IL-13 in dermal type-2 dendritic cell specialization: From function to therapeutic targeting
Abstract
Skin functions as a barrier protecting the host against physical, thermal, chemical changes as well as microbial insults. The skin is populated by several immune cell types that are crucial to host defense and to maintain self-tolerance as well as equilibrium with beneficial microbiota. Conventional dendritic cells (cDCs) are antigen-presenting cells that patrol the skin and all other nonlymphoid tissues for self or foreign antigens, and then migrate to draining lymph nodes to initiate T-cell responses. This review article describes recent developments on skin cDC specialization, focusing on the role of IL-13, a cytokine essential to allergic immune responses that is also secreted at steady state by type-2 innate lymphoid cells in healthy skin, and is required for dermal cDC differentiation. Furthermore, we contextualize how different therapeutics that block IL-13 signaling and were recently approved for the treatment of atopic dermatitis might affect cDCs in human skin.
Introduction
Dendritic cells (DCs) play an essential role in immune responses by taking up self and foreign antigens and presenting them to naïve T cells to induce their activation and proliferation. DCs also provide T cells with costimulatory molecules as well as cytokines and other accessory signals to induce T-cell differentiation into the appropriate effector phenotype. Interestingly, DCs whose developmental trajectory involves residence in nonlymphoid tissues (NLTs) before migration to the draining LN (dLN), also termed “migratory” DCs, are shaped by the NLT to acquire different phenotypes and preferentially induce different types of immune responses. This feature may be akin to the recognized tissue specialization of macrophages, which shape the NLT environment through production of different cytokines to support homeostasis and tissue function [1]. Here, we review DC tissue specialization in the context of IL-13, a cytokine that was recently found to be necessary for the normal differentiation of DCs in the dermal layer of the skin and fosters their capacity to prime Th2 responses.
DC specialization in nonlymphoid tissue
The DC family
DCs are a heterogeneous population of immune cells deriving from a common FLT3-dependent progenitor [2-6]. Within this family, the “conventional” (c)DCs are characterized by their highly efficient antigen-presenting function, and expression of CD26 and the transcription factor ZBTB46 [7, 8]. cDCs comprise two ontogenically distinct subsets, the type-1 cDCs (cDC1s) and type-2 cDCs (cDC2s) [9], that are distinguished by their properties and expression of several surface markers (summarized in Table 1 and extensively reviewed in [2]). In particular, XCR1 versus SIRPα (CD172a) expression separates cDC1s from cDC2s, respectively, across most tissues and secondary lymphoid organs of mice and humans [10]. cDC1s are very efficient at taking up apoptotic and infected cells for cross-presentation and priming of CD8+ T-cell responses [11, 12], while cDC2s are especially adept at taking up soluble and particulate antigens that they present to CD4+ T cells to induce their differentiation into effector populations with different cytokine profiles [11, 13].
| DC subset | Location | Markers | Growth factor requirement | Function |
|---|---|---|---|---|
| LN-resident cDC1s | dLN | CD26+ Flt3+ ZBTB46+ IRF8+, XCR1+, Clec9a+ | Flt3L | Antigen presentation, cross-presentation |
| LN-resident cDC2s | dLN | CD26+ Flt3+ ZBTB46+ SIRPα+, CD11b+ | Flt3L | Antigen presentation |
| Plasmacytoid DCs (pDCs) | Dermis, skin dLN | CD26+ Flt3+ ZBTB46– SiglecH+, BDCA1+ | Flt3L | IFN-I production |
| Plasmacytoid DC (pDC)-like | Dermis, skin dLN | CD26+ Flt3+ ZBTB46+ SiglecH+, BDCA1+, CX3CR1+ | FLT3L | IFN-I production, Antigen presentation |
| Migratory cDC1s (migDC1s) | Dermis, skin dLN | CD26+ Flt3+ ZBTB46+ IRF8+, XCR1+, Clec9a+ | FLT3L | Antigen presentation, cross-presentation |
| Migratory cDC2s (migDC2s) | Dermis, skin dLN | CD26+ Flt3+ ZBTB46+ SIRPα+ | FLT3L | Antigen presentation |
| Langerhans cells (LCs) | Epidermis, skin dLN | CSF1R+ CD26– ZBTB46+/– CD207high | IL-34 | Antigen presentation |
| Monocyte-derived DCs (moDCs) | Inflamed dermis | CSF1R+ CD26– ZBTB46+/– CCR2+ Ly6C+ | CSF1 | Antigen presentation |
| DC3sa) | Inflamed dermis | CD5– CD163+ CD14+ | CSF2 | Antigen presentation |
- a) Described in humans; they have not yet been identified in mice.
