Aged B cells alter immune regulation of allografts in mice
Abstract
Organ transplantation in older people is increasing, but how aging impacts B-cell responses to organ transplantation is still unknown. Here, we show that the depletion of B cells with anti-CD20 antibodies has disparate effects depending on recipient age. In young murine recipients, anti-CD20 treatment impaired the ability of immune modulation to extend skin allograft survival. In contrast, anti-CD20 treatment extended allograft survival in aged recipients treated with immune modulation. Although regulatory B-cell function and the numbers of marginal and follicular B cells were similar between age groups, a subpopulation of B cells, termed age-associated B cells (ABCs), accumulated upon aging. ABCs isolated from aged mice exhibited upregulation of CD73, CD80, CD106, and TLR2 and an increased capacity to augment T-cell alloimmunity compared to ABCs from young mice. Importantly, ABCs from aged, but not young, mice impaired the ability of immune modulation to enhance allograft survival after adoptive transfer into young transplant recipients. Our study indicates that ABCs impair the immune regulation of allografts. Thus, recipient age needs to be considered when proposing B-cell-depleting immune therapy.
Introduction
B cells produce antidonor antibodies against transplant antigens that enhance acute and chronic rejection 1-3. B cells also act as antigen present cells (APCs) to enhance cellular immunity toward an allograft 4, 5. In addition to these proimmune functions of B cells, a subpopulation of B cells, termed regulatory B (Breg) cells, has been identified that promote immune regulation by producing IL-10 2, 4, 6-9. Hence, B-cells play important and diverse roles that can either enhance or suppress immunity toward organ allografts.
The most rapid subpopulation of patients seeking organ transplants, including kidney and heart transplants, are over 65 years of age 10. Despite the increasing numbers of older patients that receive organ allografts, our understanding of how aging impacts the immune response to organ transplantation remains unclear. Studies have found that aging impacts T-cell and dendritic cell (DC) responses after organ transplantation 11-15. Yet, the role of aging on B cells following organ transplantation is unexplored.
In this study, we reveal that recipient age plays a critical role in the outcome of B-cell depletion following organ transplantation. We found that, in young (2–4 months of age) murine skin allograft recipients, B-cell depletion via anti-CD20 treatment impairs the ability of anti-CD45RB and anti-CD154 treatment to maximally extend graft survival. This observation is consistent with previous studies in humans that show that anti-CD20 precipitates acute renal allograft rejection 16. In sharp contrast, we uncovered that allografts survived longer in aged recipients (16–18 months of age) receiving anti-CD45RB and anti-CD154 when the recipients were also treated with anti-CD20. Upon aging, a subpopulation of nonfollicular, nonmarginal B cells, termed age-associated B cells (ABCs: CD19+, CD21−, CD23−, CD43−, CD93−), accumulate and exhibit enhanced activation and increased T-cell alloimmune priming capabilities. Importantly, adoptive transfer of ABCs isolated from aged mice into young skin allograft recipients impairs the ability of anti-CD45RB and anti-CD154 to extend allograft survival. Our findings indicate that, upon aging, heightened T-cell priming by the B-cell pool may impair the immune regulation of allografts, which suggests that host age impacts the response to B-cell immune depletion.
Results and discussion
To determine how aging impacts B-cell responses to organ transplantation, we evaluated how B-cell depletion influenced the immune regulation of skin allografts in young and aged murine recipients. We found that B-cell depletion via anti-CD20 antibodies has disparate effects depending on recipient age, findings that should be taken into consideration when treating aged organ recipients and recipients with other inflammatory conditions.
Recipient age critically impacts the outcome of anti-CD20 on the immune regulation of allografts
Anti-CD20 monoclonal antibodies (mAbs) have been used to deplete B cells and reduce alloantibody levels prior to transplantation 17. We found that administering 100 μg mAb against CD20 every 10 days for a total of four serial doses effectively depleted the splenic B-cell pool (CD19+ cells) in both young (2–4 months of age) and aged (16–18 months of age) C57BL/6 mice compared to isotype controls (Supporting Information Fig. 1A). Within the B-cell pool, anti-CD20 treatment ablated the follicular (CD19+, CD23hi, CD21mid), the marginal zone B-cell pool (CD19+, CD23mid, CD21hi) and the CD19+, CD23−, CD21− population in the spleens of both young and aged mice (Supporting Information Fig. 1B and C). Thus, anti-CD20 effectively ablates the B-cell pool in both young and aged mice.
