Neutrophil extracellular traps exacerbate Th1-mediated autoimmune responses in rheumatoid arthritis by promoting DC maturation
Additional correspondence: Dr. Panayotis Verginis, Division of Immunology and Transplantation, Biomedical Research Foundation, Academy of Athens, 11527 Athens, Greece
e-mail: [email protected]
Abstract
Aberrant formation of neutrophil extracellular traps (NETs) is a key feature in rheumatoid arthritis (RA) and plays a pivotal role in disease pathogenesis. However, the mechanism through which NETs shape the autoimmune response in RA remains elusive. In this study, we demonstrate that inhibition of peptidylarginine deiminases activity in collagen-induced arthritis (CIA) mouse model significantly reduces NET formation, attenuates clinical disease activity, and prevents joint destruction. Importantly, peptidylarginine deiminase 4 blocking markedly reduces the frequency of collagen-specific IFN-γ-producing T helper 1 (Th1) cells in the draining lymph nodes of immunized mice. Exposure of dendritic cells (DCs) to CIA-derived NETs induces DC maturation characterized by significant upregulation of costimulatory molecules, as well as elevated secretion of IL-6. Moreover, CIA-NET-treated DCs promote the induction of antigen-specific Th1 cells in vitro. Finally, NETs from RA patients show an increased potential to induce the maturation of DCs from healthy individuals, corroborating the findings obtained in CIA mouse model. Collectively, our findings delineate an important role of NETs in the induction and expansion of Th1 pathogenic cells in CIA through maturation of DCs and reveal a novel role of NETs in shaping the RA-autoimmune response that could be exploited therapeutically.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by persistent synovial inflammation resulting in articular cartilage and bone damage. Years before the clinical diagnosis, pathogenic autoantibodies against citrullinated proteins (ACPAs) and rheumatoid factor (RF) are detected and they are considered as serologic hallmark of the disease 1. Citrullinated antigens (Ags) and their presence in arthritic joints correlate with increased immunogenicity and concomitant disease severity 2, 3.
Dendritic cells as professional Ag-presenting cells (APCs) migrate to the lymph nodes where they process and present acquired Ags to naïve T cells and secrete cytokines resulting in skewing of naïve T cells toward T helper (Th1, Th17) 4. Emerging data have revealed that the Th1 and Th17 are of the major T-cell subsets during the course of RA but what drives their polarization and expansion is unknown.
The existence of neutrophils in RA synovial fluid (SF) particularly in early disease stages supports a fundamental role of these cells in disease pathogenesis 5-7. One of the mechanisms that has been proposed for neutrophils to cause tissue damage and autoimmune responses is through neutrophil extracellular trap (NET) formation 8-10. During the formation of NETs, neutrophil granule proteins complexed with chromatin are externalized, thus promoting differential cell activation and cytokine release 11, 12. Among the cellular events considered to be critical in NET formation are histone citrullination and decondensation catalyzed by peptidylarginine deiminase 4 (PAD4 13, 14. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide (Cl-amidine), a pan-PAD inhibitor, has previously been shown to inhibit NETs in mice and improve disease phenotypes in murine models of lupus and atherosclerosis 15, 16. NETs can exert immunostimulatory roles on different cell types and contribute in diverse disease processes such as cancer, thrombosis, and atherosclerosis 8, 17, 18. It has been shown that RA patients have an increased NETosis rate compared with both healthy and patients with osteoarthritis, while NETs’ presence correlated with levels of ACPA 11. Of interest, RA-NETs were shown to contain citrullinated proteins and enhance the inflammatory response in fibroblasts from inflamed joints 11. Although the pivotal role of NETosis in the pathogenesis of RA is emerging, the precise mechanisms involved in NET-induced autoimmune response are not known.
Herein, using the collagen-induced arthritis (CIA) mouse model, we demonstrate for the first time that NETs promote the induction of pathogenic IFN-γ-producing Th1 immune responses through modulating DC activation and maturation. Our findings reveal that the administration of Cl-amidine during CIA—a PAD activity blocker—inhibited the production of CIA-NETs, attenuated arthritis severity, and decreased Ab response to bovine collagen.