In addition to cDCs, the mouse and human DC families also include additional subsets which share some, but not all, properties of cDCs (Table 1). Plasmacytoid DCs (pDCs) are FLT3 dependent but ZBTB46 negative and secrete high amounts of type-I interferon (IFN-I), whereas plasmacytoid DC-like cells express ZBTB46 and, in addition to secreting IFN-I, can also present antigen [14]. The DC-like Langerhans cells are heterogeneous in terms of location in skin and ability to migrate to the dLN [15]; their development is independent of FLT3L but requires IL-34 and the expression of CSF1R. Monocyte-derived DCs (moDCs) [16] and the recently described CD14+ human DC3s [17], respectively, require CSF1 and CSF2 for their development. These DC populations are found during inflammation and are rare at steady state [16, 18].
Migratory cDC2s are phenotypically and functionally heterogeneous
Besides being classified into cDC1s and cDC2s, cDCs in mouse and human LNs can be further subdivided into "resident" and "migratory" subsets according to their route of entry into the LN (Table 1). LN-resident cDCs express an immature phenotype with low MHC class II (MHCII) and costimulatory molecules, and enter the LN directly via blood and high endothelial venules by a CD62L-dependent mechanism [19]. In contrast, migratory cDCs (migDCs) enter the dLN after residing for several days in NLT where they expand and differentiate into mature migDCs expressing high MHCII and costimulatory molecules [20]. Upregulation of CCR7 eventually leads mature migDCs to leave NLT to migrate to the dLN via afferent lymphatics by a CCL19/21-dependent mechanism [21, 22]. Such CCR7+ migDCs were identified in NLTs by single-cell (sc)RNAseq and termed “mature DCs enriched in immunoregulatory molecules” (mregDCs); they include both migDC1s and migDC2s [23] and represent a transient “cell state” rather than a true subset [23]. Overall, due to their different migration patterns, migDCs and resident cDCs play complementary roles in immune responses by sampling antigens that are either confined to NLT, or enter the LN via blood or lymphatic drainage, respectively.
MigDC1s are reported to express similar phenotypes and function regardless of location [12]. In contrast, migDC2s express unique markers according to the NLT site of maturation [24]. The phenotypic heterogeneity of migDC2s is observed in both mice and humans [25-27], suggesting that it is driven by the diverse tissue environments in which migDCs mature. It is especially apparent in migDC2s recovered from NLTs but also, to a lesser degree, in their respective dLNs [28, 29]. Transcriptomic analysis of migDC2 subsets from the LNs draining the skin, lung, and small intestine of naïve mice confirmed the existence of populations expressing unique gene signatures in the small intestine and skin to indicate migDC2 tissue specialization, but also revealed migDC2 populations expressing similar gene profiles despite their different tissues of origin, possibly suggesting a "default" differentiation pathway [28]. scRNAseq analyses of migDC2s isolated directly from NLTs, where tissue differences are more marked, will be essential to appreciating the diversity and tissue specialization of these populations.
The specific signals driving migDC2 diversification across tissues are gradually being characterized. In the small intestine, about 50% of lamina propria migDC2s uniquely express CD103 together with CD11b. Experiments in mice showed that the differentiation of CD103+CD11b+ migDC2s required all-trans retinoic acid (ATRA) [30] and DC expression of TGFβR1 [31]. Deletion of TGFβR1 in DCs resulted in a strong reduction of the intestinal CD103+CD11b+ population with a corresponding increase in CD103–CD11b+ migDC2s, together with decreased frequencies of Treg populations that promote tolerance to food antigens, and reduced frequencies of lamina propria Th17 cells [31]. Within the CD103+CD11b+ migDC2 subset, further diversification is associated with the precise location within the intestinal mucosa, with intra-epithelial migDC2s expressing an immature phenotype due to local high ATRA concentrations, while lamina propria migDC2s express a mature, pro-inflammatory phenotype [32].
