Esterification of olive acid oil in supercritical methanol
Abstract
The aim of this work was to produce biodiesel from olive acid oil with free fatty acid content of 91.67 wt% by using non-catalytic esterification in supercritical methanol. Design of experiments was used to evaluate the effects of various values of pressure (200–280 bar), temperature (300–380°C), methanol to acid oil volumetric ratio (0.7–1.5), and flow rate (0.4–1.2 mL/min). The optimum process conditions such as temperature of 380°C, pressure of 240 bar, volumetric ratio of acid oil to methanol of 1.12, and flow rate of 0.4 mL/min were obtained using response surface methodology (RSM) coupled with Central Composite Design (CCD). Moreover, it was found that the most effective parameters were temperature and flow rate, which was in good agreement with the experimental value. Furthermore, the fuel properties of biodiesel were upgraded, and then tested following the European standard EN 14214.
Abbreviations:
ANOVA, analysis of variance; FAME, fatty acid methyl ester; FFA, free fatty acid.
Introduction
Biodiesel (fatty acid methyl ester, FAME) is a fuel derived from triglycerides in the presence of methanol and an alkali catalyst via a transesterification reaction. To date, although transesterification has been predominant at the industrial scale due to its high conversion efficiency at reasonable cost, new catalysts have been intensely searched by researchers. Nevertheless, the usage of catalyst and low levels of impurities complicate the transesterification reaction mainly in the need to separate the catalyst from the product mixture and pre-treatments concerning water and free fatty acid (FFA), respectively.
In a more recent development, it was reported that biodiesel can be produced even in the absence of a catalyst in supercritical methanol. Naturally, this process is fast, efficient, and simple in operation allowing over 90% yields of FAMEs in just a few minutes with no need for further catalyst separation. Other advantages of the process include its good performance in the presence of water and, in particular, FFAs. Consequently, cheaper feedstocks, which often contain high FFAs, such as waste vegetable oils, acidulated soapstocks can be efficiently used. A wide range of feedstocks and process parameters have been screened for biodiesel production under supercritical conditions. In general, in these studies, since longer chained alcohols require longer treatment times, methanol is commonly used although ethanol is more environmentally friendly. However, Madras et al. 1 observed higher conversions in ethanol since the solubility parameter of ethanol is lower than that of methanol, and is closer to the solubility parameter of the oil. They investigated the transesterification of refined edible sunflower oil at various temperatures (200–400°C) at 200 bar in a molar ratio of 40:1 by using an 8 mL stainless steel reactor. They also studied the kinetics of the reaction for various reaction times (1–12 h), and monitored that the conversion did not increase significantly after 6 h. The conversions achieved for methanol and ethanol were 23 and 27%, respectively. Moreover, Warabi et al. 2 also studied the effect of alcohols such as methanol, ethanol, 1-propanol, 1-butanol, and 1-octanol on the transesterification of triglycerides and esterification of fatty acids at temperature of 300°C by using batch-type system. The results showed that reaction rates of triglycerides were slower than alkyl esterification of fatty acids for any of the alcohols employed.
According to the current literature, the majority of reports using the supercritical alcohol process adopted the batch mode. In fact, there are quite interesting results obtained by using two-step and flow tubular reactor systems. Minami and Saka 3 developed a laboratory-scale plant for the two-step supercritical methanol process involving hydrolysis of TGs in subcritical water and following methyl esterification of fatty acids in supercritical methanol at 270°C and 20 MPa within various volumetric ratios of methanol to fatty acid. It was concluded that fatty acid has an important role as acid catalyst in hydrolysis reaction of oil in subcritical water. The results of this step showed that the higher yield of FAME was achieved when the lesser amount of methanol was added to the reaction. For example, about 94 wt% of FAME could be obtained with a volumetric ratio of 0.9/1.0. In the second step, it was also found that reaction was proceeded with the autocatalytic mechanism due to dissociation of fatty acid itself in supercritical methanol. Furthermore, He et al. 4 tried to produce biodiesel from soybean oil by using flow reactor of 75 mL. The optimal reaction condition was determined as alcohol to oil molar ratio of 40:1, residence time of 25 min at 35 MPa and 310°C. The maximum yield was also found as 77% since the loss caused by the side reactions of unsaturated FAME at high reaction temperature. They also identified a critical reactor residence time of the reactants based on the reaction temperature. Bunyakiat et al. 5 also studied the continuous biodiesel production of coconut oil and palm kernel oil in the range of temperature of 270–350°C, pressure of 10–19 MPa, and methanol to oil molar ratios of 6–42. The best methyl ester conversion (96%) was obtained at a reaction temperature of 350°C, molar ratio of 42, and space time 400 s.
