Intestinal homeostasis and inflammation: Gut microbiota at the crossroads of pancreas–intestinal barrier axis
Abstract
The pancreas contains exocrine glands, which release enzymes (e.g., amylase, trypsin, and lipase) that are important for digestion and islets, which produce hormones. Digestive enzymes and hormones are secreted from the pancreas into the duodenum and bloodstream, respectively. Growing evidence suggests that the roles of the pancreas extend to not only the secretion of digestive enzymes and hormones but also to the regulation of intestinal homeostasis and inflammation (e.g., mucosal defense to pathogens and pathobionts). Organ crosstalk between the pancreas and intestine is linked to a range of physiological, immunological, and pathological activities, such as the regulation of the gut microbiota by the pancreatic proteins and lipids, the retroaction of the gut microbiota on the pancreas, the relationship between inflammatory bowel disease, and pancreatic diseases. We herein discuss the current understanding of the pancreas–intestinal barrier axis and the control of commensal bacteria in intestinal inflammation.
Introduction
The pancreas is a vital organ for both digestive and endocrine functions. It secretes pancreatic juice, which is rich in alkaline bicarbonate and various digestive enzymes (e.g., trypsin, lipase, and amylase), into the duodenum [1]. The pancreas also has an endocrine function, as it secretes various hormones (e.g., insulin, glucagon, somatostatin, pancreatic polypeptide, gastrin, etc.). In the past decades, multiple well-established communication pathways between the pancreas endocrine system and gut have been discovered. For example, the secretin-bicarbonate axis between duodenal Brunner cells and pancreatic bicarbonate excretion [2, 3]. Additionally, various intestinal peptide hormones, such as secretin, glucagon-releasing peptide, galanin, somatostatin, appetite control peptides (leptin and ghrelin), vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, and substance P, which are found in the gut and its nervous system, have been shown to directly or indirectly affect the pancreatic endocrine function [4]. This evidence strongly demonstrates an inextricable link between the pancreas and the gut. In recent years, a growing body of evidence has emerged to support that the pancreatic exocrine function affects the gut immunity, which further supports the pancreas–intestinal axis [5].
The gut hosts a vast commensal microbial community. These bacteria are often beneficial, helping metabolize food and calibrating immune responses to eliminate pathogens (e.g., bacteria, fungi, and viruses) or to create homeostatic responses to commensal organisms. Pathobionts, a subset of bacteria, exist as symbionts but can promote pathological conditions when specific genetic or environmental conditions alter their genetic expression [6]. These include Enterococcus gallinarum, Proteus mirabilis, and Escherichia coli. These bacteria can act as pathogens and translocate across the intestinal epithelial barrier, invade other tissues or organs, and cause diseases [5, 7–11]. Inflammatory bowel disease (IBD) is linked to pathobionts that exacerbate intestinal inflammation by promoting excessive host immune responses or impairing the mucosal barrier [10, 12–18]. For example, adherent invasive Escherichia coli (AIEC) not only induces epithelial IL-8 production but also induces macrophages to produce TNF-α which exacerbates inflammation [19, 20]. In addition, Proteus spp. Morganella and Providencia spp. can cause colitis by producing urease [21-23], while Mucispirillum can exacerbate gut inflammation by driving colitogenic Th1 CD4+ T cells [24].
To cope with this challenge, the gut has evolved several defenses against pathobionts. In addition to traditional defense mechanisms, the pancreas contributes to the front-line of defense against pathobionts [5]. The pancreas secretes regenerating gene (Reg) [25], secretory phospholipase A2 (sPLA2) [26], pancreatic lipase [27], trypsin [28], and glycoprotein 2 (GP2) [5], which protect the gut through anti-microbial effects by inducing bacterial lysis [25, 29], protecting the epithelial layer [25], balancing intestinal microbiota [27], regulating bacterial translocation [5], and activating other germicidal proteins (e.g., Reg3α/γ and α-defensin) [30, 31].