Results
Enhanced spontaneous NETs formation in PMNs from RA patients and CIA bone marrow
NETs have been demonstrated to be a prominent feature in RA polymorphonuclear cells (PMNs) 11. Thus, we sought to assess NETosis in a cohort of RA patients with high inflammatory burden (clinical characteristics are in Table 1). We found that RA PMNs display increased propensity to spontaneously form NETs compared to control PMNs isolated from healthy donors (Fig. 1A and B). Specifically, a significant increase in NET formation in RF (+) as compared to RF (–) RA patients was demonstrated (Supporting Information Fig. 1A). We next assessed whether RA inflammatory milieu could drive NET formation. In order to assess the capacity of soluble factors to induce NETosis, PMNs were isolated from healthy donors and treated with 2% serum or SF isolated from RA patients or healthy serum. It was found that both RA serum and SF are potent inducers of NETosis in healthy PMNs (Fig. 1C). Furthermore, serum or SF derived from RF (+) patients induced higher NETosis as compared to the serum or SF from RF (–) RA patients (Supporting Information Fig. 1B and C). Collectively, the RA inflammatory burden is associated with the increased rate of spontaneous NET release. In addition, the existence of autoantibodies further augments NETosis, which is in accordance with previous reports 11.
| Variable | RA (n = 30) | Control (n = 15) |
|---|---|---|
| Age (mean ± SD) | 63 ± 12.63 | 54 ± 22 |
| Gender (% females) | 67% | 52% |
| Disease duration (months, mean ± SEM) | 44.4 ± 6.55 | |
| RF (% positive and mean titer ± SEM, IU/mL) | 47% (229.2 ± 15.77) | |
| ACPA (% positive and mean titer ± SEM, U/mL) | 37% (329.1 ± 133.6) | |
| ESR (mean ± SEM, mm/h) | 40.6 ± 3.64 | |
| CRP (mean ± SEM, mg/dL) | 4.5 ± 1.64 | |
| Disease activity score 28 joints (DAS28) | 6.3 ± 0.78 | |
| Medications (% taking) | ||
| Naïve to treatment | 23% | |
| Methotrexate | 45% | |
| Leflunomide | 20% | |
| Biologic agents | 45% | |
| Glucocorticoids | 43% |
- ESR, erythrocyte sedimentation rate, CRP, C-reactive protein.
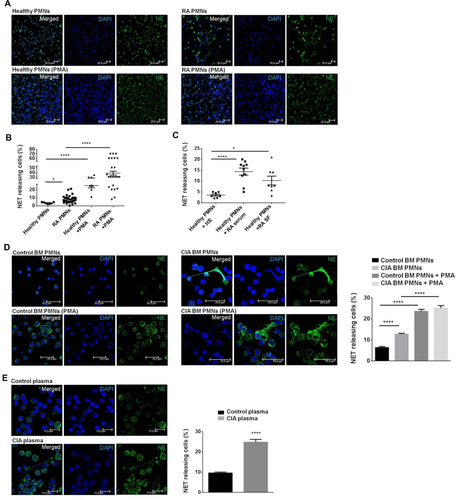
To delineate the role of NETs in the adaptive immune responses, we first sought to establish whether aberrant NETosis also occurs in CIA mouse model. Thus, we found that BM PMNs from CIA diseased mice (d35–45 post collagen injection) had higher numbers of spontaneously released NETs, characterized as DNA structures complexed with neutrophil elastase (NE) compared to BM PMNs isolated from naïve mice (Fig. 1D). Moreover, costaining of DNA with anticitrullinated H3 revealed that externalized NETs contained citrullinated epitopes (Supporting Information Fig. 2).
Next, we asked whether the inflammatory environment of CIA could induce NET formation. To this end, BM PMNs from naïve DBA/1 mice were treated with 2% CIA or naïve mouse plasma. Interestingly, CIA plasma induced significantly elevated NET release compared to naïve plasma that failed to induce NETosis, as evidenced by the colocalization of DNA with NE (Fig. 1E). Collectively, these results provide evidence for an increased NET formation by PMNs in CIA model and corroborate human data that NETs are driven by CIA inflammatory milieu.
Cl-amidine treatment during CIA reduces clinical disease activity and joint destruction
PAD4 inhibition has been shown to prevent NETs 15, 16 and thus Cl-amidine is considered as an inhibitor for NET formation. Cl-amidine is a highly specific inhibitor of PAD4 and related intracellular and extracellular PAD isozymes 19. Indeed, in vitro Cl-amidine treatment prevented hypercitrullination of histone H3 in naïve PMNs as shown by Western blot (Supporting Information Fig. 3). Given that citrullination (catalyzed by PAD4) is also an important mechanism during RA pathogenesis, we sought to investigate the phenotypic and immunological impact of PAD4 inhibition—as an inhibitor of NETosis—in the CIA model.