Some economic results were obtained by Marchetti and Errazu 6, and they employed a process simulator to analyze different scenarios and to evaluate productivity, raw material consumption, economic competitiveness, and environmental impacts of each process. The economic analysis showed that supercritical technology is suitable to be used but not financially profitable. The return over investment rate is the lowest; the payback time is too high. It should be noted that biodiesel is still far more expensive than conventional petroleum-derived diesel due to the higher feedstock and processing costs. Therefore, efforts should be focused on novel processes and using lower-cost feedstocks. In view of the circumstances, the supercritical alcohol process is also capable of using lower-cost feedstocks with high FFA content; reaction takes a shorter reaction time.
In the recent past, Haas et al. 7 achieved maximum esterification of soybean acid oil containing 59.3 wt% FFA at 65°C and a molar ratio of total fatty acid/methanol/sulfuric acid of 1:15:1.5 for 26 h. Today, in this work, FAMEs from olive acid oil in a continuous tubular reactor by using supercritical alcohol process is possible within few minutes.
Materials and methods
Materials
Acid oil, a by-product of olive oil refining, was kindly supplied by Verde A.Ş, and stored at laboratory conditions. It has greenish-yellow color and pasty form at laboratory conditions. It contains 91.67 wt% FFA and 3.76 wt% total unsaponifiable material which consists of 75.28% of squalene. The profile of fatty acids of acid oil is 8.81% of palmitic acid (C16:0), 1.35% of palmitoleic acid (C16:1), 2.75% of stearic acid (C18:0), 73.80% of oleic acid (C18:1), 7.77% of linoleic acid (C18:2), 0.03% of linolenic acid (C18:3), 0.36% of arachidic acid (C20:0), 0.31% of gadoleic acid (C20:1), and trace amounts of other fatty acids. It was used without further treatment. All chemical reagents used were also analytical grade.
Experimental apparatus
Experiments were performed using a bench-scale reaction system consisting of a high pressure pump (Autoclave Engineers), a tubular-flow reactor of 6 mL which was located in a tubular electrical furnace of 75 cm length, which was connected with PID controller (±1°C), a magnetic stirrer with temperature controller, and a back pressure regulator (BPR). The schematic diagram of the experimental setup used was shown in Fig. 1. In this system, the residence time of reactants in the tubular reactor could be regulated by adjusting the flux of the high-pressure pump (between 0.10 and 9.99 mL/min). The reaction temperature could also be adjusted by a controller of the electrical furnace in the range of 25–1250°C.
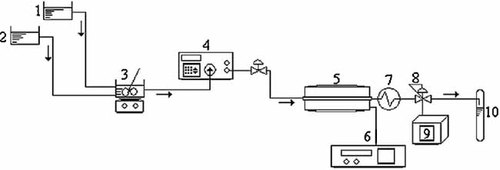
Schematic diagram of the experimental apparatus: (1) methanol tank, (2) acid oil tank, (3) magnetic stirrer with temperature controller, (4) high pressure pump, (5) a tubular reactor located in the tubular electrical furnace, (6) PID controller, (7) cooling in environmental conditions, (8) BPR, (9) digital pressure gauge, and (10) sample collection.
Design of experiments and statistical analysis
In this study, a five-level-four-factor Central Composite Design (CCD) was used to provide information regarding the interior of the experiment region. Hence, the effects of temperature (x1), pressure (x2), methanol to acid oil volumetric ratio (x3), and flow rate of the mixture (x4) on the ester content (yFAME) was evaluated. The coded levels as −2, −1, 0, 1, and 2, and the natural values of the factors set were shown in Table 1. The experimental sequence was randomized in order to minimize the effects of the uncontrolled factors. Moreover, six replications were performed at the center point to allow the estimation of the pure error. The complete design matrix of the experiments performed, requiring 30 experiments, was also shown in Table 2.