We then examined the impact of B cells on the efficacy of anti-CD45RB, an agent that blocks signal one between APCs and T cells 18, and anti-CD154, a signal two blocker 19, to maximally enhance BALB/c skin graft survival in young and aged C57BL/6 mice. Anti-CD45RB and anti-CD154 have been shown to extend graft survival in murine transplant recipients 18, although aged recipients do not exhibit the maximal extension of graft survival observed in young recipients 20. Treatment with anti-CD45RB and anti-CD154 reduced the total B-cell pool by 50% seven days after skin allograft transplantation in both young and aged recipients (Supporting Information Fig. 1D). Additionally, compared to isotype controls, the B-cell pool remained ablated at 3 weeks posttransplantation in young and aged mice that received perioperative anti-CD45RB, anti-CD154, and a skin allograft with anti-CD20 mAb (Supporting Information Fig. 1E). These data show that anti-CD20 mAb is highly effective at ablating B cells in murine recipients treated with anti-CD45RB and anti-CD154.
In young skin allograft recipients, we found that B-cell depletion with anti-CD20 mAb impaired the ability of anti-CD45RB and anti-CD154 to maximally extend allograft survival compared to an isotype control of anti-CD20 (Fig. 1A). (The isotype control did not impact the efficacy of anti-CD45RB and anti-CD154 to extend allograft survival; there was no significant difference in graft survival times between young mice that received anti-CD45RB and anti-CD154 with the isotype control compared to young mice that received anti-CD45RB and anti-CD154 without the isotype control (Fig. 1A)). In contrast, in aged recipients, B-cell depletion led to a small but significantly enhanced ability of anti-CD45RB and anti-CD154 to extend allograft survival compared to aged recipients that received anti-CD45RB, anti-CD154, and an isotype control of anti-CD20 (Fig. 1B). B-cell depletion alone in aged recipients did not impact the tempo of skin allograft rejection (Fig. 1B), an expected finding given the immunogenicity of full major histocompatibility complex (MHC) skin graft rejection, which is a T-cell-dependent process 21. Thus, these results indicate that recipient age critically impacts the outcome of B-cell depletion on the immune regulation of allografts. Specifically, with an immunoregulatory regimen that exhibits an age-dependent effect on graft survival 20, B-cell depletion enhances immune regulation in aged recipients but impairs it in young recipients.
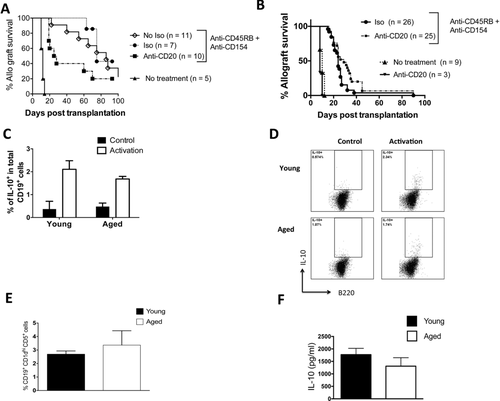
Recipient age alters the impact of B-cell depletion on the immune regulation of skin allografts. (A) Young C57BL/6 mice received BALB/c skin transplants with either no further treatment or treatment with anti-CD45RB, anti-CD154, and anti-CD20 mAb or an isotype control to anti-CD20 mAb. Allograft survival was assessed. p = 0.006: isotype, median survival time (MST) 75 days versus anti-CD20, MST = 25 days (Log-rank). p < 0.0001: anti-CD45RB, anti-CD154 versus no treatment (MST = 12 days). p = 0.5: isotype control + anti-CD45RB, anti-CD154 versus anti-CD45RB, anti-CD154 + no isotype control (MST = 86 days). Pooled data from two independent experiments. (B) Aged C57BL/6 mice received BALB/c skin transplants with either no further treatment, treatment with anti-CD20 only, or treatment with anti-CD45RB, anti-CD154, and anti-CD20 mAb or an isotype control to anti-CD20 mAb. Allograft survival was assessed. p = 0.05, isotype (MST = 26 days) versus anti-CD20 (MST = 30 days) (Log-rank). p < 0.0001 anti-CD45RB, anti-CD154 versus no treatment (MST = 10 days). Anti-CD20 treatment alone, MST = 8 days. Pooled data from six independent experiments. (C, D) Aged and young splenic B cells were enriched by magnetic negative separation and stimulated with LPS, PMA, and ionomycin at the indicated doses (see methods). (C) The proportion of IL-10-producing cells was assessed after intracellular cytokine staining (pooled data from two independent experiments, n = 12 biological replicates/group). (D) Representative flow cytometric plots gated on B220+ cells are shown. (E) Spleens of aged and young mice were stained with fluorescent antibodies, and data were acquired via flow cytometry. The percentage of CD19+, CD1dhi,and CD5+ within total splenic B-cell pool was measured (n = 6 mice/group). The data are pooled from two independent experiments. (F) Enriched B cells from the spleens of young and aged mice 1 week after skin allograft transplantation and treatment with anti-CD45RB and anti-CD154. The cells were stimulated with LPS, and IL-10 was measured by ELISA (n = 4 biological replicates/group). Nonactivated cells did not produce IL-10 (not shown). Data are from a single representative experiment from a total of three independent experiments, each experiment yield consistent results. (C, E, and F) Data are shown as mean + SEM.