The dermal layer of murine skin also harbors a unique migDC2 subset, the CD11blow migDC2s [33], which are genuine cDCs as demonstrated by their FLT3 dependence [34] and expression of CD26 and ZBTB46 ([10, 35] and our unpublished results). However, unlike other migDC2s, the differentiation of CD11blow migDC2s requires expression of the transcription factors KLF4 [35] and STAT6 [28]. KLF4 promotes the early development of all cDC2s, a function it carries out in cooperation with IRF4 [35]. In addition, together with STAT6, KLF4 enables migDC2s to respond to IL-13 secreted by dermal type-2 innate lymphoid cells (ILC2s) at steady state [28]. Since ILC2s in lung and small intestine do not express IL-13 at steady state [28, 36], this IL-13 dependence explains at least in part why the CD11blow migDC2 subset is found only in skin. As is the case for CD103+CD11b+ intestinal migDC2s, the factors promoting phenotypic specialization also shape functional activity, with innate IL-13 skewing the antigen presenting function of CD11blow migDC2s away from Th17 to favor Th2 differentiation [28, 35]. It has not been demonstrated whether this skewing of Th differentiation supports essential skin homeostasis. However, it is plausible that Th2 responses might play a useful role in skin by sensing external threats via IgE-dependent mast cell activation [37], IL-31-dependent itch responses [38, 39], or by promoting tissue repair to quickly restore barrier function [40]. Ultimately, how the IL-13-dependent CD11blow migDC2s might support optimal skin function will require further study.
IL-4 and IL-13 receptors and their signaling
The cytokines IL-4 and IL-13 are produced during helminth infections and allergies and play a key role in orchestrating effector Th2 immune responses. In both mice and humans, IL-4 and IL-13 signal through related heterodimeric receptors which share the same IL-4Rα signaling subunit, Janus kinase (JAK)1, and STAT6 signaling components (Fig. 1) (reviewed in [41]). The “type-I” IL-4 receptor (IL-4R) is composed of one IL-4Rα chain and the common γ chain (γc), is mainly expressed on immune cells, and binds only IL-4. The IL-13 receptor (IL-13R), also termed “type-II” IL-4R as it also binds IL-4, is a dimer of one IL-4-binding IL-4Rα subunit and one IL-13-binding IL-13Rα1 subunit, and is expressed by cells of hematopoietic and non-hematopoietic origins except lymphocytes. IL-13Rα1 and γc contribute to signaling through their association with JAK2 and Tyrosine kinase (TYK)2, or JAK3, respectively (Fig. 1). IL-13 can also bind with high affinity to a second receptor, IL-13Rα2, initially classified as a decoy receptor but now thought to have functional activity [42]. Although it can be expressed in certain conditions [43], IL-13Rα2 is generally not expressed on cDCs and will not be further considered.
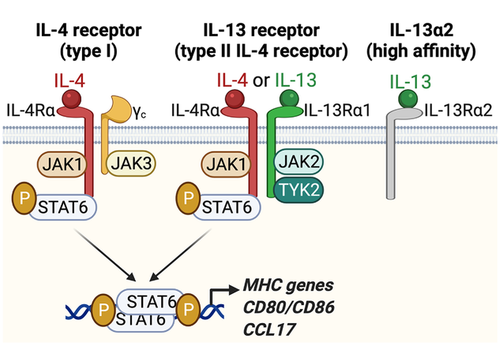
Due to the distinct expression patterns of their receptors, IL-4 and IL-13 drive different aspects of type-2 immunity. IL-4 is essential to the regulation of lymphocyte responses as it is required for the maintenance of Th2 cell fate and IgE class switching in B cells. In addition, IL-4 and IL-13 act on nonimmune cells regulating many aspects of Th2 inflammation including cell traffic through endothelia, mucus production at epithelial surfaces, tissue repair, and fibrosis [44]. IL-4 and IL-13 also regulate the function of myeloid cells through both IL-4R and IL-13R and the cooperative activity of the transcription factors STAT6 and KLF4 [45]. Classically, IL-4 and IL-13 are used to induce alternative activation of macrophages in vitro while also inhibiting pro-inflammatory polarization induced by exposure to Toll-like receptor (TLR) ligands and/or interferon (IFN)-γ [46]. The typical markers of macrophages activated with IL-4 and IL-13 are molecules with regulatory functions or associated with type-2 immunity such as the mannose receptor 1 (MRC1 or CD206), Arginase 1, RELMα, IL-10, TGFβ1, and CCL17. Alternatively-activated macrophages are mainly associated with wound healing and tissue repair [47].
