Enzymatic removal of 3-monochloro-1,2-propanediol (3-MCPD) and its esters from oils
Abstract
3-Monochloro-1,2-propanediol (3-MCPD) is a contaminant in processed food well known for about 30 years. More recently, this compound has observed attendance due to its occurrence as fatty acid esters in edible oils and products derived from them. In this study, the first enzymatic approach to remove 3-MCPD and its esters from aqueous and biphasic systems by converting it into glycerol is described. First, 3-MCPD was converted in an aqueous system by an enzyme cascade consisting of a halohydrin dehalogenase from Arthrobacter sp. AD2 and an epoxide hydrolase from Agrobacterium radiobacter AD1 with complete conversion to glycerol. Next, it could also be shown, that the corresponding oleic acid monoester of 3-monochloropropanediol-1-monooleic-ester (3-MCPD-ester) was converted in a biphasic system in the presence of an edible oil by Candida antarctica lipase A to yield free 3-MCPD and the corresponding fatty acid. Hence, also 3-MCPD-esters can be converted by an enzyme cascade into the harmless product glycerol.
Practical applications: Since several reports have been recently published on the contamination of foods with 3-MCPD and its fatty acid esters, there is a great demand to remove these compounds and an urgency to find useful methods for this. In this contribution, we present an easy enzymatic way to remove 3-MCPD and its esters from the reaction media (i.e., plant oil) by converting it to the nontoxic glycerol. The method requires neither high temperature nor organic solvents.
Abbreviations:
3-MCPD, 3-monochloro-1,2-propanediol; 3-MCPD-ester, 3-monochloropropanediol-1-monooleic-ester; CAL-A, Candida antarctica lipase A; EH, epoxide hydrolase; HHD, halohydrin dehalogenase.
Introduction
3-Halogen-1,2-propanediols and their mono- and diester, in particular 3-monochloro-1,2-propanediol (3-MCPD) and its fatty acid esters are known contaminants in foods 1, 2. There have been several studies on the toxicity and carcinogenicity of 3-MCPD, which found 3-MCPD to be carcinogenic to rats when given at high doses 3, 4. In another study, dietary administration of 3-MCPD increased the incidence of Leydig-cell and mammary gland tumors and of benign kidney tumors 5. β-Chlorolactic acid and oxalic acid are metabolites of 3-MCPD and the accumulation of oxalic acid was related to nephropathy 4. It is therefore crucial to remove 3-MCPD and its derivatives from food including fats and oils. From the current knowledge, about 3-MCPD the Joint FAO/WHO Expert Committee on Food Additives (JECFA) deduced in 2001 a provisional maximum tolerable daily intake (PMTDI) of free 3-MCPD of 2 µg/kg body weight for humans 6.
3-MCPD was originally found in acid hydrolyzed vegetable protein (acid-HVP) 7, and this was also thought to be the main source of 3-MCPD in food. Examples are soups, savory snacks, and gravy mixes 8. Another well known source of 3-MCPD are soy sauces 9. More recently, many other products were identified to contain 3-MCPD without a relation to acid-HVP, like roasted cereals, fermented sausages, and toasted bread 2, 8, 10. Recently, edible oils were found to contain significant amounts of 3-MCPD in its fatty acid esters form, but only very little amounts of free 3-MCPD 1. These compounds are believed to be formed during refining of vegetable oils, in particular during deodorization at temperatures above 240°C. In further studies, 3-monochloropropanediol-1-monooleic-ester (3-MCPD-esters) were also found among others in the crust of white bread, doughnuts, salty crackers, human breast milk, vegetable fats, smoked meat, and infant and baby foods 1, 11-13. Especially the occurrence of 3-MCPD-esters in baby and infant foods concerns the general public and enforces the demand of investigation on this topic.