Conversely, gut microbes also affect the pancreas. For example, IBD patients are at higher risk for multiple pancreatic diseases, including pancreatitis [32], pancreatic cancer [33], and type II diabetes [34]. Furthermore, bacteria can translocate to the pancreas under pathological conditions [35, 36]. With the increase in intestinal microbes and by compromising the epithelial barrier function, pancreatitis is more severe and may lead to acute necrotizing pancreatitis (ANP) [37, 38].
Taken together, understanding the communication loop between the pancreas and gut reveals why pancreatic disease is often associated with intestinal diseases and may lead to the development of therapeutic targets for intractable pancreatic and intestinal diseases.
Pathobionts and related disease
Pathobionts and IBD
IBD is a chronic inflammatory disorder that includes ulcerative colitis (UC) and Crohn's disease (CD) [39]. It has been shown that IBD patients show dysbiosis in the gut [40]. However, it is unclear whether dysbiosis plays a causal role or is just the result of inflammation, and hard to specify the particular organism which is associated with inflammatory disease. In this section, we show some organisms which have been implicated with the pathogenicity of IBD (Table 1). By adhering to epithelial cells, E. coli is frequently recovered from CD patients [41-43]. AIEC adheres to the epithelium via type1 pili (FimH) on the surface of AIEC isolates, interacting with cell adhesion molecule 6 (CEACAM6) receptors [44]. AIEC elicits epithelial IL-8 expression by binding flagella, leading to TNF-α production by macrophages and enhanced inflammation [19, 20]. P. mirabilis is another well-studied pathobiont [9]. Colonization experiments of mice with DSS showed severe colitis than control [10, 45]. Zhang et al. identified P. mirabilis by urease gene-specific gene amplification because Proteus. spp are widely recognized to produce urease activity [10]. A recent study showed that urease genes got involved in the pathogenesis of CD [21]. Bacterial urease hydrolyzes host-derived urea in the colon into ammonia which is the preferred nitrogen source for E. coli [21]. This leads to a predominance of Proteobacteria, including AIEC [21] and the exacerbation of colitis, which implies that, in addition to P. mirabilis, other urease-producing bacteria (e.g., Morganella and Providencia spp.) can expand and contribute to colitis [22, 23]. Rare species (e.g., Bilophila wadsworthia) usually proliferate in response to diets that are high in certain saturated fats and have been implicated with inflammatory disease [12, 46, 47]. Saturated fats promote taurine-conjugation of hepatic bile acids, which increases the availability of organic sulfur used by sulfate-reducing microbes like B. wadsworthia. Subsequently, expansion of B. wadsworthia activates dendritic cells to release IL-12p40, which induces Th1 CD4+ T cells that mediate colitis [12]. Several studies reported that Mucispirillum spp. is high in the intestine of colitis model mice [48-50]. And in recent years, Mucispirillum spp. was shown to be involved in intestinal inflammation in immunodeficient mice [24]. Defects of NOD2 cytochrome b-245 beta chain (CYBB) (important for microbial clearance) can result in the accumulation of Mucispirillum schaedleri [24]. Caruso et al. found that Mucispirillum appears to enhance colitogenic Th1 CD4+ T cells, as observed in NOD2 −/– CYBB−/− mice gavage with Mucispirillum [24]. Enterotoxigenic Bacteroides fragilis has also been detected in intestinal mucosa of IBD patients and reported to acerate inflammation of colitis mice [51-53]. Cao et al. revealed the underlying mechanism of enterotoxigenic B. fragilis-mediated intestinal inflammation [54]. Enterotoxigenic B. fragilis-facilitated T-helper type 17 cell differentiation by downregulating exosomal miR-149-3p, which suppresses the expression of IL17A and receptor-related orphan receptor (ROR) C in CD4 T cells [54].