| Variables | Levels | ||||
|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |
| Temperature (°C), x1 | 300 | 320 | 340 | 360 | 380 |
| Pressure (bar), x2 | 200 | 220 | 240 | 260 | 280 |
| Methanol/acid oil volumetric ratio, x3 | 0.7 | 0.9 | 1.1 | 1.3 | 1.5 |
| Flow rate of mixture (mL/min), x4 | 0.4 | 0.6 | 0.8 | 1.0 | 1.2 |
| Run | x1 | x2 | x3 | x4 | yFAME |
|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | 77.05 |
| 2 | 1 | −1 | −1 | 1 | 75.78 |
| 3 | −1 | 1 | −1 | 1 | 70.72 |
| 4 | 1 | 1 | −1 | −1 | 85.34 |
| 5 | −1 | −1 | 1 | 1 | 63.04 |
| 6 | 1 | −1 | 1 | −1 | 81.09 |
| 7 | −1 | 1 | 1 | −1 | 73.97 |
| 8 | 1 | 1 | 1 | 1 | 70.90 |
| 9 | 0 | 0 | 0 | 0 | 76.97 |
| 10 | 0 | 0 | 0 | 0 | 77.64 |
| 11 | −1 | −1 | −1 | 1 | 65.43 |
| 12 | 1 | −1 | −1 | −1 | 81.45 |
| 13 | −1 | 1 | −1 | −1 | 72.83 |
| 14 | 1 | 1 | −1 | 1 | 76.77 |
| 15 | −1 | −1 | 1 | −1 | 71.35 |
| 16 | 1 | −1 | 1 | 1 | 68.89 |
| 17 | −1 | 1 | 1 | 1 | 59.50 |
| 18 | 1 | 1 | 1 | −1 | 81.24 |
| 19 | 0 | 0 | 0 | 0 | 76.96 |
| 20 | 0 | 0 | 0 | 0 | 77.15 |
| 21 | −2 | 0 | 0 | 0 | 62.46 |
| 22 | 2 | 0 | 0 | 0 | 84.64 |
| 23 | 0 | −2 | 0 | 0 | 70.03 |
| 24 | 0 | 2 | 0 | 0 | 74.63 |
| 25 | 0 | 0 | −2 | 0 | 77.08 |
| 26 | 0 | 0 | 2 | 0 | 70.49 |
| 27 | 0 | 0 | 0 | −2 | 84.33 |
| 28 | 0 | 0 | 0 | 2 | 64.59 |
| 29 | 0 | 0 | 0 | 0 | 77.53 |
| 30 | 0 | 0 | 0 | 0 | 75.97 |
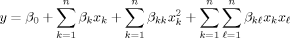 ()
() are the terms of main effects and the interaction effects, respectively. β0 is the intercept, βk, βkk, and βkl are the coefficients of linear, quadratic, and interaction effects, respectively.
are the terms of main effects and the interaction effects, respectively. β0 is the intercept, βk, βkk, and βkl are the coefficients of linear, quadratic, and interaction effects, respectively.The quality of the fit of the model, generated by Statistica software version 6.0 (StatSoft, Inc.), was also evaluated using the coefficients of determination and analysis of variance (ANOVA).
Esterification in supercritical methanol
It may be mentioned here that acid oil was used to convert to esters without any pre-treatment. Nevertheless, olive acid oil was heated to 35°C since it was in a pasty form at laboratory conditions, and premixed with methanol at a constant stirring rate of 200 rpm before fed into the tubular reactor. In these experiments, no catalyst was used. Experiments were performed based on experimental design, and operating conditions such as temperature (300–380°C), pressure (200–280 bar), methanol to acid oil volumetric ratio (0.7–1.5), and flow rate of the mixture (0.4–1.2 mL/min) were selected.
The esterification reactions were carried out in flow system (Fig. 1), and samples were collected in a glass vial placed at the reactor output after the steady-state condition was reached. Then, the product mixture was placed in a separating funnel. The upper phase consisting of unreacted methanol and acid oil was removed from the lower phase consisting of excessive methanol, biodiesel, and water. Finally, both phases were individually evaporated under vacuum at 80°C to separate the excessive methanol. However, it was visually observed crystalline particulates in crude FAME after a very short time. Indeed, the high unsaponifiable content (3.76 wt%) in the samples causes this kind of crystal formation. However, crude FAME samples without post-treatments were analyzed by GC.