Aging does not alter Breg function
In islet and cardiac allograft models, CD45RB and anti-CD154 are known to act via Breg cells to enhance graft survival 6, 22. Thus, an increased potency of Breg function in young recipients than in aged recipients could explain the different effects of B-cell depletion observed between young and aged transplant recipients. Production of the immune suppressive cytokine, IL-10, is a consistent hallmark of Breg function that limits T-cell immunity 23, 24. However, we found no difference in the proportion of IL-10-producing B cells upon ex vivo stimulation with lipopolysaccharide (LPS) in the spleens of young and aged mice prior to transplantation (Fig. 1C and D). We also found no difference in the proportion of CD19+, CD1dhi, CD5+, cells that contain putative Breg populations 7, between the spleens young and aged mice (Fig. 1E). (Note that IL-35 a marker of IL-10-producing B cells was not assessed 25). After mice were transplanted and treated with anti-CD45RB and anti-CD154, we found no difference in IL-10 production in the spleens of young and aged mice (Fig. 1F). Thus, we did not find that Breg function or numbers are altered upon aging.
Aging increases the accumulation of ABCs that exhibit immune memory and activation phenotypes
As we did not identify an alteration in Breg number or function between young and aged mice, we next examined other populations of B cells, including follicular and marginal zone B cells. We did not find increases in the total CD19+ B cells pool or in the subpopulations of follicular or marginal zone B cells between the spleens of young, aged, and advanced aged (24 months of age) mice (Fig. 2A). However, we found an increase in a CD19+, CD23−, CD21−, CD43−, CD93− population of B cells known as ABCs 26, 27 (Fig. 2 and Supporting Information Fig. 2A). We found low proportions (<1% BrdU+ cells) of proliferating cells within the ABC population of both young and aged mice. We also found similar proportions of apoptotic (Annexin V+) cells in splenic B-cell subpopulations in young and aged mice (Supporting Information Fig. 2B). These results suggest that neither differences in cellular proliferation nor the degree of apoptosis explain the increased numbers of ABCs in the spleens of aged mice.