| Pathobionts | Pathogenesis | References |
|---|---|---|
| Adherent-Invasive E. coli (AIEC) | Induce severe colitis in CEABAC10 transgenic mice expressing human CEACAMs | [17] |
| Proteus mirabilis | Induce pro-inflammatory pathways in gnotobiotic mice | [10] |
| Bilophila wadsworthia | Increase incidence of colitis in susceptible Il10−/− mice | [12] |
| Mucispirillum schaedleri | Trigger early-onset spontaneous TH1-type intestinal inflammation in NOD2−/–CYBB−/− mice | [24] |
| Enterotoxigenic Bacteroides fragilis | Accelerate DSS–induced colitis | [13, 14] |
| Saccharomyces cerevisiae | Worsen intestinal disease by enhancing gut epithelial leakage in colitis mice | [16] |
| Debayomyces hansenii | Impair mucosal healing in biopsy injury in mice | [15] |
| Enterococcus faecalis | Induce chronic colitis in susceptible mono-associated Il10−/− mice | [18] |
- Abbreviations: CEACAM, carcinoembryonic antigen-related cell adhesion molecule; CYBB, cytochrome b-245 beta chain; NOD2, nucleotide-binding oligomerization domain-containing protein 2.
Pathobionts that translocate to the pancreas
Pathobionts invading the epithelium can translocate to underlying layers and be disseminated to other organs (e.g., to the mesenteric LN via lymphatics or liver via the portal vein, and the pancreatic duct en route to the pancreas) [36, 55, 56]. Translocations trigger innate and adaptive immune responses and induce inflammation of colonized tissues or organs [57] (Table 2).
| Bacteria | Disease | Tissue | References |
|---|---|---|---|
| E. gallinarum | Systemic lupus erythematosus | Mesenteric veins, mesenteric lymph node (MLN), liver, spleen | [7] |
| Autoimmune hepatitis | Mesenteric veins, MLN, liver, spleen | ||
| Severe experimental pancreatitis | Blood, pancreas, spleen | [58] | |
| Primary sclerosing cholangitis | MLN | [8] | |
| P. mirabilis | Primary sclerosing cholangitis | MLN | |
| Enterococcus | Stroke | Blood, lung | [59] |
| Alcoholic hepatitis | Liver | [60] | |
| Enterococcus, E. coli | Cirrhosis | MLN | [11] |
| Acute pancreatitis | Pancreas | [35] | |
| E. faecalis | Inflammatory bowel disease | Mesenteric fat, omentum | [61] |
| Enterocolitis | MLN | [62] | |
| Lactobacillus spp. | Myeloid leukemia | Blood, MLN, spleen | [63] |
| Systemic lupus erythematosus | MLN, Liver, Spleen | [64] | |
| Clostridium innocuum | Adipose hyperplasia in enteritis site of Crohn's disease | Creeping fat of mesenteric adipose tissue | [65] |
| E. hirae | Promoting cyclophosphamide in the treatment of cancer | MLN, spleen | [66] |
| Lachnospiraceae | Hepatocellular carcinoma | Liver | [67] |
| Candidatus Arthromitus sp. (SFB) | Cirrhosis | MLN, ascitic fluid, blood | [68] |
Translocated bacteria, especially Proteobacteria in the pancreas, are associated with pancreatic ductal adenocarcinoma—a major tumor of this organ [55]. In patients with these tumors, Proteobacteria account for nearly 50% of the intrapancreatic microbiome in pancreatic ductal adenocarcinoma (PDAC)-bearing patients [55]. Dysbiosis in the pancreatic microbiome induces tumor-promoting macrophage differentiation via activation of TLR2 and TLR5 by lipopolysaccharides and flagellins, respectively [55]. Other research suggests that translocation to pancreatic LNs can induce type 1 diabetes (T1D) in mice. In more detail, using a streptozotocin-induced diabetes model Costa et al. demonstrated that NOD2 receptor activation by bacterial muramyl dipeptide drives differentiation of pathogenic Th1 and Th17 cells, which in turn contribute to T1D [69]. It is believed that both NOD2 activation and the release of autoantigens by the β cells damaged by the streptozotocin act synergistically to reach a threshold of β cell death that leads to hyperglycemia and consequently diabetes [69]. Although the pathogenesis of autoimmunity against cells remains to be established, gut bacteria may be involved in T1D, as this relationship may contribute to comorbid IBD and type 2 diabetes [70]. It should be noted that in addition to bacterial translocation due to the break of the epithelial barrier, gut microbiota may have a direct influence on type 2 diabetes via an effect on immune regulation [71, 72].