We initially examined the effect of Cl-amidine administration in BM-derived CIA-NETs. To address this, we administered Cl-amidine intraperitoneally (i.p., or DMSO as vehicle control) daily from d21 through d45 after priming with bovine type II collagen (bCII, Fig. 2A). We found that NETs release was significantly decreased in CIA-Cl-amidine administered mice compared to CIA-DMSO animals as assessed by the costaining of DNA with NE (Fig. 2B). Importantly, CIA-Cl-amidine administered mice experienced significantly decreased severity and disease onset as compared to vehicle control administered mice. Specifically, mice receiving daily Cl-amidine exhibited >50% decrease in clinical disease score on days 25–32 compared to mice administered vehicle control (CIA-DMSO, Fig. 2C). Representative histological analyses of proximal knee joints were performed in two mice per group at the end of each experiment, at d32 and 39 post immunization. Specifically, in the proximal knee joint severe arthritis with pannus formation was observed in CIA-DMSO mice (Fig. 2D upper panel and lower panel; grade 4), as compared to moderate arthritis in CIA-Cl-amidine group (Fig. 2D upper panel and lower panel; grade 1). In addition, CIA-Cl-amidine-treated mice exhibited decreased intensity of inflammation and better integrity of bone structure in the proximal joint compared to the CIA-DMSO group (Fig. 2D). Collectively, these results suggest that Cl-amidine treatment reduces NET formation by BM PMNs, attenuates clinical disease severity, and decreases joint inflammation during the effector phase of the disease in CIA model.

Another hallmark of RA pathogenesis and CIA is the generation of autoantibodies. To this end, plasma from bCII-injected mice treated with Cl-amidine or vehicle control (DMSO) was analyzed for the presence of total IgG Ab to bCII. Notably, Cl-amidine treatment significantly attenuated the anti-bovine CII IgG Abs levels compared with DMSO vehicle control at the end point of each experiment (d32 to d45 post immunization, Fig. 2E). These results demonstrate that Cl-amidine treatment decreases the humoral response to bCII. In summary, the above findings provide evidence that Cl-amidine administration in the CIA model inhibits NETosis and attenuates disease phenotype and arthritis progression.
Cl-amidine treatment diminishes IFN-γ-producing Th1 cells and attenuates DC maturation
Next, we examined whether the attenuated disease pathology in the CIA after Cl-amidine administration could be attributed in decreased Th1 and/or Th17 immune responses. To address this, flow cytometry was performed on draining LN (dLN) cells (dLNCs) from CIA mice treated with Cl-amidine daily for 12 days after the first immunization with CII. Interestingly, the frequency of CD4+ T cells was decreased in CIA-Cl-amidine administered mice compared to CIA-vehicle control mice (Fig. 3A) and this was accompanied by significantly decreased frequencies of IFN-γ-expressing CD4+ T cells by dLNCs in CIA-Cl-amidine. IL-17-expressing CD4+ T cells were also decreased but did not reach statistical significance (Fig. 3A).
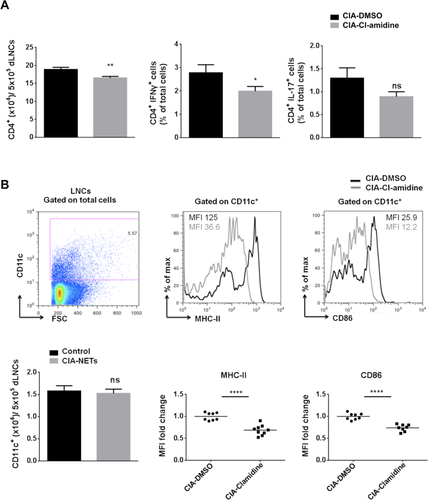
T-cell activation, polarization, and proliferation in vivo require Ag presentation by professional APCs. DCs serve as the best candidate that upon maturation provide the necessary costimulatory molecules and secrete proinflammatory cytokines to instruct T-cell responses 20, 21. Therefore, we examined whether Cl-amidine administration in CIA could also affect DC maturation. Interestingly, it was found a significantly decreased expression of MHC class II (MHC-II) and CD86 costimulatory molecules expressed by CD11c+ DCs isolated from dLNs upon Cl-amidine treatment compared to DMSO vehicle group (Fig. 3B). Taken together, these data demonstrate that treatment of CIA mice with Cl-amidine attenuated the Th1 immune responses and the maturation of DCs in the dLNs.