Analysis methods
The content and fatty acid profile of crude FAME was determined by using Varian 450-GC equipped with a FID, and Varian Select™ Biodiesel for FAME column (part no: CP9080; 30 m × 0.32 mm × 0.25 µm) according to EN 14103. The operating temperatures for injector, detector, and oven were isothermal at 250, 250, and 210°C, respectively. The split ratio of 100 mL/min, and helium as a carrier gas (83 kPa) were used. Methyl heptadecanoate was used as an internal standard, and 1 µL sample was injected. Moreover, the fuel properties of crude FAME samples were determined according to EN 14214.
Results and discussion
Fatty acid profile in crude FAME
Some fatty acids are beneficial to the performance of biodiesel although this effect has not been conclusively confirmed with careful engine testing. For instance, oleic acid is probably the best, while linoleic is less desirable and linolenic acid is most undesirable. Tropical oils such as palm oil due to the contents of higher saturated fatty acids tend to have significant problems with cold-weather performance.
In this study, quality was also investigated depending on the fatty acid profile in crude FAME. There are three main kinds of fatty acids available in the crude FAME samples such as palmitic (C16:0), oleic (C18:1), and linoleic (C18:2) acid. The content of linolenic acid (C18:3) methyl ester, which has negative effects on oxidative stability and cold flow properties of biodiesel, was found almost in trace levels, as expected. The results also showed that oleic acid methyl ester was the main component, holding 44–66 wt% of biodiesel.
Optimization of reaction conditions by response surface methodology
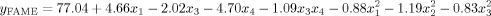 ()
()The ANOVA for reduced quadratic model is also provided as shown in Table 3. The F-value of 26.29 with a very low probability value (p-value < 0.01%) implies that the model is significant. Moreover, a high value of correlation coefficient, R (=0.98), indicates a close agreement between predicted value and actual value of response. As a result of statistical analysis, it was observed that the temperature and flow rate are the main effects on the ester content. While the temperature causes positive variation (+4.66) the flow rate is effective in negative form (−4.70) on the FAME content (%). In contrast, since the pressure factor within the selected operating conditions is statistically insignificant (p-value > 0.05) it is not available in equation (Table 4).
| Sum of squares | df | Mean | F | p-Level | |
|---|---|---|---|---|---|
| Regression | 1250.20 | 14.00 | 89.30 | 26.29 | 0.000000052 |
| Residual | 50.95 | 15.00 | 3.40 | ||
| Total | 1301.15 |
| Coefficient | Std. error | p-Level | |
|---|---|---|---|
| Intercept | 77.04 | 0.752413 | 0.000000 |
| x1 | 4.66 | 0.376206 | 0.000000 |
| x2 | 0.68 | 0.376206 | 0.089340 |
| x3 | −2.02 | 0.376206 | 0.000077 |
| x4 | −4.70 | 0.376206 | 0.000000 |
| x1x2 | 0.43 | 0.460757 | 0.364711 |
| x1x3 | 0.06 | 0.460757 | 0.898104 |
| x1x4 | −0.02 | 0.460757 | 0.969497 |
| x2x3 | −0.29 | 0.460757 | 0.534324 |
| x2x4 | 0.14 | 0.460757 | 0.759147 |
| x3x4 | −1.09 | 0.460757 | 0.032558 |
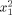 |
−0.88 | 0.351909 | 0.024049 |
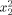 |
−1.19 | 0.351909 | 0.004142 |
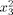 |
−0.83 | 0.351909 | 0.033197 |
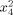 |
−0.66 | 0.351909 | 0.081848 |
- Bold values are statistically insignificant (p value > 0.05).
The optimal values of the selected variables, which were obtained by solving the regression equation (Eq. 2), were as follows: temperature of 380°C, pressure of 240 bar, methanol to acid oil volumetric ratio of 1.12 (or molar ratio of 9.45), and flow rate of mixture of 0.4 mL/min. The theoretical FAME content predicted under the above conditions was 92.25%.
In order to verify the prediction of the model, the optimal reaction conditions were applied to an extra experiment. The FAME content was obtained as 92.30%. This figure shows that well agreement within the estimated value of the model equation, and also the repeatability in the experimental setup is extremely high. Because the experiments were performed in the laboratory where the temperature is in the range of 14–21°C and the moisture is in the range of 48–54%, and at different times.