IL-4 and IL-13 signaling in cDC differentiation
IL-4/IL-13 signaling has been shown to be involved in the differentiation of thymic cDC populations as well as migDC2 populations in dermis.
Bone marrow (BM)-derived early thymic progenitors (ETPs) are pluripotent stem cells that develop into either lymphoid or myeloid cells within the thymus. IL-4/IL-13 signaling in ETPs activates both STAT6 and STAT1. While STAT6 inhibits the lymphoid fate, STAT1 guides ETPs toward the cDC1 fate by increasing IRF8 expression [48, 49]. Therefore, IL-4/IL-13 in the thymus act at a much earlier stage of lineage differentiation compared to skin, where they are thought to target cells that are already committed to the migDC2 lineage. More recently, in a mechanism akin to that observed in the skin, Th2 cytokines were shown to also activate a population of thymic SIRPα+CD301b+ cDC2s that mediate clonal deletion but are not necessary for Treg differentiation [50]. Although the source of IL-13 in the thymus has not been determined, thymic invariant natural killer T cells are known producers of IL-4 [51].
As discussed above, IL-13 is also expressed at steady state by ILC2s in mouse dermis [52] and is secreted independently of microbiota and the alarmins TLSP, IL-25, or IL-33 [28, 36] to induce the differentiation of dermal cDC2 precursors into CD11blow instead of CD11bhi migDC2s [28]. Interestingly, the lack of IL-13 signaling resulted in correspondingly increased numbers of CD11bhi migDC2s in skin and dLN, but did not fully ablate CD11blow migDC2 differentiation with the few remaining CD11blow cells maintaining a degree of transcriptional identity when compared to CD11bhi by either bulk or scRNAseq technologies [28]. In addition, CD11bhi migDC2s expressed similar transcriptional profiles in WT and STAT6 knock-out (KO) mice, suggesting that the differentiation of this population was not affected [28]. Together with the observation that IL-13 treatment could rescue the differentiation of dermal CD11blow migDC2s in IL-13-KO mice, but did not induce CD11b downregulation in all dermal migDC2s, or in migDC2s in other tissues [28], these data suggest that differentiation into the CD11blow phenotype may require the cooperation of IL-13 signaling together with additional and yet unidentified signals specific to the dermal niche.
The impact of IL-13 signaling on migDC2 function
Several mouse studies have documented how IL-13 affects the phenotype and function of migDC2s. In skin, IL-13-dependent differentiation into CD11blow migDC2s was associated with many changes in gene expression compared to CD11bhi migDC2s [28]. Transcripts for several pattern recognition receptors (PRRs) including Tlr1, Tlr2, Tlr6, and Tlr11 were expressed at a significantly lower level in CD11blow migDC2s compared to CD11bhi, as were the expression of the TLR chaperone transcript Unc93b1 and the fungal receptors Clec7a and Clec4n (respectively, encoding Dectin-1 and Dectin-2). Decreased expression of these microbial sensors likely explains the reduced upregulation of transcripts for the proinflammatory cytokines Il12a, Il23a, Il12b, and Il1b in CD11blow migDC2s that had taken up inactivated mycobacterial or fungal pathogens compared to CD11bhi [28, 53]. Interestingly, decreased PRR expression had no impact on the upregulation of CD86, PDL2, and CCR7 after exposure to microbial signals, with these markers being expressed at similar or higher levels on the CD11blow compared to CD11bhi migDC2s [53]. Similar IL-4/IL-13-dependent changes in cDC cytokine production were also observed in a mouse model of atopic dermatitis (AD) [54] and in neonatal type-2 immune responses [55], in which IL-13 from mast cells or basophils, respectively, downregulated the expression of IL-12 by cDCs. Similarly, IL-4 blockade enhanced IL-12 production by intratumoral antigen-bearing cDC1s thereby reducing tumor burden [56]. On the other hand, IL-4Rα-dependent signaling in moDCs was necessary for Th1 responses in Leishmania major-infected mice [57], while signaling in cDC1s exacerbated Th1 inflammation in cerebral malaria [58]. These latter findings may be consistent with the reported ability of IL-4 to increase bioactive IL-12 in murine spleen cDCs [59], suggesting that the impact of IL-4/IL-13 signaling on IL-12 expression in DCs may be subset and context dependent.