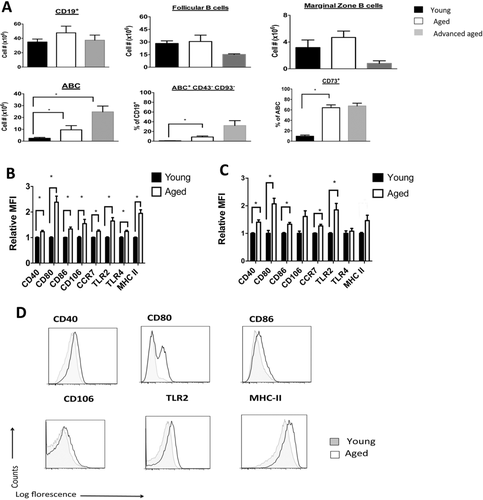
Aging increases the accumulation of ABCs that exhibit memory and activation phenotypes. (A) The spleens of young (2–4 months of age), aged (16–18 months of age), and advanced aged mice (22–24 months of age) were harvested and stained with appropriate fluorescent antibodies, and data were acquired via flow cytometry. Absolute cell number or proportions are shown. *p < 0.01, n = 4–6 mice/group, representative of one experiment that was repeated two times with consistent results. (B) Enriched splenic B cells from nontransplanted and nontreated young and aged mice were stained with the indicated fluorescent antibody and gated on the ABC subpopulation. Relative median fluorescence intensity is normalized to average median fluorescence intensity of young mice. *p < 0.01, (t-test) between young and aged groups, n = 16 mice/group. (C) Enriched splenic B cells were obtained 12 days after skin allotransplantation and treatment with anti-CD45RB and anti-CD154 in young and aged recipient mice. Staining is per (B). *p < 0.01, (t-test) n = 6 mice/group. (B, C) Data are shown as mean + SEM and are representative of pooled data from either four (B) or two (C) independent experiments. (D) Representative flow cytometry plots gated on ABC subpopulation from nontransplanted mice from B are shown (gating is shown in Supporting Information Fig. 2A). Plots are representative of four independent experiments.
We found that ABCs isolated from aged mice exhibited a three-fold higher proportion of CD73 and CD80 positive cells, markers of memory B cells 28, 29, as compared to ABCs from young mice (Fig. 2A and Supporting Information Fig. 2C and D). CD73hi ABCs also coexpressed CD80 (a memory B-cell marker 29 and costimulatory molecule) with CD73hi ABCs from aged mice exhibiting a higher expression of CD80 as compared to CD73hi ABCs from young mice (Supporting Information Fig. 2E). Additionally, ABCs isolated from aged mice exhibited a two-fold increase in MHC class II, and a 10% increase in TLR4 compared to ABCs isolated from young mice, respectively (Fig. 2B and D). In contrast, within the follicular and marginal zone B-cell populations, only MHC Class II expression was higher in the aged B-cell subpopulations compared to the young ones (Supporting Information Fig. 2F). Besides TLR4, these changes in ABCs persisted after aged and young mice were transplanted and treated with anti-CD45RB and anti-CD154 (Fig. 2C). These data indicate that ABCs exhibit an increase in activation and an immune memory phenotype upon aging.
Aging enhances priming of allogeneic T cells in vitro
As ABCs from aged mice exhibited increases in CD80 and MHC class II, which can enhance priming of T-cell function 30, we next assessed the alloimmune T-cell priming function of aged and young B cells. Enriched splenic B cells (total CD19+ population) from aged C57BL/6 mice exhibited an enhanced ability to induce both BALB/c CD4+ and CD8+ T-cell proliferation in vitro compared to B cells purified from young C57BL/6 mice (Fig. 3A and Supporting Information Fig. 3A). Furthermore, splenic B cells that were enriched from aged transplant recipients treated with anti-CD45RB and anti-CD154 exhibited an enhanced ability to activate allogeneic T-cells ex vivo compared to splenic B cells isolated from similarly treated young mice (Fig. 3B).