Homeostatic defense by pancreatic mediators in the intestine
As an organ directly connected to the gut, the pancreas was once known for its digestive functions. However, increasing evidence suggests that it also has a role in fighting off invading pathobionts and maintaining the balance of the intestinal flora. The pancreatic exocrine function has been shown to contribute significantly more to the composition of intestinal microorganisms than any other host factor in individuals without pancreatic disease [73, 74]. On the other hand, in individuals in whom the pancreatic exocrine function is compromised or those who have undergone total/partial resection of the pancreas, the intestinal microbes may be in a state of dysbiosis. For example, harmful Klebsiella spp. are enriched, while Faecalibacterium prausnitzii and Roseburia spp. decreased, which are thought to have potential anti-inflammatory effects [75, 76].
Pancreatic proteins
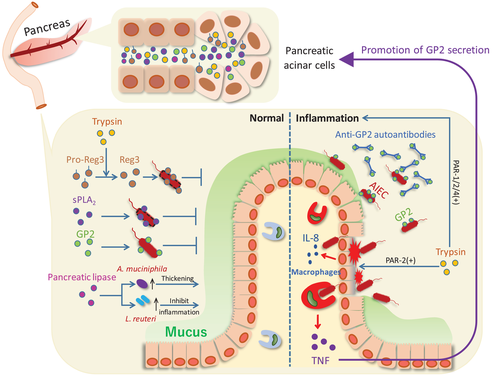
Reg is part of the C-type lectin family [77] and plays a role as an antibacterial protein, which is mainly produced by pancreatic acinar cells and secreted into the intestine to prevent bacterial invasion [25]. Reg is involved in various pathological processes (e.g., tumorigenesis and diabetes), and provides beneficial antibacterial functions that protect the host [25]. The increased expression of Reg2, 3β, 3γ in the pancreas occurs in mice with colitis, suggesting communication between the pancreas and inflamed colon [5]. Reg3, a core Reg family member, is a multifunctional antimicrobial peptide [77]. It not only causes bacterial membranes to perforate [25] but also limits the interaction between bacteria and the intestinal epithelium [78] (Fig. 1). Reg3γ mainly targets Gram-positive bacteria, while Reg3β targets Gram-negative organisms [79-81]. Gene-manipulated mice lacking Reg3γ showed altered bacterial distribution in their inner mucus, causing an increase in direct interactions between bacteria and epithelial cells, leading to severe ileal inflammation [82]. Another study on ethanol-induced alcoholic steatohepatitis showed that Reg3β and Reg3γ could limit bacterial retention on the intestinal surface, thereby reducing pathobiont translocation caused by the disruption of the intestinal barrier by ethanol [83].
Pancreatic PLA2 enzymes
sPLA2 is the largest subfamily of PLA2 enzymes [26]. Based on a cluster analysis of varying propeptide sequences, specific disulfide bonds, and C-terminal extension sequences of these subtypes, human sPLA2 is divided into three groups: type I/II/V/X, type III, and type XII [84]. Evidence suggests that subtypes IB, IID, V, X, and XIIB are expressed in the pancreas [26, 85, 86].
Among the subtypes, IIA sPLA2 is known for its potent antibacterial action. IIA sPLA2 is mainly found at the barrier site, after secretion by prostatic epithelial, lacrimal gland, and other cells [87]. IIA sPLA2 has a high affinity for phospholipid glycerol, which is rich in bacterial membranes while having a low affinity for phosphatidylcholine, found in mammal cell membranes [87, 88]. Even though there is no established evidence of the pancreatic expression of IIA sPLA2—also called nonpancreatic type—it is reasonable to suspect that the structural similarity of the V and X subtypes confers similar antibacterial effects (Fig. 1). Weaker antibacterial activity of subtypes V and X than IIA has been reported [29], but they are also relatively bactericidal against Gram-positive bacteria [29]. sPLA2 in group X showed significant bactericidal activity against L. monocytogenes and S. aureus [29], while group V displayed a bactericidal effect on Micrococcus luteus treated with lysozyme to lyse the cell wall [89]. It is worth mentioning that, slightly different from II A sPLA2, groups V and X have strong hydrolytic ability for PC, suggesting a potential anti-inflammatory function [90].