Until now, though, there are no studies about the toxicity of 3-MCPD-esters. Thus, it remains unclear whether the esters of 3-MCPD show similar characteristics as 3-MCPD itself. However, there are hints that they are at least partly hydrolyzed in the human intestinal system, since in an in vitro study (an intestinal model) 3-MCPD-mono and -diesters were hydrolyzed by pancreatic lipases 14. Therefore it was recommended by the German Bundesinstitut für Risikobewertung (BfR) and the European Food Safety Authority (EFSA) to treat the esters like free 3-MCPD in terms of toxicity 15. It was also recommended by the BfR to decrease the amount of 3-MCPD-esters in food, in particular in baby milk powder.
There are several physico–chemical methods described in literature to reduce the amount of 3-MCPD 1, 2, 14. A disadvantage of these approaches is that they need high temperatures and that the concentration of 3-MCPD can even increase. Furthermore, these treatments may lead to unintended changes in the quality of the oils, like isomerization, degradation, or exchanges of fatty acids or in the spectrum of antioxidants. Bel-Rhlid et al. 16 described a whole cell system with Saccharomyces cerevisiae to convert racemic (R,S)-3-MCPD with 68% conversion. The reaction needed for this conversion a rather long incubation time of 48 h. The enzymes responsible for this conversion were not identified and it was not shown, whether also 3-MCPD-esters were converted.
In this work, we present an enzymatic way to degrade 3-MCPD via glycidol to glycerol, which is a nontoxic compound that is permitted in food. We also show that in principle also 3-MCPD-esters can be removed by the use of lipase to produce the free 3-MCPD followed by subsequent enzymatic conversion to glycerol.
Materials and methods
Chemicals and enzymes
Racemic glycidol, 3-MCPD, and HEPES were purchased from Sigma–Aldrich (Steinheim, Germany). Halohydrin dehalogenase (HHD) HheA from Arthrobacter sp. AD2 and epoxide hydrolase (EH) EchA from Agrobacterium radiobacter AD1 were purchased from Codexis (Jülich, Germany). Candida antarctica lipase A (CAL-A) and C. antarctica lipase B (CAL-B) were purchased from Novozymes (Bagsværd, Denmark). For reactions in a biphasic system commercially available edible oil (olive oil) was used as nonpolar phase. Before applying the enzymes in biocatalytic reactions, both HheA and EchA were diafiltrated three times with HEPES-buffer (50 mM, pH 8, 4°C) using centrifugal filters (Amicon Ultra, exclusion size: 10 kDa) from Millipore (Billerica, MA, USA).
Synthesis of the 3-monochloropropanediol-1-monooleic-ester (3-MCPD-ester)
3-MCPD (10.0 mmol, 1.1 g) and oleic acid (10.7 mmol, 3.02 g) were dissolved in 30 mL methyl tert-butyl ether (MTBE), and approximately 5 g of activated molecular sieve were added to remove water generated during the esterification. The reaction was started by adding 300 mg CAL-B and the mixture was stirred overnight at 37°C in a nitrogen atmosphere. After TLC analysis indicated full conversion, the reaction was stopped by filtration. The precipitate was washed with MTBE and the filtrate concentrated by rotary evaporation. The product was obtained in quantitative yield (4.1 g) and its structural identity and purity was confirmed by NMR spectroscopy and GC-MS.
GC analyses
GC analyses were performed on a Hewlett Packard 5860 Series II Gas chromatograph equipped with an FID using a nitro-terephthalic acid derivated PEG capillary column (30 m × 0.25 mm). The temperature program was as follows: 5 min at 180°C with a subsequent gradient of 10°C/min up to 220°C. Ten-microliter samples were removed from the enzymatic reaction and centrifuged before injection. From the centrifuged sample, 0.4 µL was directly injected into the GC device. Quantification was carried out using DMSO as an internal standard. DMSO was added to all reactions in a concentration that resembled the starting concentration of the substrate. Glycidol, 3-MCPD, and DMSO were detected with retention times of 3.4, 4.8, and 7.8 min, respectively. The response factors were determined to be f3-MCPD/DMSO = 0.417 and fglycidol/DMSO = 0.583 with a SD of 7.4 and 3.5%, respectively. Response factors were remeasured after incubation for 24 h at 30°C to ensure stability of 3-MCPD and glycidol toward autohydrolysis.