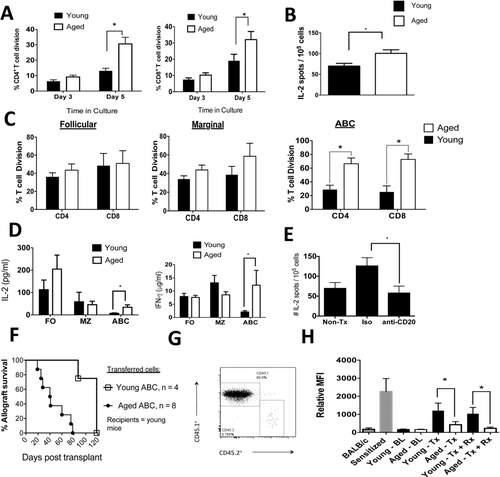
Aged B cells enhance anti-donor T-cell immunity and impair the immune regulation of allografts. (A) The total B-cell pool (CD19+ cells) was enriched by negative magnetic separation from young and aged mice. Cells were irradiated and cultured with allogeneic, (BALB/c), enriched T cells that were stained with violet proliferation dye. The percentage of dividing cells is from pooled data from three independent experiments (n = 12–14 biological replicates/age group). *p < 0.01 (t-test). (B) The total B-cell pool (CD19+ cells) was enriched by negative magnetic separation from young and aged mice 12 days post skin allotransplantation and treatment with anti-CD45RB and anti-CD154. The B cells were irradiated and cultured with allogeneic T cells ex vivo for 12 h. IL-2 production by T cells was measured via ELISPOT. *p = 0.01 (t-test). N = 7 biological replicates/age group. T cells that were not stimulated ex vivo produced < 10 spots/105 cells. Data are pooled from two independent experiments. (C) B cells were FACS sorted into follicular cells, marginal cells, or ABCs (see Supporting Information Fig. 3A for representative FACS plots), irradiated, and cultured with allogeneic T cells as per A. A representative experiment is shown (n = 3 biological replicates/age group/experiment). *p < 0.01 (t-test). Consistent results were observed in four independent experiments. (D) MLC culture supernatants from C above were harvested, and IL-2 and IFN-γ production was measured by ELISA. *p < 0.02 (Mann–Whitney). The data shown are pooled from three independent experiments with three biological replicates/experiment. (E) Aged C57LB/6 mice were transplanted with BALB/c skin allografts, treated with anti-CD45RB, anti-CD154, and anti-CD20 mAb or an isotype control. At three weeks posttransplantation, spleens were obtained, and T cells were enriched by magnetic negative selection and cultured with irradiated donor spleen cells. The number of IL-2-producing T cells was measured via ELISPOT (n = 6 mice/group). *p = 0.03 (t-test). Data are pooled from two independent experiments. (F) Young CD45.1+ mice received 1 × 106 splenic FACS-purified ABCs via tail vein injections from young or aged CD45.2+ mice on day −2 relative to skin allograft transplantation. Mice received anti-CD45RB and anti-CD154 as described in section Materials and methods. The difference between the experimental groups was statistically significant, p = 0.0006 (Log-rank). Data shown are pooled from two independent experiments. (G) ABCs were isolated from aged CD45.2+ mice and transferred into young CD45.1+ mice that were transplanted with a BALB/c skin graft and then treated with anti-CD45RB and anti-CD154. One hundred days after transfer, the spleens were obtained and the proportion of CD45.2+ cells was determined after appropriate staining and data acquisition via flow cytometry. Plots are gated on lymphocytes and are representative of a single experiment. (H) Serum was obtained from mice, diluted, and cultured with allogeneic donor T cells. After the cells were washed, they were stained with an anti-IgG fluorescent antibody, and the data were acquired via flow cytometry. BL, baseline; Tx, transplantation; Rx, anti-CD45RB + anti-CD154. *p < 0.01. Data are pooled from two independent experiments. (A–E, H). Data are shown as SEM.
We then further purified aged and young splenic B cells into follicular, marginal, and ABC subtypes via fluorescence-activated cell sorting (FACS; Supporting Information Fig. 3B) and cultured the purified subpopulations with allogeneic (BALB/c) CD4+ and CD8+ T cells. ABCs, but not marginal or follicular B cells, purified from the spleens of aged C57BL/6 mice exhibited an age-dependent enhancement in the ability to induce T-cell alloimmune proliferation (Fig. 3C). ABCs purified from aged mice also increased IL-2 and IFN-γ production by allogeneic T cells compared to ABCs purified from young mice, whereas follicular and marginal zone B cells did not exhibit age-dependent increases in IL-2 and IFN-γ production (Fig. 3D). IL-17 was detected at low levels in this assay with no differences noted between the cell populations upon aging (Supporting Information Fig. 3C). Together, these data show that ABC exhibit enhanced in vitro T-cell alloimmunity upon aging.
Aged B cells exhibit enhanced IL-2 T-cell alloimmune responses in vivo
As anti-CD20 mAb ablates all B-cell subpopulations examined (Supporting Information Fig. 1B), we tested the requirement for B-cells in anti-donor T-cell priming in vivo. We transplanted aged C57BL/6 mice with a BALB/c skin graft and administered anti-CD45RB and anti-CD154 along with the anti-CD20 mAb or an isotype control of anti-CD20 mAb. We found that, 3 weeks after transplantation, anti-CD20 treatment reduced IL-2 anti-donor T-cell responses in aged recipients but not in isotype control-treated aged recipients (Fig. 3E). In similarly treated young transplant recipients, anti-CD20 treatment did not alter the anti-donor IL-2 response (Supporting Information Fig. 3D). These data reveal that B-cell depletion reduces IL-2 antidonor T-cell responses in aged skin allograft recipients who receive anti-CD45RB and anti-CD154.