Pancreatic lipase
A recent study reported additional functions of pancreatic lipase. Investigating the mechanisms by which pancreatic enzyme replacement therapy works, pancreatic lipase, in addition to its promoting fat and protein-absorption functions, has positive effects on the gut microbiota [27] (Fig. 1). 16S ribosomal RNA gene amplicon analyses of microorganisms in the cecum, transverse colon, and stool of pancreatic lipase-treated mice reveal Akkermansia muciniphila and Lactobacillus reuteri, essential probiotics in the intestine, were increased in relative abundance [27]. However, the underlying mechanism remains to be determined.
A. Muciniphila is believed to break down mucin into propionic and acetic acids and send immune and metabolic signals to the host [91]. Ethanol-induced intestinal barrier dysfunction is associated with a decrease in A. muciniphila [92]. A. muciniphila supplementation enhances the intestinal barrier function by increasing numbers of goblet cells, mucin two mucus layer thickness, and tight junction protein [92], which prevent pathobiont translocation and inflammation [93]. Furthermore, L. reuteri is effective in inhibiting intestinal inflammation [94, 95]. Thus, it follows that pancreatic lipase indirectly protects the gut barrier through the balancing of gut microbial communities. Notably, pancreatic lipase may indirectly affect colitis in other ways, aside from balancing the microbiome. For example, in pancreatitis patients, the lipase exocrine secretion from the pancreas is often insufficient [96]. Lipase insufficiency was reported to worsen the consequences of malabsorption in CD [97]. The above evidence indicates that pancreatitis may cause or aggravate colitis, but the underlying association remains to be demonstrated.
Trypsin
As the primary enzyme secreted by the pancreas, trypsin performs digestive functions and regulates the activities of other proteins. Inactive pro-Reg3α/γ can be converted into an active form by trypsin hydrolysis [31] (Fig. 1). Moreover, in humans, pro-α-defensin can be activated by trypsin hydrolysis [30]. α-defensin has a bactericidal effect by causing bacterial membrane perforation through electrostatic interaction [30].
Several serine proteases, including trypsin, affect immune responses [98]. Their dysregulated hydrolysis can lead to structural and functional changes of the mucosal barrier, as well as inflammation [98, 99] (Fig. 1). For example, the protease-activated receptor (PAR) family is widely expressed in the GI tract, and PAR-1, 2, and 4 activity can be regulated by serine, which causes inflammation [100]. Moreover, PARs can be expressed on the apical and basolateral sides of intestinal epithelial cells; thus, some (PAR-1 and 2) may be activated by serine proteases (e.g., trypsin). This will disrupt tight junctions and affect intestinal permeability [100, 101]. Several recent studies shifted from expression profiles to proteolytic activity, finding that increased hydrolytic activity of serine proteases is associated with irritable bowel syndrome and IBD [28, 102]. In addition to barrier damage, high proteolytic activity seems to be associated with decreased microbial diversity; however, the causal relationship remains unclear [28]. Thus, pancreatic trypsin may impact many aspects of intestinal disease development.
In conclusion, as an organ physically and directly connected to the gut, the pancreas is an essential digestive organ that also secretes various defensive mediators (e.g., Reg, sPLA2, pancreatic lipase, and trypsin) that contribute to intestinal homeostasis. Continuous exploration of new pancreatic-derived defense mediators will further our understanding of the pancreas–gut axis and may provide novel therapies for diseases such as IBD.
Novel front-line of defense by pancreatic mediators in the intestine
GP2 is the most abundant zymogen granule membrane protein secreted by pancreatic acinar cells [5]. It is anchored to the zymogen granule membrane by a glycosylphosphatidylinositol [5, 103]. GP2 is demonstrated to be constitutively released by glycosylphosphatidylinositol hydrolysis, independent of digestive signals and bacterial stimulation [5, 104]. We previously reported that microfold cells (M cells) in the follicle-associated epithelium of Peyer's patches express GP2 [5, 105–107]. To investigate the primary source of intestinal GP2, we examined the precise GP2 distribution in the digestive system and generated conditional GP2-deficient mice. Results suggested that intestinal GP2 is primarily of pancreatic origin and can spread throughout the small intestine and colon lumen [5].