Reactions in an aqueous system
Enzymatic hydrolysis of 3-MCPD was performed in glass vials with a reaction volume of 200 µL in HEPES (50 mM, pH 8) buffer at 30°C in a thermomixer. The starting concentration of the substrate (3-MCPD) was 10 mM and the amount of HheA used was 10 µg. Five milligrams of EchA was added either from the beginning of the reaction together with HheA or after consumption of 3-MCPD (see Section 3). Samples were taken at various intervals as described and the reaction progress was monitored by GC analyses. Controls were performed to investigate the stability of 3-MCPD and glycidol without addition of any enzyme. Even after 24 h incubation time, no significant changes in their concentrations could be determined.
Reactions in a biphasic system
Enzymatic hydrolysis of 3-MCPD in a biphasic system was performed essentially as described above, except that the reaction volume was increased to 1 mL and olive oil was added to serve as nonpolar phase. 3-MCPD was added to a final concentration of 10 mM (in the aqueous phase). Different ratios of polar to nonpolar phases were examined to study the effect of this factor. The reaction was performed at 30°C in a thermomixer and samples were taken and analyzed as described above.
In a similar manner, reactions using the 3-MCPD oleic acid ester were performed. The concentration of the 3-MCPD-ester (in the olive oil phase) was 50 mM. As above, several reactions with different ratios of polar to nonpolar phase were prepared. The enzymatic hydrolysis was started by addition of lipase CAL-A at 30°C in a thermomixer. Samples were taken from the aqueous phase and analyzed as described above. Potential autohydrolysis of the 3-MCPD-ester was studied in the same system in the absence of CAL-A.
Results and discussion
The basic concept for the enzymatic removal of 3-MCPD and its fatty acid esters is shown in Fig.1. The fatty acid monoester of 3-MCPD can first be hydrolyzed by a lipase to furnish the free 3-MCPD. This is then converted by an HHD to the corresponding epoxide glycidol, which is finally hydrolyzed by an EH to glycerol.
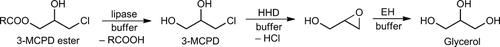
Enzymatic degradation of 3-MCPD and its fatty acid ester. A 3-MCPD fatty acid ester is first hydrolyzed by a lipase. The resulting free 3-MCPD is than converted into the epoxide by an HHD followed by hydrolysis using an EH.
Conversion of 3-MCPD in an aqueous system
In order to proof this concept, it was first verified whether the HHD and the EH are capable to perform the two-step conversion of 3-MCPD to glycerol in an aqueous system. For this, both HheA and EchA were added simultaneously to the reaction mixture at the beginning of the reaction. Both enzymes were diafiltrated prior to use to ensure that possible interfering compounds like chloride ions as well as glycerol present in the commercial preparation were removed. It is obvious from Fig. 2a, that the combined use of both enzymes did not lead to complete conversion as after 7.5 h the remaining concentrations of 3-MCPD and glycidol were determined to 2.9 and 6.8 mM, respectively, which corresponds to a conversion of approximately 70%. Even after 24 h no complete conversion of 3-MCPD and glycidol was observed (data not shown), which was attributed to possible inactivation of the EH by 3-MCPD.
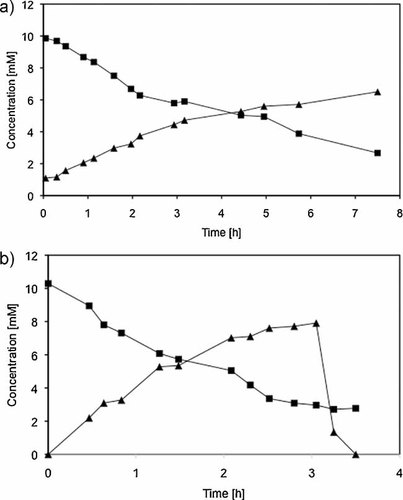
Conversion of 3-MCPD (squares) to glycidol (triangles) in an aqueous system. (a) HHD and EH were added simultaneously at the beginning of the reaction and (b) the EH was added after 3-MCPD had been significantly consumed.