ABCs isolated from aged mice impair the ability of anti-CD45RB and anti-CD154 to extend allograft survival after adoptive transfers into young transplant recipients
To determine if ABCs from aged, nontransplanted, nontreated mice were sufficient to impair the immune regulation of allografts, we adoptively transferred FACS-sorted ABCs from either young mice or aged CD45.2+ mice into young CD45.1+ mice. Two days after adoptive transfer, mice were transplanted with a skin allograft and treated with anti-CD45RB and anti-CD154. On the day of transplantation, we noted that there were between 0.6–0.8% of CD45.2+ cells in the peripheral blood of recipients. Furthermore, in a separate cohort of young recipient CD45.1+ mice, the engraftment of adoptively transferred ABCs within the spleen was similar between transferred ABCs purified from young or aged mice (0.4–0.6% of splenic ABCs). We found that the transfer of aged, but not young, ABCs impaired the ability of anti-CD45RB and anti-CD154 to extend allograft survival in young recipients (Fig. 3F). At 100 days posttransplantation, aged ABCs remained detectable (0.76% of spleen cells) within the spleen of the recipients (Fig. 3G). These data provide direct in vivo evidence that ABCs from aged mice impair the immune regulation of allografts.
Aged transplant recipients exhibit lower alloantibody levels than young transplant recipients
B cells respond to transplant antigens by producing donor-specific antibodies (DSAs). Prior to transplantation, we found low and similar plasma IgG DSA levels between young and aged C57BL/6 mice. Two weeks after BALB/c skin transplantation, both aged and young mice exhibited increases in the serum levels of anti-donor IgG alloantibodies; however, the aged recipients exhibited significantly lower levels than their younger counterparts (Fig. 3H), which is consistent with previous vaccine studies that show that aging impairs antibody production 31. Administration of anti-CD45RB and anti-CD154 did not impact alloantibody levels, and the reduced antidonor IgG levels in the aged recipients persisted (Fig. 3H). These data indicate that aging impairs the production of DSAs; thus, increased DSA production is unlikely to be a mechanism by which aging influences B cells to enhance antidonor responses.
Concluding remarks
In summary, our study indicates that the accumulation of ABCs, likely due to a series of environmental exposures, may override the immunoregulatory functions of B cells in aged transplant recipients. In addition to these quantitative changes, our work indicates that upon aging, ABCs also exhibit qualitative changes, such as an upregulation of costimulatory molecules, which enhances the priming of allogeneic T cells. However, it is possible that ABCs directly interact with immunosuppressive cells, such as Breg cells, to impair immune regulation; this possibility will require future investigation. The age of the patients is not always stated in clinical studies, such as one reporting that B-cell depletion precipitated acute allograft rejection 16. Our study indicates that recipient age should be considered when investigating the immune response to B-cell depletion following organ transplantation and possibly in the treatment of other inflammatory conditions.
Materials and methods
Mice, transplantation, and reagents administered in vivo
Young (2–4 months), aged (16-18 months), and advanced aged (22–24 months) C57BL/6 (H2b) mice were obtained from the National Institutes of Aging rodent colony. Young donor BALB/c mice (H2d) and C57BL/6 CD45.1+ were purchased from the Jackson Laboratory (Bar Harbor, ME, USA).
Full-thickness BALB/c skin was transplanted to the dorsum of C57BL/6 recipients as described previously 32. Recipients were treated with anti-CD45RB (clone HB220, 100 μg intraperitoneally on days −1, 0, 1, 2, 5, and 8 relative to transplantation) and anti-CD154 (clone MR1, 250 μg intraperitoneally on days 0, 2, 6, and 8 relative to transplantation). Both antibodies were purchased from BioXcell (Lebanon, NH, USA). Complete loss of the skin graft area was considered as rejection. B-cell depletion was accomplished using an anti-CD20 monoclonal antibody (four doses of 100 μg/dose administered on days −5, +5, +15, and +25 relative to transplantation). The anti-CD20 antibody (clone 5D2) was obtained from Genentech (San Francisco, CA, USA).