Effect of selected factors on the properties of FAME
Influence of methanol/acid oil volumetric ratio
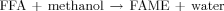 ()
()Since the esterification reaction is an equilibrium reaction, the ratio of alcohol to FFA is one of the main factors. As the reaction proceeds, water and methyl ester are formed until the equilibrium is reached. Methyl ester yield can be increased if water is removed from the reaction mixture during the esterification reaction. However, in this study, since supercritical alcohol can virtually esterify FFAs or transesterify triglycerides no sensitivity to water content, this conventional mentality does not consider. Moreover, an increase in the ratio of alcohol to oil in catalyst-free reactions provides greater contact between substrates, consequently favoring reaction conversion. The optimal value is defined as about 40:1 in the literature 4, 9, 10. Since methanol is a reactant in the reaction, higher methanol content will cause the reaction equilibrium to move to the side of production. Naturally, this can increase the biodiesel yield. Additionally, a higher molar ratio of methanol to oil means more oil can be dissolved in methanol due to the polarity of methanol under its supercritical state. Conversely, when fatty acids were preferred instead of triglycerides, oil needs less amount of alcohol consumption. This finding in this study well adjusted with the results of Minami and Saka 3 that were used two-step supercritical methanol process.
Influence of temperature
It is clearly found that temperature significantly affects both the conversion to FAME and the fatty acid profile of FAME. The temperature should be higher than 360°C to achieve higher than ester content of 80%. As a proof, the optimal temperature was found as 380°C in this study. Whereas, according to the literature, higher temperatures, typically above 350°C, can favor backward reaction of glycerol with methyl esters and may cause product degradation, thus decreasing the production of fatty acid esters 4, 11. In this study, since feedstock used contains negligible amount of glycerides there is no undesired thermal decomposition reaction due to glycerol formation. Therefore, even higher temperature of 380°C may be easily selected as reaction temperature. Furthermore, excess amounts of methanol reduces the critical temperature of the mixture, however, in this study, as mentioned earlier, alcohol consumption is much less due to usage of acid oil.
Influence of flow rate
According to previous studies, the biodiesel yield is directly connected with the residence time for reactants. The yield decreased with the increase of residence time after a critical point of residence time 4. However, the critical point of residence time can be changed depending on reaction temperature; namely, residence time is moved forward with the increase of reaction temperature. The same approximation was observed in this study although the reaction systems were somewhat different; there was a premixing with alcohol and acid oil in this study.
Influence of pressure
In the literature, a few investigations are available about the effect of pressure on the transesterification conversion using the supercritical method. Some of them show that conversion rises along with an increasing pressure. For example, He et al. 4 observed a positive effect in the range of 10–40 MPa, with the best condition found at 35 MPa. Conversely, Bunyakiat et al. 5 and Warabi et al. 2 found that pressure cannot have a significant influence on the reaction rate. In this study, experimental data show that the pressures over 220 bar insignificantly increase the conversion. This is statistically proved on the reduced model, and also found that optimum value of the pressure participates in the center point within operating conditions selected.
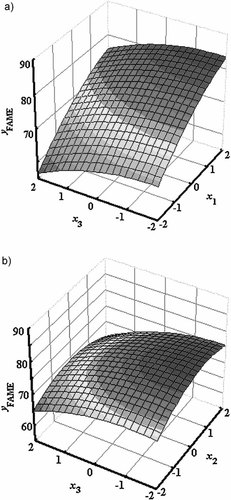
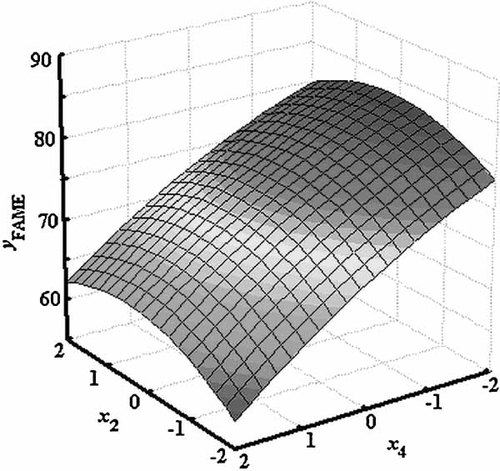
To accomplish this, the 3D response surfaces were plotted in Figs. 2-4, keeping each time the missing factor at its center level. It is deduced from Fig. 2 that the maximal acquisition of FAME contents was achieved with a high reaction temperature, lower methanol-to-oil volumetric ratio, and higher pressures than 220 bar. Figure 3 represents the effects of flow rate and pressure on the methyl ester content. The effect of pressure was basically visible for higher pressures than 240 bar and lowest flow rates. Otherwise, conversion to FAME does not occur. Figure 4 shows that there is available reversible effect for temperature and pressure on conversion of acid oil to FAME.