GP2 production has been demonstrated to be increased in colitis [5]; however, studies on the role of pancreatic GP2 in intestinal inflammation have been limited. To determine the cause of the increased GP2 expression in colitis, we established an in vitro acinar cell culture system based on Ptf1aCre-ERTM-tdTomato mice. We found that TNF-α, an inflammatory cytokine associated with colitis, is the leading cause [5].
Although the exact function of GP2 in intestinal inflammation is unknown, the homolog of GP2, Tamm–Horsfall protein (THP), has been studied extensively. THP is the most prevalent protein in mammalian urine [108]. Previous studies showed that THP binds specifically to FimH expressed by E. coli and Salmonella typhimurium [109]. FimH is essential for bacteria to bind to epithelial cells; thus, THP can prevent bacteria from attaching to and invading urothelial cells [110]. Inspired by the THP studies, we determined that GP2 has a similar protective effect on the intestinal epithelium. We detected more inflammation and mucosa-associated commensal bacteria (e.g., AIEC) in pancreas-specific GP2-deficient colitis mice in comparison to normal mice [5]. In colitis, TNF-α was shown to upregulate GP2, which specifically binds to FimH on the surface of symbiotic E. coli, preventing epithelial attachment and penetration [5]. The addition of recombinant GP2 significantly reduced bacterial invasion [5]. We also demonstrated that under inflammatory conditions—once GP2-mediated bacterial inhibition is broken—translocated GP2-conjugated bacteria induce GP2 autoantibodies, which neutralize GP2 and exacerbate inflammation [5]. Other reports indicate that GP2 inhibits the secretion of pro-inflammatory cytokines (e.g., CXCL8, IL-17, TNF-α) and reduces the activation and proliferation of inflammatory T cells [103]. In summary, GP2 is an essential member of the first line of defense against adhesive and invasive symbiotic bacteria during gut inflammation (Fig. 1).
Pancreatic-intestine axis in inflammation
Increased risk of pancreatic dysfunction in IBD patients
IBD patients may have a higher risk of multiple pancreatic diseases, including pancreatitis [32], pancreatic cancer [33], and type II diabetes [34, 111].
Although the hazard of acute pancreatitis brought by other diseases is not always excluded, the higher incidence in IBD patients was confirmed [112]. However, the question is whether IBD itself increases the risk of pancreatitis because many other factors associated with the incidence of pancreatitis, such as diabetes [113], obesity [114], alcohol-related disease, biliary stones [115], and dugs [115], may not be well excluded. Yet, according to a novel cohort study, approximately 20% of pancreatitis in IBD patients is idiopathic (second to drug-induced pancreatitis, which accounts for 50%), and it also points out that a significantly increased rate of acute pancreatitis recurrent was found in UC patients [32]. However, another meta-analysis suggested that the incidence of acute pancreatitis in CD patients is higher than that in UC patients [116]. In conclusion, IBD patients face a higher risk of pancreatitis, but if IBD directly increases pancreatitis incidence needs more research.
Additionally, IBD is reportedly associated with pancreatic cancer and diabetes. Although a previous meta-analysis indicated an unchanged risk of pancreatic cancer in IBD patients [117], a 442 IBD patient-based study reported that the incidence of pancreatic cancer was increased in IBD patients [33]. Consequently, maybe it is too early to confirm the relationship between pancreatic cancer and IBD. Additionally, a nationwide study also shows that IBD patients have a higher overall risk of cancer, including pancreatic cancer [118].
With respect to diabetes, a nationwide cohort study from Denmark showed a higher risk of type 2 diabetes in IBD patients [70]. Notably, research has found that the mutation of some autoimmune-related genes overlaps between IBD and type 1 diabetes patients, including protein tyrosine phosphatase non-receptor type 2 (PTPN2) and PTPN22, which negatively regulate T-cell activation [119]. Therefore, the association between T1D and IBD may be involved in immune dysfunction. Especially, mutations of PTPN2 and PTPN22 were revealed to alter the composition of intestinal microbes in IBD patients [120]. This raises the possibility that overlapping genes between IBD and diabetes may contribute to the connection to the commensal microbes in the gut.