The Yale University Institutional Animal Care and Use Committee approved the use of animals in this study.
Cell sorting, flow cytometry, adoptive transfer, and ELISA
The following monoclonal, fluorescently tagged antibodies were obtained from Biolegend (CD19, TCRβ, CD5, CD21, CD23, CD43, CD93, CD73, CD40, CD80, CD86, MHC-II, TLR2, TLR4, B220, CD1b, CD5, TIM-1, and IL-10). Data were acquired on a BD LSRII flow cytometer and analyzed using FlowJo Software (TreeStar). For intracellular cytokine staining, 1 × 106 spleen cells were activated using phorbol 12-myristate 13 acetate (PMA) (50 ng/mL), ionomycin (500 ng/mL), and LPS (10 μg/mL) (Sigma) for 4 h as previously described 7. Golgi stop was added to the cultures 1 h before staining with the indicated antibodies. For all flow cytometric analysis, cells were gated on a propidium iodide negative population. Magnetic negative cell enrichment (>95% purity, as assessed by flow cytometric staining) of splenic B cells (CD19+) and T cells was performed using kits from Stemcell Technologies (Vancouver, BC, Canada). In specific cases, B-cell subpopulations (Supporting Information Fig. 3B) were further sorted by FACS on a BD FACS Aria II cell sorter. FACS-purified splenic ABCs were transferred at a dose of 1 × 106 cells/mouse on day −2 relative to transplantation. Enriched B cells (1 × 106 cells/well) were stimulated with 10 μg/mL LPS for 2 days, and IL-10 (eBioscience, San Diego, CA, USA) concentrations were measured in the culture supernatants via an enzyme-linked immunosorbent assay (ELISA). IL-2 (eBioscience) concentrations were measured in mixed lymphocyte culture (MLC) culture supernatants via ELISA.
MLC
As described above, B cells were enriched from the spleens of young or aged C57BL/6 mice and irradiated (1000 RAD). These cells (2 × 105 cells/well) were then cultured in complete RPMI media at a ratio of 1:2: B cells:CD4+ or CD8+ T-cells that were enriched from BALB/c mice. T cells were labeled with violet proliferation dye 450 (BD Biosciences, San Diego, CA, USA) prior to coculture with irradiated B-cells. Proliferation was assessed at days 3 and 5 of culture. Prior to flow cytometry cells were stained with antibodies against TCRβ, CD4, and CD8. Cells were gated on TCRβ+, CD4+, or TCRβ+ CD8+ and proliferation was determined by comparing the values to nonactivated (i.e., T cells not stimulated with B cells) control T cells.
ELISPOT
Recipient T cells (1 × 105 cells/well) and irradiated donor splenic cells (5 × 105 cells/well) were cultured in 96-well ELISPOT plates coated with the antibody of interest. After 18 h of culture, the plates were treated with the reagents according to manufacturer's instructions (BD Biosciences). Plates were read on an AID ELISPOT reader and analyzed using AID ELISPOT software (AID, Strassberg, Germany).
Measurement of alloantibodies
Serum was obtained from mice and diluted 1:100 in PBS. The serum was incubated with enriched allogeneic T cells (target T-cells; 2 × 105 cells/well) in 96-well plates. After incubating for 1 h at 4ºC, the cells were washed twice with PBS, resuspended, and cultured with anti-mouse IgG FITC (Jackson Immuno Research West Grove, PA, USA) on ice for 30 min. The cells were washed twice and resuspended in PBS, and then data were acquired on a flow cytometer. The degree of fluorescence was reported as median fluorescence intensity as a measure of antidonor antibody attachment to the target T cells.
Statistics
Means between indicated experimental groups were statistically analyzed by parametric tests (two-way Student t-test) or nonparametric tests (Mann–Whitney), and survival analyses were performed via a log-rank test using Graphpad Prism Software. A p-value < 0.05 was considered significant. Error bars shown in figures represent the standard error of the mean (SEM).
Acknowledgments
This study was supported by the NIH grant AG028082 to D.R.G.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- ABC
-
- age-associated B cell
-
- Breg
-
- regulatory B cell
-
- DSA
-
- donor-specific antibodies
-
- MST
-
- median survival time