The effects of factors on the ester content: (a) ester content versus temperature and methanol/acid oil volumetric ratio; (b) ester content versus pressure and methanol/acid oil volumetric ratio.
The effects of flow rate and pressure on the ester content.
The effects of temperature and pressure on the ester content.
Analysis of crude FAME samples
The crude FAME samples obtained followed by reaction time in the ranges of 2–5 min is a clear yellow liquid. The fatty acid profile of samples was analyzed by GC, and it was clearly seen that the biodiesel obtained mainly contained three kinds of FAMEs. Although the stability of biodiesel depends on the fatty acid profile of feedstock used for the biodiesel production, the number and position of the double bonds, and forming by-products such as acids, esters, aldehydes, ketones, lactones, etc., cause auto-oxidation. Therefore, oxidation stability is one of the issues affecting the storage of biodiesel. In this study, this factor was measured by using Rancimat 743 according to EN 14214. It was not found to be in reasonable agreement with standard value (>6 h) due to high unsaponified content and FFAs of about 2% in the crude FAME. At the optimal condition, oxidation stability was found as 1.83 h which does not satisfy EN 14214 requirements. Moreover, kinematic viscosity is useful for monitoring the fuel quality of biodiesel during storage. It was found 4.71 cSt at 40°C well adjusted with standard value (3.5–5.0 cSt at 40°C). The density of crude biodiesel was also measured as 0.85 g/mL at 15°C. The water content is also an important factor since water can promote microbial growth, lead to tank corrosion, and participate in the formation of emulsions. In this study, it was measured as 202.88 ppm by Karl Fischer Titrator (Mettler Toledo DL39, USA).
Upgrading of biodiesel quality
Typical steps process in commercial biodiesel production may include pre-treatments such as filtration of solid precipitates; dewatering, refining, and degumming; followed by esterification or transesterification; and recovery of methanol from biodiesel and glycerin by distillation; then post-treatments such as removing of trace catalyst, soaps, and glycerin; filtering out adsorbent fines, sterol glucosides, and other solids. In this study, we focus our attention on the evaluation of acid oil as biodiesel, and determination of reaction conditions. Upgrading of crude biodiesel is beyond the scope of this study. However, biodiesel was treated with bleaching earth and calcium hydroxide at 80°C, and then coarsely filtered. Consequently, the methyl ester content of 96.6% was achieved (Table 5).
| Property | Method | Unit | Olive acid oil methyl ester | EN 14214 standard |
|---|---|---|---|---|
| Ester content | EN 14103 | % | 96.6 | >96.5 |
| Density at 15 °C | ISO 3675 | kg/m3 | 0.86 | 0.86–0.90 |
| Acid number | EN 14104 | mg KOH/g | 0.18 | <0.5 |
| Oxidation stability | EN 14112 | h | 1.74 | >6 |
| 12.08a) | ||||
| Viscosity at 40 °C | ISO 3104 | mm2/s | 4.68 | 3.5–5.0 |
| Iodine number | EN 14111 | g/100 g | 62.28 | <120 |
| Sulfated ash | ISO 3987 | g/100 g | <0.01 | <0.02 |
| Water content | EN ISO 12937 | mg/kg | 424.02 | <500 |
| Linolenic acid methyl ester | EN 14103 | % | 0.11 | <12 |
| Higher calorific value | – | MJ/kg | 39.6 | Not specified |
| Lower calorific value | – | MJ/kg | 37.9 | Not specified |
- a) By the addition of antioxidant of 0.3%.
Conclusions
The esterification of FFAs with supercritical alcohol provides a chance to produce cheap alternative fuels, which could reduce air pollution and protect the environment and save time and catalyst. In this study, olive acid oil containing FFA up to 90% was used as feedstock since the cost of feedstock accounts for more than 70% of the biodiesel production cost. Moreover, biodiesel production was performed using supercritical methanol since the conventional transesterification processes were not the feasible route. Consequently, biodiesel was obtained as ester content of 96.6%.
Acknowledgements
The authors would like to gratefully acknowledge TÜBİTAK (project no 107M243) for the financial support and Verde A.Ş. (www.olioverde.com.tr) who graciously provided olive acid oil.
The authors have declared no conflict of interest.