CIA-derived NETs increase the maturation of myeloid DCs
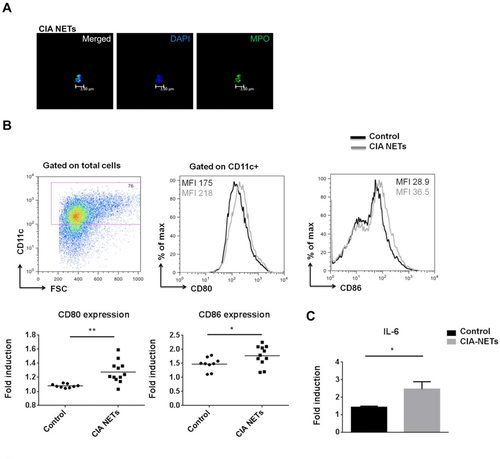
To provide direct evidence for a role of NETs in DC maturation, we generated BM-derived DCs (BMDCs) from naïve DBA/1 and treated them with supernatants containing CIA-NETs (Fig. 4A) or control supernatants. We first examined the expression of costimulatory molecules on BMDCs (CD11c+) in the presence or absence of CIA-NETs. We observed a significant upregulation of both CD80 and CD86 costimulatory molecules in the presence of CIA-NETs as compared to control (Fig. 4B). Moreover, assessment of proinflammatory cytokines in culture supernatants revealed a vast increase of IL-6 by DCs in the presence of NETs (Fig. 4C). TNF-α production was also increased but did not reach statistical significance (data not shown). These results indicate that NETs are capable of directly activating DCs as shown by the induction of costimulatory molecules and proinflammatory cytokine.
NETs potentiate the immunostimulatory effect of BMDCs in shaping Ag-specific Th1 immune responses
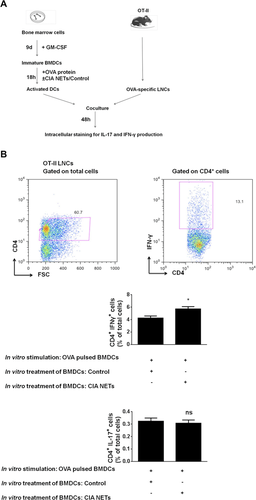
To assess the functional importance of our findings, we applied the OT-II mouse model. We performed coculture experiments of CIA-NET-treated ovalbumin (OVA) pulsed BMDCs with OT-II dLNCs as shown in Figure 5A. To this end, WT BMDCs were pulsed with OVA in the presence of CIA-NETs or control supernatants. Forty-eight hours later, the intracellular IL-17 and IFN-γ production by CD4+ T cells was assessed. Interestingly, CIA-NET-treated OVA-pulsed BMDCs significantly induced IFN-γ production by CD4+ OT-II-LNCs as compared with control-treated OVA-pulsed BMDCs (Fig. 5B). Notably, no significant difference was observed in IL-17 production by CD4+ T cells found in OT-II-dLNCs either in the presence of CIA-NETs or control (Fig. 5B). These findings provide evidence for the increased capacity of NET-treated DCs to promote autoreactive Th1 immune responses.
Human RA-NETs enhance the maturation of monocyte-differentiated DCs
Since NETs are a prominent feature in RA, we sought to examine whether RA-NETs could induce the maturation of human DCs. To this end, we exposed human monocyte-differentiated DCs (moDCs) to RA-NETs and compared their effect to that from supernatants derived from control PMNs. RA-NETs increased the expression of HLA-DR and CD86 costimulatory molecule by DCs as compared to control (Fig. 6A). Moreover, assessment of proinflammatory cytokines in culture supernatants revealed that RA-NETs significantly increased IL-6 and TNF-α cytokine release as compared to control supernatants (Fig. 6B). Overall, our data provide evidence for an immunostimulatory role of human RA-NETs in APCs.

Discussion
Although it is becoming apparent that NETs have a significant impact on chronic autoimmune inflammatory diseases such as lupus, psoriasis, atherosclerosis, and arthritis 11, 12, 22-24, the mechanism involved in NET-mediated adaptive autoimmune responses remains elusive. Herein, we provide new insights into the NETs-mediated induction of T-cell autoreactivity that contribute to the immunopathogenesis of RA. Specifically, we identified that CIA-NETs favor the induction of Th1 immune responses through DC activation. In human, RA-NETs also augmented the inflammatory properties of moDCs. Importantly, inhibition of NETosis in vivo using Cl-amidine, a pan-PAD inhibitor, significantly diminished CIA severity and delayed disease onset. Additionally, Cl-amidine treatment decreased the expression of MHC-II and CD86 costimulatory molecules on DCs and Th1 immune responses during the initial steps of the disease course.