To overcome this limitation, the EH was added only after significant consumption of 3-MCPD occurred (Fig. 2b). Indeed, a rapid consumption of glycidol took place and after only 30 min it could not be detected anymore. The reasons for the residual concentration of 3-MCPD after 24 h remain unclear, but this could be due to low stability of the HHD used.
Conversion of 3-MCPD in a biphasic system
After it could be confirmed that the HHD and the EH are able to convert 3-MCPD to the final product glycerol, the concept was transferred to a biphasic system containing olive oil as model oil, because 3-MCPD (and in particular its esters) are found in edible oils. As 3-MCPD, glycidol and glycerol are all highly soluble in the aqueous phase, the limitation in this biphasic system is rather the enzyme stability in the presence of the highly hydrophobic oil and the interface between both phases. Hence, the conversion of 3-MCPD was measured in three different systems with distinct ratios of both phases (Table 1). The data show that HheA is even active at low concentrations of water in the system and shows good conversions. The same holds true for the EchA, which was able to convert the glycidol released by HheA within short time to glycerol. As refined edible oils are one of the most important sources for contamination with 3-MCPD and their processing should avoid large amounts of water (or buffer), it is very important that both enzymes were found active even at only 5% water content.
| Amount of water in oil phase (% v/v) | 3-MCPD (mM) | Glycidol (mM) |
|---|---|---|
| Incubation with HHD | ||
| 50 | 2.6 | 6.3 |
| 20 | 1.4 | 7.2 |
| 5 | 0 | 7.7 |
| After addition of EH and 1 h incubation | ||
| 50 | 2.6 | 0.7 |
| 20 | 1.4 | 0 |
| 5 | 0 | 0 |
Conversion of a 3-MCPD fatty acid ester in the biphasic system
As already pointed out in the introduction, the major amount of 3-MCPD occurs as ester derivative and we have therefore synthesized the 3-MCPD-1-monooleate serving as model compound. Its hydrolysis catalyzed by a lipase at the interface oil/water is expected to yield the water soluble free 3-MCPD, which can then be converted to glycerol according to Fig. 1. Again, three systems with distinct amounts of water (50, 20, and 5% v/v) in the olive oil phase were prepared. CAL-A was added to start the reaction and the conversion was determined by GC analysis (Table 2). The results indicate that CAL-A is very active against the ester, independently of the amount of water in the system. The released free 3-MCPD was further converted to glycerol as described under Section 3.2. Hence, the combination of the three enzymes enables the conversion from the 3-MCPD-ester via the free 3-MCPD and glycidol to glycerol. Thus, these experiments provided a proof-of-concept for this novel approach. Further experiments are needed to transfer the concept to other plant oils contaminated with 3-MCPD or its esters and to up-scale the results obtained in this model system.
| Water (% v/v) | Conversion (%) |
|---|---|
| 50 | 99.5 |
| 20 | 98.9 |
| 5 | 100.0 |
Conclusions
It could be shown that the combination of an HHD and an EH EchA enables the easy removal of the food contaminant 3-MCPD to the nontoxic product glycerol via glycidol. As the reaction also occurs in a biphasic system in the presence of an oil, it resembles a very mild method for the detoxification of edible oils for nutrition.
Acknowledgements
We thank the German Research Foundation (DFG, grant Bo1862/4-1) for financial support, Dr. Aurelio Hidalgo (UAM-CSIC, Madrid, Spain) for useful discussions and Anita Gollin (Institute of Biochemistry, Greifswald, Germany) for help with chemical synthesis.
The authors declare no conflict of interest.