Gao et al. [60] also reported lower PRR expression in PDL2hi BM-DC populations that were preferentially associated with Th2 responses. They found that Tlr1, Tlr2, Tlr6 as well as Tlr4 and Tlr5 transcripts were expressed at a lower level in PDL2hi compared to PDL2lo cells from GM-CSF BM-DC cultures, while Tlr9 transcripts were higher. In addition, PDL2hi BM-DCs treated with LPS or CpG expressed lower levels of Il6, TNFa, Il1b, and Il12a compared to PDL2lo BM-DCs. Given the heterogeneity of BM-DC cultures [61], it is unclear whether the reported differences between PDL2hi and PDL2lo populations were due to differential TLR expression in distinct DC subsets, as proposed by the authors, or differential expression in the macrophage versus DC populations present in those cultures. Notwithstanding the uncertainties, all these studies associate reduced DC responsiveness to microbial stimuli with an increased ability to prime Th2 responses.
In addition to dampening Th1 and Th17 polarization, IL-13 signaling in migDC2s appears to be a positive driver of Th2 differentiation (Fig. 2). As mentioned above, steady-state dermal IL-13 drives the differentiation of a population of mouse CD11blow migDC2s that are necessary for Th2 differentiation [28, 35]. In the lung, Halim et al. were the first to show that, after inhalation of protease allergens, IL-33-dependent ILC2-derived IL-13 is necessary for migDC2 upregulation of CCR7, migration to dLN, and for the upregulation of CD40 and Th2 cell priming in dLN [62] (Fig. 2). As the specific DC signals that drive Th2 differentiation in LN are unknown, it is not possible to test their expression and dependence on IL-13 signaling. The described reduced levels of microbial PRR and proinflammatory cytokines might promote Th2 differentiation by either preventing the production of cytokines that inhibit Th2 differentiation such as IFNγ, or by enabling migDC2s to acquire a “default” activation phenotype. Alternatively, CD11blow migDC2s [34] appear especially responsive to the Th2-promoting cytokine thymic stromal lymphopoietin (TSLP) [63], although the molecular basis of this increased responsiveness is undefined. Unlike spleen cDCs which require IL-4 signaling to express functional TSLP receptor in vivo [64], migDC2 populations from skin, lung, or small intestine all express the necessary transcripts for TSLP-dependent signaling regardless of STAT6 expression [28], suggesting that the increased responsiveness of CD11blow migDC2s to TSLP is not due to higher receptor expression but is likely mediated via other mechanisms. Finally, IL-13 induces expression of CCL17 by migDC2s to support the recruitment of effector Th2 cells into lung or skin [65]. CCL17 is also upregulated by skin-dLN migDC2s exposed to allergens [66] and might promote interaction between antigen-loaded migDC2s and CD4+ T cells during Th2 differentiation in LN.