Here we show some clinical works to discuss the relationship between pancreas diseases and IBD. However, the mechanism beyond this connection is not well known. It could relate to immune alterations, gut microbes alterations, or IBD complications [115].
Bacterial translocation and the gut-pancreas loop
Gut microbiota translocating into the pancreas disrupts local homeostasis. The analysis of acute pancreatitis patients’ feces revealed an increase in E. coli and Enterococcus and a KEGG pathway analysis of 16s rRNA sequencing data also described enhancement of invading E. coli into epithelial cells [121]. Using an acute pancreatitis (AP) mouse model, the same research group confirmed the correlations between the gut microbiota burden and AP severity [94]. ANP is a severe form of AP [37]. In other studies, an E. coli MG1655 mono-colonized ANP rat model showed more severe pancreatic injury in comparison to a normal ANP rat model with significant upregulation of the TLR-4-mitogen-activated protein pathway and activation of the endoplasmic reticulum stress pathway in intestinal epithelial cells [38]. These data suggest that a heavy intestinal bacteria burden and barrier disruption aggravate the severity of pancreatitis. Whenever the intestinal barrier is disrupted by inflammation, the risk of bacterial translocation is increased. Supporting this, adherent E. coli strains were isolated from the mucosa of CD patients [122] and E. coli was also detected in the pancreas of AP model rats [123]. Besides, E. coli was found to have increased in the blood of both the CD and UC patients in comparison to the healthy group [123]. This raises the possibility that commensal bacteria enter the pancreas when the gut barrier is disrupted by colitis. To determine whether gut bacteria translocate into the pancreas and contribute to AP development, Li et al. conducted in situ hybridization on pancreatic sections from an acute pancreatitis model [35]. They found that E. coli only existed in affected mice with severe pancreatic injury [35]. To confirm the relationship between E. coli translocation and AP severity, they conducted experiments on germ-free (GF) mice [124], and pseudo-GF mice (an animal model with the loss of most gut bacteria). The number of E. coli in the pancreas was reduced in pseudo-GF mice and absent in GF mice in comparison to SPF mice [35]. Since AP was less severe when the recovery of gut microbiota was reduced (as it was in the NLPR3-deficient AP mouse model), they proposed that NLPR3 may be a crucial factor in the gut microbiota-pancreatitis axis. To clearly understand the gut-pancreas microbial loop, more information on the translocation of gut microbiota into the pancreas and how it impacts pancreatic homeostasis is required.
Notably, the normal pancreas also harbors bacteria from the gastrointestinal tract. Human pancreas tissue-based data showed significantly higher rates of Ancinobator and Pseudomonas in the normal pancreas in comparison to the pancreas of patients with pancreatitis or PDAC. The same study reported an increased number of E. coli in pancreatitis and increased Propionibacterium in PDAC samples [125].
Potential mechanisms through which gut microbes are transported to the pancreas are not well known. One possibility that has been suggested is by the pancreatic duct [126]. Another possibility is transfer from the distal gastrointestinal tract via the bloodstream through the portal vein. It is well known that inflammatory diseases, such as IBD, are associated with higher intestinal permeability [127]. Increasing permeability of the gut also leads to increased translocation of commensal bacteria [128].
A recent study [129] strengthens this hypothesis using butyrate (a metabolite that enhances the gut barrier [130] and protects the host gut from pathogen invasion [131]) to relieve ANP. van den Berg et al. [129] found that the oral administration of butyrate to ANP model mice enhanced the intestinal barrier function and reduced the numbers of bacteria in both blood and the pancreas. Importantly, they noted that the reduced numbers of bacteria in the blood and pancreas were associated with the protective effect of butyrate, since colonization of Proteobacteria in the cecum, specifically Escherichia/Shigella, was completely prevented. Moreover, they showed that the intravenous injection of butyrate did not have the same effect as oral administration, indicating that the reduced bacterial load in the pancreas and blood due to the administration of butyrate does not directly affect the survival of bacteria that are already present in the pancreas and blood—rather, it suppresses their translocation from the intestine.