The high importance of CD4+ T cells in RA pathogenesis has been supported by genetic studies showing an association between genes important for T-cell activation and function (such as HLA-DR, PTPN22, CTLA4, CD40, etc.) and the risk for RA 25, 26. In addition, the clinical effectiveness of T-cell-directed therapies (CTLA4Ig) 27 further supports the important role of these cells in disease progression. Finally, adoptive transfer of collagen-specific T cells into severe combined immunodeficiency (SCID) recipients can cause disease 28. It is well established that both Th1 and Th17 responses contribute in RA pathogenesis 29, 30. Our study demonstrates that NETs promote induction of IFN-γ-producing Th1 cells in vitro, whereas they do not appear to affect Th17-mediated responses. Of note, the clinical efficacy of anti-IL-17 agents has been highly variable in inflammatory arthritides, showing effectiveness in psoriasis and psoriatic arthritis but moderate to weak in RA 31. Although it has been shown that IL-17A may induce NETosis in the context of RA 11, that NETs may also be loaded with IL-17A in different human diseases (thrombosis, Alzheimer's disease) 32, 33, or that they may indirectly activate Th17 cells in atherosclerotic plaques 34, in our study there is no evidence of a Th17 polarizing ability of NETs. Consistently, in our experiments Cl-amidine administered during the priming of the arthritogenic response resulted in the decrease of IFN-γ-producing CD4+ T cells in the dLNs but not of IL-17-producing cells. This is further supported by the increased IFN-γ production by Ag (OVA) specific T cells cocultured with NET-treated BMDCs in the presence of OVA. Thus, we propose that recognition of NETs by APCs generates an inflammatory signal that favors Th1 induction.
DCs are characterized as professional APCs and they have been shown to be indispensable for T-cell activation and proliferation 20. Of note, it was previously demonstrated that PMNs prone to form NETs were shown to activate myeloid DCs (CD11c+ CD11b+ CD45RA−) 35. However, whether DC activation was mediated by NETs or other PMN-related molecules was not addressed. Our findings provide evidence for a direct immunogenic ability of NETs to increase the DC costimulatory molecules, CD80 and CD86 as well as the secretion of IL-6. In addition, spontaneously released RA-NETs increased the maturation of human DCs. Finally, further evidence was obtained upon Cl-amidine administration during CIA that was accompanied by downregulation of DC costimulatory molecule expression in dLNs. The molecular mechanism via which NETs are recognized by DCs remains unknown. It is likely that NETs as DNA-containing structures may activate DCs via Toll-like receptor 9 (TLR9), or other endosomal DNA-sensors. In line with this, it has been shown that phorbol myristate acetate (PMA) induced NETs efficiently triggered plasmacytoid DC activation through TLR9 22.
It is well established that NETosis could be specifically inhibited by Cl-amidine administration (PAD inhibition). Studies in murine lupus showed that Cl-amidine inhibited NETs and protected mice against disease-related organ damage and associated autoimmunity, evidence that strengthens our findings 15, 16. In support, our findings demonstrate that Cl-amidine administration reduced NET formation, ameliorated arthritis severity, and delayed disease onset and this was accompanied by reduced anti-CII antibody titers. The molecular mechanism through which Cl-amidine reduces CIA severity is currently under investigation. Accumulating evidence suggests that citrullination is an important mechanism in NET formation contributing to the chromatin decondensation and facilitate NET release 36, 37. In support of this notion, it has been shown that a PAD4-null strain of mice failed to produce NETs 38, 39. NETs may also function as additional pool of citrullinated antigens. It has been shown that active PAD isoforms are found attached on NETs from RA SF PMNs, which may contribute to the generation of extracellular autoantigens in RA. Our findings demonstrate that the levels of spontaneous NETosis in human are correlated with increased autoantibodies (RF positivity) and the RA inflammatory environment enriched in autoantibodies increases NETosis by control PMNs more avidly than an autoantibody poor environment. In support, it has been shown that RA sera, SF, RF, and IgG fractions purified from RA patients with high levels of ACPAs significantly enhanced NET formation and led to distinct protein content in the NETs 11. Therefore, we propose that Cl-amidine inhibits NETs release and the externalization of citrullinated antigens, which could activate citrulline Ag-specific autoreactive T cells, thus leading to disease perpetuation. Finally, in mice, the presence of proinflammatory cytokines such as IL-6 and TNF-α, which are abundant in CIA sera 40, 41, might influence NET formation upon PMN signaling. This is in line with our recent report in lupus-prone mice that IL-6 demonstrated to drive NET release by granulocytic cells 24.