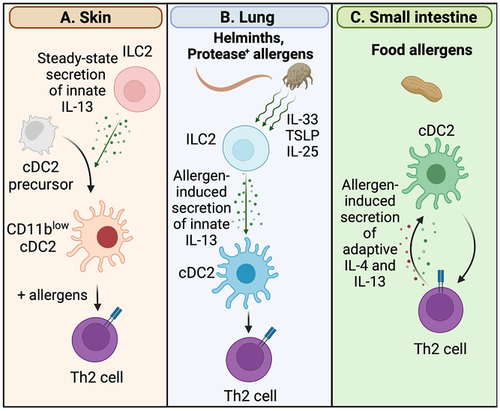
Whether IL-13 signaling in migDCs is important in the induction of Th2 immune responses in the intestinal tract is yet to be formally demonstrated, however, evidence suggests that, together with IL-4, this cytokine might at least facilitate the continuation and propagation of Th2 responses. In mice, IL-4+ Th2 cells specific for ingested antigens were found to "educate" antigen-presenting DCs to more efficiently prime Th2 responses of different specificities [67]. IL-4 and IL-10 were necessary but not sufficient for DC education, suggesting that additional T-cell-derived signals were also involved [67]. In humans, peanut allergen-specific Th2 cells drove the IL-4Rα-dependent differentiation of CD23+ DCs, which may be more efficient at presenting allergens through IgE/CD23-dependent uptake, and CD209+ moDCs which stimulate Th production of IL-4 and IL-13 more efficiently than their CD209-negative counterparts [68] (Fig. 2). These studies imply that existing food allergies may foster further allergic responses in the intestine.
Together with studies showing that the deletion of IL-4Rα in DCs and monocytes impaired Th2 responses to the parasite Schistosoma mansoni [69], as well as the differentiation of Th2 cells and infiltration of eosinophils into the lung in a model of allergic airway inflammation induced by house dust mite [70], these observations suggest that IL-13 skews DC function toward a Th2 stimulatory function, regardless of tissue location and of whether IL-13 is produced at steady state or induced by cytokine signaling or antigen recognition.
IL-13 and cDC2s in human skin in health and disease
IL-13 in healthy human skin
The finding that IL-13 is necessary for the differentiation of skin migDC2s in mice raises the question of whether similar mechanisms are also operating in the skin of healthy humans. Freshly explanted human ILC2s were reported to produce low levels of IL-13 protein in vitro [71, 72]. In addition, IL13 transcripts were detected in human skin [73, 74] and could be traced to populations of ILC2s and T cells [28, 73]. The latter finding was confirmed in a recent article [75] reporting that ILC2s represented about 70% of the total ILC population in skin and were enriched in skin compared to blood suggesting preferential entry and/or differentiation. In healthy donors, ILCs were mainly found in the upper dermis, where migDCs are most abundant [76], and approximately 15% expressed IL13 transcripts. Thus, molecular and histological evidence suggest that, similar to mice, ILC2s in human skin are a steady-state source of IL-13 and may be located in the vicinity of dermal migDCs.
High-dimensional analyses of human DCs from lymphoid tissues and NLTs including skin have shown that skin migDC2s harbor unique phenotypic and transcriptomic profiles compared to cDC2s in other tissues [25, 27]. While these analyses did not identify distinct subsets within skin migDC2s, they confirmed an influence of the skin environment on local migDC2s. An unbiased analysis of publicly available datasets to identify potential drivers of the skin-specific signature showed that migDC2s from healthy human skin express an IL-4/IL-13 gene signature compared to cDC2s in blood, spleen, and lung, with core enrichment genes such as IRF4 and CCL22 also highly expressed in mouse CD11blow migDC2s [28]. As these analyses were carried out on bulk RNA data, they cannot establish whether the IL-4/IL-13 signature was expressed by all migDC2s from human skin or by only a selected subset as observed in mice. Nonetheless, together with data suggesting that IL-13-producing ILCs are present in healthy human skin, these analyses suggest that the differentiation of human and mouse dermal DC2s is likely driven by the same cytokine signals.
IL-13 in atopic dermatitis and inflammatory skin diseases
While the significance of IL-13 expression in skin at steady state is only starting to be appreciated, the high expression of IL-13 transcripts and protein in the lesional skin of AD patients are well described. AD is a common skin inflammatory disease characterized by an itchy rash [77, 78]. It is especially frequent in children and is associated with dysbiosis, loss of skin barrier function due to genetic variation, and increased incidence of other types of allergic disease including food allergies, asthma, and rhinitis [79]. The lesional skin of AD patients harbors increased numbers of IL-13+ CD4+ Th2 cells reacting to specific allergens and ILC2s responding to cytokines/alarmins including TSLP and IL-33. These CD4+ T cells and ILC2s also express additional Th2 cytokines including IL-5 and IL-4, with ILC2s also acquiring expression of Th17 cytokines including IL-22 and IL-26 [75]. Dysbiosis of the skin microbiome is commonly observed in AD, especially a predominance of Staphylococcus aureus which increases during disease flares [78]. It remains unclear whether dysbiosis is a driver of disease, or a consequence of the increased skin permeability and reduced antibacterial responses caused by the dominant type-2 environment. DC populations including migDC2s are consistently increased in AD lesions, and express an activated phenotype with increased expression of the type-2 chemokines CCL17 and CCL22, and the chemokine receptor CCR7, compared to healthy controls [74, 80]. Activated HLA-DRhiCCR7hiFSCN+ mregDCs expressing high CCL17 have also been identified in AD lesions [18], together with DC3s and Langerhans cells with a proliferative phenotype [81].