Utilizing gut microbiota, such as probiotics, in the treatment of pancreatitis is still controversial. Studies have implied that the administration of Lactobacillus plantarum by internal feeding [132], or nasojejunal tube [133] had a positive effect with respect to reducing pancreatitis sepsis. Another research group pointed out that treatment with a widely used probiotic, Bifidobacterium, reduces the gastrointestinal dysfunction [134]. In contrast, newer research revealed that the combination of Lactobacillus and Bifidobacterium therapy failed to prevent infectious complications in pancreatitis patients [135]. However, the author of that study also noted that nutrient overabundance and delayed administration time may have influenced the failure of their probiotic treatment [135].
There are many gaps in our knowledge pertaining to the pancreas-intestine axis. Although some pancreatic diseases, like diabetes, pancreatitis, and pancreatic cancer, are more prevalent in the presence of intestinal inflammation, few studies have confirmed the mechanistic role of gut bacteria. Other microorganisms may translocate while microbial metabolites targeting the pancreas could also contribute to disease.
Conclusion
A new front has opened in the pancreas–intestinal axis as the evidence of this connection emerges. The pancreas maintains intestinal homeostasis by releasing different proteases or other substances into the gut. Conversely, the commensal microbes and neurons activated by gut microbes also alter the function and homeostasis of the pancreas. In-depth exploration of the pancreas–intestinal axis is therefore of great importance. This would provide a better understanding of the mechanisms of various normal physiological functions and disease development. This field of research is advancing rapidly. Novel therapies that alleviate the social burden of intractable pancreatic and intestinal diseases may be developed in the near future.
Acknowledgements
This work was supported by grants from The Ministry of Education, Culture, Sports, Science, and Technology (MEXT) for LEADER (Y.K.); Japan Agency for Medical Research and Development (AMED) PRIME (20gm6010012h0004/20gm6210024h0001) (Y.K.) and Project Focused on Developing Key Technology for Discovering and Manufacturing Drugs for Next-Generation Treatment and Diagnosis “The next-generation drug discovery and development technology on regulating intestinal microbiome” (NeDDTrim) (JP21ae0121040) (H.K.); Japan Society for the Promotion of Science (JSPS) for Grant-in-Aid for Scientific Research S (18H05280) (H.K. and Y.K.) and Scientific Research B (19H03450) (Y.K.), Challenging Research (Exploratory) 21K19494] (Y.K.), Funds for the Promotion of Joint International Research (18KK0432) (Y.K.), Danone Institute of Japan Foundation (Y.K.), The Naito Foundation (Y.K.), Hoyu Science Foundation (Y.K.), Waksman foundation of Japan (Y.K.), Yamada Science Foundation (Y.K.), IMSUT International Joint Research Project K003, RO1 (AI079145-08) (P. B. E.), Future Medicine Funds at Chiba University (Y.K.), and the Chiba University-UC San Diego Center for Mucosal Immunology, Allergy, and Vaccines (cMAV) (H.K., P. B. E.).
Conflict of interest
The authors declare no commercial or financial conflict of interest.
References
Abbreviations
-
- AIEC
-
- adherent invasive E. coli
-
- ANP
-
- acute necrotizing pancreatitis
-
- AP
-
- acute pancreatitis
-
- CD
-
- Crohn's disease
-
- CYBB
-
- cytochrome b-245 beta chain
-
- GF
-
- germ-free
-
- GI
-
- gastrointestinal
-
- IBD
-
- inflammatory bowel disease
-
- IBS
-
- irritable bowel syndrome
-
- M cells
-
- microfold cells
-
- PAR
-
- protease-activated receptor
-
- PC
-
- phosphatidylcholine
-
- PDAC
-
- pancreatic ductal adenocarcinoma
-
- PG
-
- phospholipid glycerol
-
- Reg
-
- regenerating gene
-
- sPLA2
-
- secretory phospholipase A2
-
- T1D
-
- type 1 diabetes
-
- THP
-
- Tamm–Horsfall protein