In summary, our findings provide evidence for the first time to our knowledge for a novel role of NETs in shaping the RA-related autoimmune responses in vitro by promoting induction of IFN-γ-producing Th1 cells. Inhibition of NET release significantly attenuated CIA and led to decreased stimulatory properties of antigen-bearing DCs. Our findings provide new insights into the pathogenic role of NETs in RA that could lead to the development of new targeted therapeutic approaches.
Materials and methods
Human subjects
Peripheral blood samples were obtained from patients diagnosed with RA according to the 1987 American College of Rheumatology (ACR) criteria 42, followed by the Rheumatology Clinic of the University Hospital of Crete. SF was collected from active RA patients, centrifuged at 800 × g for 15 min at 4°C and supernatants were stored at –80°C until used. Serum was also isolated from patients or healthy donors. The study was approved by the Ethics Committee of the University Hospital of Heraklion, University of Crete School of Medicine, and all participants gave informed consent.
Reagents
Fluorescent-conjugated monoclonal antibodies to CD80 (16-10A1), CD86 (GL-1), CD11c (N418), CD4 (RM4-5), CD25 (pc61), and CD44 (IM7) were all from Biolegend; Ly6G (1A8) and IFN-γ (XMG1.2) from BD Pharmingen; MHC-II (M5/114.15.2) from Miltenyi; IL-17 (eBio17B7) and brefeldin A from eBioscience. Dulbecco's modified Eagle's medium (DMEM-glutamax, Gibco), RPMI-1640 (Gibco), fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL), all were from Gibco, Carlsbad, California, USA; PMA, ionomycin, saponin, and A23187 calcium ionophore were from Sigma-Aldrich. Murine PMNs were isolated with Percoll (Sigma) and cultured in RPMI-1640 supplemented with l-glutamine, 2% bovine serum albumin (Gibco), and 10 mM HEPES (Gibco). PBS tablets were from Gibco.
For immunofluorescence the following primary antibodies were used: polyclonal anti-NE Ab (Abcam), polyclonal anti-human myeloperoxidase (anti-MPO, Dako), anticitrullinated H3 (Abcam), CF488-labeled anti-rabbit Ab (Biotium), CF555-conjugated goat anti-rabbit IgG (H + L) Ab (Biotium) and DAPI (Sigma).
Mice
Male DBA/1J and C57BL/6 (B6) mice (7- to 12-week-old males) were obtained from the SPF facility of the Institute of Molecular Biology and Biotechnology (IMBB Heraklion Crete, Greece). OT-II TCR transgenic mice were obtained from Biomedical Research Foundation of the Academy of Athens. All procedures were in accordance to institutional guidelines and were approved by the Greek Federal Veterinary Office.
CIA induction, treatment groups, and scoring system
For induction of CIA, DBA/1J mice were injected intradermally at the base of the tail with 100 μL complete Freund's adjuvant (Sigma), containing 200 μg bCII (MD Biosciences) and 250 μg inactivated Mycobacterium tuberculosis (H37Ra; Difco) on d0, followed by a boost immunization on d21 43. Each experimental group consisted of eight mice that were treated daily after the d21 booster injection through sacrifice on d35–45 with one of three interventions: no injection, PBS/DMSO (vehicle control), or 10 mg/kg Cl-amidine (kindly provided by P. R. Thompson). All injections were given i.p. and doses were calculated for the average weight of the group (20 g). Mice were scored five times a week from d21 according to the following scoring key: 0 points if no visible clinical symptoms, 1 point per swollen toe (regardless of whether this includes one or two joints (exclusive digit I in both front paws), 5 points for involvement of knuckles (metatarsal/metacarpal), or 5 points for involvement of wrist (tarsal/carpal), giving a total score of 9 and 10 per paw for front and hind paws, respectively. Blood was collected by retro-orbital aspiration at the end of the study and the isolated plasma was stored at –80°C for later batch processing. At d35–45, all animals were sacrificed by anesthesia with diethyl-ester and cervical dislocation.
Histological examination
Tissue was fixed in 4% buffered formalin solution, routinely processed, and embedded in paraffin. Bone was previously decalcified in Schaefer solution. Four micrometer thickness sections were stained with hematoxylin & eosin (H&E) and evaluated by a Nikon Eclipse E-400 light microscope. Histological grading of arthritis of the proximal limb joint (knees) from each animal was performed according to the following five-scale grading system: 0 = normal histology of the knee, 1 = normal synovium or mild synovial reaction with scarce inflammatory cells, 2 = moderate synovial reaction and moderate density of inflammatory cells, 3 = severe synovial hyperplasia and dense population of inflammatory cells, 4 = pannus formation associated with articular cartilage erosion. Histopathology pictures were captured using a Nikon Digital sight, DS-SM, photographic system.