AD shares some features with another inflammatory skin disease, psoriasis (PS), which also presents with itchy skin lesions. Unlike AD, psoriatic lesions are characterized by the infiltration of Th17 cells with overexpression of IL-23, IL-17, and IL-22 [82]. Although 81% of AD dysregulated genes are shared with PS [83], the two conditions are rarely reported to co-exist in the same patient [84], and when they do, each type of lesion upregulates the respective cytokines characteristic of each disease [85]. Due to the different cytokine environment, migDC2 populations in lesional PS skin are also distinct from those found in AD: CCL17 and CCL22 are not upregulated while, consistent with the Th17 profile, IL23 and IL1B-expressing pro-inflammatory DC3s are enriched [18]. Interestingly, genome-wide association studies indicate an opposing influence of polymorphisms in the IL13 locus and in other Th2-related genes such as the RAD50/Th2 Cytokine Locus Control Region on AD and PS, with IL-13 and/or RAD50 activity positively correlating with the risk of AD [86, 87] while the correlation with PS is negative [88, 89]. These opposing effects of IL-13 are also consistent with a number of case reports of psoriatic lesions appearing in patients treated with Dupilumab, a biological agent that blocks IL-13 (and IL-4) signaling [90, 91]. Together, these data suggest that sufficient IL-13 is necessary for the maintenance of skin health and local immune homeostasis.
Overall, observations showing different DC phenotypes and T-cell cytokine profiles in AD and PS, and the opposing impact of IL-13 on these diseases, have striking similarities with the observations in mice [28] that IL-13 modulates dermal migDC2 differentiation and functional activity, promoting allergic immune responses while inhibiting anti-microbial immunity. This similarity may suggest a role of IL-13-dependent regulation of migDC2 function in the propensity to AD and PS, and that manipulating the IL-13 signaling pathway in DCs might offer useful treatment opportunities. Clearly, IL-13 can also affect other cell types in skin, including macrophages and especially nonimmune cells such as keratinocytes and stromal cells, which also play an important role in the pathogenesis of AD and PS [92].
Treatment and therapeutic targeting of IL-4R and IL-13R signaling
Several recently generated therapeutic monoclonal antibodies block IL-4 and/or IL-13 signaling with varying degrees of specificity by binding to the IL-4Rα subunit of both the IL-4 and IL-13 receptors [93], binding IL-13 to prevent dimerization of the IL-13Rα1 and IL-4Rα subunits [94], or blocking IL-13 binding to the IL-13Rα1 and IL-13Rα2 subunits [95] (Table 2). Each of these treatments have demonstrated improved symptoms and patient quality of life in moderate to severe AD while suppressing objective measures of type-2 inflammation. The efficacy of IL-13-binding monoclonals in AD may imply that blocking the activity IL-13 is sufficient at reducing AD inflammation, and that the additional blockade of IL-4 provides little further benefit compared to blocking IL-13 alone [94]. This result is consistent with IL-13 being found in AD lesions at higher levels than IL-4, and better correlating with the severity of disease (reviewed in [96]). The important role of IL-13 as the driver of allergic tissue inflammation also extends to mouse studies of oxazolone-dependent experimental AD [97].
| Administration route | Agent | Target | Reference |
|---|---|---|---|
| Subcutaneous | Dupilumab | IL-4Rα (blocks IL-4 binding to IL-4R and IL-13R) | [93] |
| Tralokinumaba) | IL-13 (blocks binding to IL-13Rα1 and IL-13Rα2) | [95] | |
| Lebrikizumabb) | IL-13 (prevents formation of the IL-13Rα1/IL-4Rα heterodimer) | [94] | |
| Topical | Ruxolitiniba) | JAK1, JAK2 | [100] |
| Delgocitiniba) | JAK1, JAK2, JAK3, TYK2 | [101] | |
| Tofacitinibc) | JAK1 JAK3 | [102] | |
| Oral | Baricitinibd) | JAK1 JAK2 | [103] |
| Abrocitinibe) | JAK1 | [104] | |
| Upadacitinibe) | JAK1 | [105] |
- a) FDA approved Sept./Dec. 2021.
