with PLs. According to C. Sanchez-Moreno 39, this property is generally higher in bulk structures. And it is possible that the unsaturated fatty acids would be stabilized by the long alkyl chain. Another explanation may be the higher hydrophobicity of policosanyl phenolates than that of their starting reactants. Due to the excellent potential of policosanyl phenolates in lipid oxidation inhibition, other experiments were used to confirm in this study.
3.4 Inhibition of lipid oxidation in linoleic acid model system
The so called TOTOX value is an indicator to evaluate the lipid total oxidation, and provide a comprehensive overview of the oxidation process. It is a combination of PV and p-AnV, which are both indicators of lipid oxidation, but reflect the oxidation level at the early and later stages, respectively. In this study, linoleic acid was used as the oxidation source to explore the effect on lipid oxidation supplemented by the samples, because the model system made it simpler to analyze the changes in each stage of lipid oxidation.
The oxidation of linoleic acid is shown in Fig. 5(A). At the beginning, the PV dramatically increased, because hydrogen was abstracted from the linoleic acid, resulting in lipid free radicals, which in turn reacted with molecular oxygen to form lipid peroxyl radicals. The peroxyl radicals were unstable and in turn abstracted hydrogen atoms from the linoleic acid to form a hydroperoxide and a new alkyl free radical. Interactions of this type would continue for 10 40–100 41 cycles. But a substantial drop appeared after 1 day because the above process was ended by the formation of non-radical products resulting from combinations of two radical species 42, 43. During above period, the accumulation rate of p-AnV was rapidly increasing. In the second week, both PV and p-AnV increased slowly, indicating that the oxidation process was completed. The total oxidation trend of linoleic acid is shown as the TOTOX values in Fig. 5(A), which reflected slow-fast-slow increases and exhibited maximum oxidation on the 14th day.
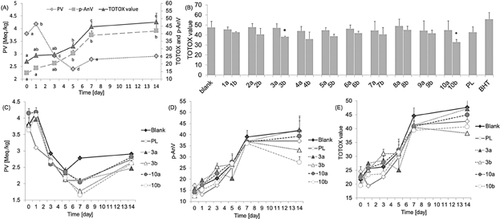
The inhibition effects of synthesized compounds (100µM) on lipid total oxidation were compared with those of their corresponding starting reactants, and the results are shown in Fig. 5(B). Phenolic acids and policosanols showed no inhibition effect on lipid oxidation. After esterification with PLs, however, compounds 3b and 10b became efficient inhibitors. As shown in Fig. 5(C–E), in the first 5 days, the PV trends of 3a, 3b, 10a, and 10b were the same with blank; therefore, we do not know the detailed effect of the chemicals via the PVs because there was a dynamic balance between the early and later stages. However, sustained decreases to day 7 were occurred in the PVs, indicating that the compounds efficiently inhibited the formation of peroxides, and 3b and 10b were better than starting reactants. Moreover, declines in p-AnV, which were different from that for the blank, occurred in 3b and 10b after 7 days, illustrating that the synthesized esters made the terminal oxidation products unstable and prone to decomposition. In terms of total oxidation, the inhibition effects occurred after 1 wk.
3.5 Antioxidant activity in cooked ground pork model system
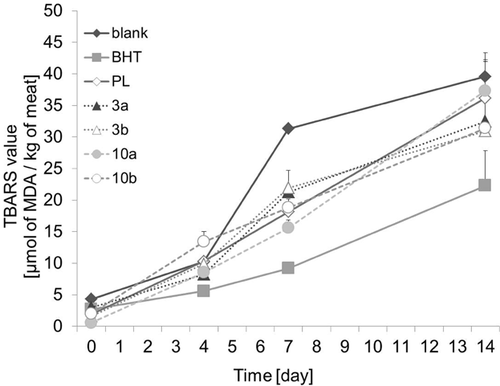
The antioxidant activities of the potential products were determined in cooked ground pork, which provides an excellent model for assessing the effectiveness of antioxidants. In food oxidation processes, MDA is the major oxidation product and causes deterioration in the food and an off-flavor. The MDA concentration in cooked pork with and without antioxidants was measured by monitoring the formation of TBARS. The results are shown in Fig. 6. During the storage period, all meat samples showed an increased content of MDA over time as a result of lipid oxidation. However, the samples with added antioxidants had a lower MDA level than the blank reference samples after 7 days. Also, a better antioxidant effect of 3b and 10b was obtained over their starting reactants after 14 days. Though all samples showed activities which are much lower than BHT, but the activities of the two policosanyl products are significant higher than blank, policosanols, and/or their corresponding phenolic acids. Therefore, 3b and 10b showed moderate and durable antioxidant activity compared with the others, which suggests that they could be used as alternative food antioxidants.
4 Conclusions
In this study, ten policosanyl phenolates were successfully synthesized through a two-step chemoenzymatic route. In this process, the yields of policosanyl phenolates were enhanced by using vinyl phenolates as intermediates, but were influenced by different functional groups of the phenolates. The physicochemical and biological properties of phenolic acids were changed after lipophilization with policosanols. The hydrophobicity of the policosanyl phenolates was higher than that of the phenolic acids. However, the antioxidant properties of the policosanyl phenolates were worse in ABTS, but better in PV than those of their corresponding phenolic acids. Policosanyl p-coumarate (3b) and policosanyl 5-phenylvalerate (10b) showed a moderate and durable inhibition effect on lipid oxidation, both in the linoleic acid system and in the cooked pork system. This suggests that they have the potential to be used as alternative food antioxidants. The availability of a synthetic approach for the preparation of these phenolipids will greatly facilitate further investigations of their biological properties. Investigation into the other potential health benefits of policosanyl phenolates is currently underway.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2012R1A1A2008842) and the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2009-0094071), Hallym University Research Fund (HRF-201404-007).
The authors have declared no conflict of interest.