Measurement of type II collagen-specific antibodies in plasma
Plasma samples were collected at the peak of the disease and the levels of anti-CII IgG were measured by ELISA as described 43. Horseradish peroxidase conjugated anti-mouse immunoglobulin IgG (Millipore) was used as secondary antibody and 3,3’,5,5’-tetramethylbenzidine as substrate.
Isolation of PMNs
Human and mouse PMNs were isolated as previously described 44, 45. Viability of human PMNs was measured 99% by trypan blue dye exclusion and mouse BM PMNs were more than 85% Ly6G-positive by flow cytometry.
Generation, assessment, and quantification of NETs (human, mouse)
Primary human PMNs (healthy or RA) or mouse PMNs from bCII-injected or control mice were seeded onto coverslips coated with 10% poly-l-lysine (Sigma-Aldrich). Primary human PMNs were cultured for 3 h at 37°C, 5% CO2 and murine BM-derived PMNs incubated for 4 h at 37°C, 5% CO2. Cells were fixed with 4% parafolmadehyde, blocked with 5% BSA, and were permeabilized with 0.5% Triton X-100. DNA was stained with 300 nM DAPI. Protein staining was with anti-NE or with anti-MPO primary Ab for 1 h at RT, followed by 1-h incubation (RT) with CF488-labeled or with CF555-conjugated secondary Ab. Three washes with 0.5% BSA/PBS were performed in between all stainings. After staining, coverslips were mounted on Mowiol 4–88 (Sigma-Aldrich) and were observed under confocal microscope (Leica SP8). NETs were manually quantified by two blinded observers. Decondensed nuclei (stained with DAPI), which also stained positively for NE or MPO, were considered NETs. The percentage of NETs was calculated as the average of at least five fields, normalized to the total number of cells. DNA was quantified with a PicoGreen dsDNA kit (Invitrogen).
For NETs isolation, human RA PMNs (1.5 × 106) or murine CIA BM-derived PMNs (2 × 106) were seeded in 6-well tissue culture plates and cultured for 3 or 4 h, respectively. Wells were carefully washed once with prewarmed medium and supernatants were vigorously collected and spun at 50 × g for 5 min at 4°C to remove intact cells. Thus, the supernatant obtained after centrifugation (RA-NETs or CIA-NETs) were lyophilized resuspended in the appropriate volume of medium (3× condensed) and were used for the coculture experiments.
Generation of BMDCs and DC-NETs coculture experiments
DCs were generated from BM progenitors of DBA1/J mice and B6 mice 46 and RBC were lysed with NH4Cl. On days 0, 3, 6, and 8, cultures were supplemented with fresh complete DMEM glutamax containing 20% X63Ag8 supernatant (derived from a murine granulocyte-macrophage colony-stimulating factor (GM-CSF) secreting cell line; kindly provided by B. Stockinger, National Institute of Medical Research, London, UK 47. After 9 days of culture, nonadherent cells were harvested and purity was assessed based on CD11c expression by flow cytometry. DCs were subsequently cultured in the presence or absence of 5% of concentrated supernatants derived from bCII-injected (CIA-NETs) or naïve (control) mice. Culture supernatants and cells were collected 18 h after stimulation and cytokine levels were determined by ELISA. Cells were acquired after surface staining on a FACS Calibur (BD Biosciences).
Assessment of OVA-specific T-cell responses and intracellular cytokine staining
LNs from OT-II transgenic mice were excised and single-cell suspensions (4 × 105 cells/ 200 μL/well) were subsequently cultured in complete DMEM medium in the presence or absence of DCs (105 cells) previously pulsed with OVA endotoxin free (20 μg/mL, Hyglos) and/or 5% of concentrated CIA-NETs or control supernatants 48. Forty-four hours later LNCs were restimulated with PMA (50 ng/mL) and ionomycin (2 μg/mL) for 6 h at 37°C, 5% CO2, and brefeldin A (10 μg/mL) was added for the last 2 h. Following staining of surface markers, cells were fixed with parafolmadehyde and permeabilized with 0.5% saponin buffer 49. Cells were then incubated with IL-17 and IFN-γ mAbs and acquired on FACS Calibur.