- b) Phase II completed.
- c) Under review.
- d) European Medicines Agency (EMA) approved 2020, FDA approval pending.
- e) FDA approved, Jan. 2022.
- * Information updated May 2022.
The impact of IL-4Rα blockade on AD inflammation has been characterized by bulk and single-cell transcriptomic studies as well as through biomarker analyses [80, 93]. These studies found normalization of the lesional skin transcriptome, including in keratinocytes, and reduced disease biomarkers in serum. However, even after 1-year treatment, the levels of serum IL-13 remained high, and there were significant proportions of disease-associated mregDCs and Th2 cells expressing IL13 and IL22 in skin [80]. These findings suggest that some degree of immune activation was persisting in the skin of treated patients, which is presumably independent of IL-4/IL-13 signaling.
Downstream IL-4R and IL-13R signaling via phosphorylation-dependent activation of the JAK/STAT pathway has provided additional therapeutic targets in the treatment of patients with AD. JAK inhibitors are small therapeutic molecules that block the enzymatic activity of receptor-associated tyrosine kinases and were recently reviewed in [98]. A range of JAK inhibitors (Table 2) in Phase II and III clinical trials have demonstrated significant improvement in AD patient outcomes and several oral and topical formulations are now approved for treatment of AD. Although these agents do not affect only the IL-4R/IL-13R signaling pathways and have wide-ranging effects, their topical use is effective and associated with fewer adverse effects in patients with AD [99]. How JAK inhibitors affect the function of skin migDC2s is currently unknown.
Conclusions and future perspectives
The first identification of IL-13 as a cytokine that conditions migDC2s to instruct Th2 differentiation was in the context of airway ILC2s activated by allergen proteases [65]. While the pro-Th2 function of IL-13 is conserved in skin, its regulation by allergens is not: skin IL-13 is produced at steady state regardless of allergens, alarmins or microbiota, and drives the differentiation of a dermal CD11blow migDC2 subset specialized for Th2 induction [28]. The constitutive expression of IL-13 opens several interesting questions on the role of IL-13 in skin function and allergy development: first, whether IL-13 secretion by dermal ILC2s is indeed constitutive or is regulated by yet-to-be-identified signals, and whether it acts in concert with other factors specific to the skin environment to support the differentiation of CD11blow migDC2s. These studies could generate interesting information on how IL-13 production is regulated by environmental factors. Second, the role of IL-13 in licensing migDC2s to prime Th2 responses may provide useful leads into the key molecular changes that enable DCs to induce Th2 differentiation. The identification of these changes has eluded immunologists for many years. And, last but not least, why is IL-13 made in skin at steady state? How are Th2 immune responses advantageous to skin to justify the presence of a mechanism that specifically promotes their priming? The answers to these questions will provide new insight on the immune functions of skin and how they contribute to maintaining issue homeostasis and susceptibility or resistance to inflammatory skin disease.
Acknowledgements
This work was supported by a Health Research Council of New Zealand Independent Research Organization grant to the Malaghan Institute of Medical Research.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Author contributions
OL, MB, and FR wrote the manuscript and edited and approved the final draft.
Open Research
Data availability statement
None.
References
Abbreviations
-
- AD
-
- atopic dermatitis
-
- dLN
-
- draining LN
-
- ETP
-
- early thymic progenitor
-
- ILC
-
- innate lymphoid cell
-
- migDC
-
- migratory cDC
-
- moDC
-
- monocyte-derived DC
-
- NLT
-
- nonlymphoid tissue
-
- PS
-
- psoriasis
-
- TSLP
-
- thymic stromal lymphopoietin