Generation of moDCs and moDC-NETs coculture experiments
Monocytes were positively selected from human peripheral blood of healthy volunteers using CD14 MicroBeads (Miltenyi Biotec) according to the manufacturer's instructions and cells were cultured in complete RPMI 1640 medium for 5 days with the addition of human GM-CSF and recombinant IL-4 (both from Peprotech) at d0 (100 ng/mL) and d3 (50 ng/mL). After 5 days of culture, nonadherent cells were harvested and moDCs were subsequently cultured in complete RPMI medium in flat-bottom 96-well plates at a density of 4 × 105 cells/200 μL/well in the presence or absence of 5% of concentrated supernatants derived from RA (RA-NETs) or healthy PMNs (control). Culture supernatants and cells were collected 18 h after stimulation. Cytokine levels were determined by ELISA and the cells were acquired after surface staining on a FACS Calibur.
Western blot analysis
Control BM PMNs were lysed on ice in SDS lysis buffer (2% SDS, 62.5 mM Tris pH 6.8, 5% 2-mercaptoethanol, 10% glycerol) supplemented with Complete Protease inhibitor cocktail (Roche) and centrifuged at 13 000 × g for 30 min at 4°C. Whole-cell lysates (40 μg protein) were subjected to SDS-PAGE electrophoresis on 15% gels and then transferred to an Immobilon-Psq membrane (Millipore). Membranes were blocked with 5% BSA in TBST and then incubated with anti-MPO (1:200) as loading control and anticitrullinated H3 (1:100). Detection was performed using HRP-linked Abs (Cell Signaling Technology) and enhanced chemiluminescent (ECL) detection reagents (Amersham Biosciences).
ELISA
Detection of mouse IL-6 and TNF-α Duo set (R&D systems) and human IL-6 and TNF-α (eBioscience) in culture supernatants harvested at the indicated time was performed by sandwich ELISA following the manufacturer's recommendations. Light absorbance at 450 nm was measured using the ELx800 Biotek.
Flow cytometry
Cells were stained for extracellular markers for 20 min at 4°C in PBS/5% FBS. Cells were acquired on a FACS Calibur and analyzed using the FlowJo software (Tree Star).
Statistics
Statistical analysis was performed with Prism software version 6.01 (GraphPad Software). Student's t-test (two-tailed, 95% confidence interval) was used, unless indicated otherwise. p-Values less than 0.05 were considered statistically significant.
Acknowledgments
All authors were involved in drafting the article and revising it critically for important intellectual content, and all authors approved the final version to be published. P.V. and P.S. had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design was done by G.P., K.K., P.V., and P.S. Acquisition of data was done by G.P., K.V., E.D., P.V., and P.S. Analysis and interpretation of data were done by G.P., K.K., E.D., G.B., K.R., D.B., P.V., and P.S.
We thank G. Hamilos and K. Girtzimanaki for helpful discussions and T. Alissafi, M. Ioannou, E. Kontaki, and A. Raptopoulou for providing technical support. We also thank nurses (P. Rapsomaniki, M. Terizaki, and E. Kambouraki) and clinicians (A. Fanouriakis, A. Repa) from the Rheumatology clinic of the University Hospital of Heraklion and the personnel of the Confocal Microscopy Facility of the Institute of Molecular Biology and Biotechnology (Heraklion, Crete, Greece) for their expertise. This work was supported by the Greek General Secretariat of Research and Technology Grants “Synergasia” Action of the Operational Program “Education and Lifelong Learning,” ARISTEIA II 3468 (to P.V.), European Union project Innovative Medicine Initiative 6 (BeTheCure) contract number 115142-2 (to P.V. and D.B.), the Hellenic society for Rheumatology, the Pancretan Health Association, and Celgene award for investigative rheumatology. G.B. was supported by TransPOT, FP7–2011 REGPOT-1.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
References
Abbreviations
-
- ACPAs
-
- anticitrullinated peptide antibodies
-
- bCII
-
- bovine collagen-II
-
- BMDC
-
- BM-derived DC
-
- CIA
-
- collagen-induced arthritis
-
- Cl-amidine
-
- N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-l-ornithine amide
-
- dLN
-
- draining LN
-
- dLNC
-
- dLN cell
-
- MHC-II
-
- MHC class II
-
- MPO
-
- myeloperoxidase
-
- NE
-
- neutrophil elastase
-
- NETs
-
- neutrophil extracellular traps
-
- PADs
-
- peptidylarginine deiminases
-
- PMNs
-
- polymorphonuclear cells
-
- RA
-
- rheumatoid arthritis
-
- RF
-
- rheumatoid factor
-
- SF
-
- synovial fluid




