Chronic Trichuris muris infection alters hematopoiesis and causes IFN-γ-expressing T-cell accumulation in the mouse bone marrow
Abstract
Proinflammatory cytokines produced during immune responses to infectious stimuli are well-characterized to have secondary effects on the function of hematopoietic progenitor cells in the BM. However, these effects on the BM are poorly characterized during chronic infection with intestinal helminth parasites. In this study, we use the Trichuris muris model of infection and show that Th1 cell-associated, but not acute Th2 cell-associated, responses to chronic T. muris infection cause a major, transient expansion of CD48−CD150− multipotent progenitor cells in the BM that is dependent on the presence of adaptive immune cells and IFN-γ signaling. Chronic T. muris infection also broadly stimulated proliferation of BM progenitor cells including CD48−CD150+ hematopoietic stem cells. This shift in progenitor activity during chronic T. muris infection correlated with a functional increase in myeloid colony formation in vitro as well as neutrophilia in the BM and peripheral blood. In parallel, we observed an accumulation of CD4+, CD8+, and CD4−CD8− (double negative) T cells that expressed IFN-γ, displaying activated and central memory-type phenotypes in the bone marrow during chronic infection. Thus, these results demonstrate that Th1 cell-driven responses in the intestine during chronic helminth infection potently influence upstream hematopoietic processes in the BM via IFN-γ.
Introduction
There is a continuous turnover of blood cells in the BM that is critically important for replenishing leukocyte populations that become activated and depleted during an immune response. Infectious stimuli are known to significantly perturb overall immune homeostasis with respect to hematopoiesis in the BM (recently reviewed by Zaretsky et al. 1). Consequent hematopoietic stress induced by inflammation during infections can suppress the output of blood cells from the BM that can result in conditions such as cytopenia 2. One specific mechanism of pathogens exerting effects on HSCs is the ligation of TLRs that can negatively impact HSC survival and function 3. Additionally, cytokines produced during an infection can also feedback onto the hematopoietic system. The proinflammatory cytokine IFN-γ, typically associated with cell-mediated immune responses against intracellular pathogens, has been shown to have an influence on hematopoietic progenitor cells in terms of differentiation, self-renewal, and downstream effector functions 4. Specifically, IFN-γ can alter myelopoiesis and activate HSCs during infections with intracellular organisms 5, 6. Another study suggested that IFN-γ has a negative impact on HSC proliferation and self-renewal and thus may play a critical role in hematopoietic stress during chronic inflammation 7. Additional studies have implicated IFN-γ affecting the BM such as during viral lung infections and during colitis 8, 9. However, the role of IFN-γ in modulating hematopoiesis in the context of intestinal infections has not been thoroughly investigated.
Intestinal helminths are some of the most prevalent and pervasive infectious agents of humans that infect billions of people globally, causing significant morbidity in affected populations 10. During host anti-helminth responses or as a result of helminth-mediated immunomodulation, there are likely diverse effects on hematopoiesis in the host organism. However, the impact of helminth-elicited intestinal immune responses—such as the systemic effect of helminth-induced inflammatory cytokines—on hematopoietic responses is currently poorly characterized. Although some studies indicated a correlation between intestinal helminth infections and anemia 11, 12, no studies have been carried out to investigating the potential effects of these infections on the BM in humans that could help better characterize the systemic consequences on the host. In this study, we investigate the effect of helminth-driven intestinal inflammation using the Trichuris muris model in which we can control whether the infection induces a Th1 or Th2 cell-mediated intestinal immune response. We identify a significant effect of persistent, Th1 cell-associated T. muris infection on hematopoietic progenitor populations in the BM that is absent during transient Th2 cell-associated T. muris infection. This effect on upstream hematopoietic progenitor cells peaks on day 21 postinfection even though peripheral blood neutrophilia was evident at later time points of the chronic worm infection. We further show that this infection-mediated effect on hematopoiesis is dependent on both IFN-γ and adaptive immunity, correlating with an accumulation of IFN-γ-expressing T cells in the BM. These results highlight that inflammatory signals originating from the intestine during persistent helminth infections can distally effect the hematopoietic compartment in the BM through the local induction of inflammatory cytokines.
Results
Chronic T. muris infection induces increased LSK cells in the BM
To assess the systemic impact of localized intestinal immune responses on hematopoiesis, we employed the T. muris model of helminth infection. The T. muris model is a well-characterized system that allows the induction of either a Th1 cell-driven persistent infection (low dose) or a Th2 cell-driven acute infection (high dose) in the large intestine of most strains of wild-type mice 13. Using this infection model, we could compare the effects of intestinal Th1 and Th2 cell-dependent immune responses on hematopoiesis. As expected, low dose (LD) T. muris infection of C57BL/6 mice resulted in a persistent worm burden 21 days postinfection and remained chronically infected 35 days postinfection (Fig. 1A). In contrast, high dose (HD) T. muris infection resulted in few or no detectable worms in the cecum, consistent with a protective Th2 cell response. Th1 and Th2 cell-dominant responses, for the LD and HD infections, respectively, were confirmed by analyzing T. muris-specific IgG responses and cytokine expression in the colon (see Supporting Information Fig. 1). Isolation and enumeration of total BM cells from these mice showed that LD, but not HD T. muris infection resulted in a significant reduction in total cellularity in the BM (Fig. 1B). Next, we assessed whether specific changes to the composition of major cell populations in the BM occur in response to LD or HD T. muris infections. Since LD T. muris infection is associated with an IFN-γ-driven Th1 cell responses 13 and that IFN-γ has been shown to induce the expression of Sca-1 on immune cells 14, we assessed Sca-1 expression on hematopoietic cells in the BM of infected mice. The frequency and absolute number of total CD45+ BM cells expressing Sca-1 was increased twofold in LD T. muris-infected mice compared to naive controls (Fig. 1C). Conversely, mice that received a HD T. muris infection did not have elevated Sca-1 expression on hematopoietic cells in the BM. We further analyzed lineage-negative cell populations in the BM and found that only LD T. muris infection caused a significant increase in the frequency and absolute number of Sca-1+c-Kit+ (LSK) cells compared to naive and HD T. muris-infected mice (Fig. 1D). Together, these data show that a persistent LD T. muris infection induces significant and broad changes in the hematopoietic compartment in the BM that are absent after a transient HD T. muris infection.
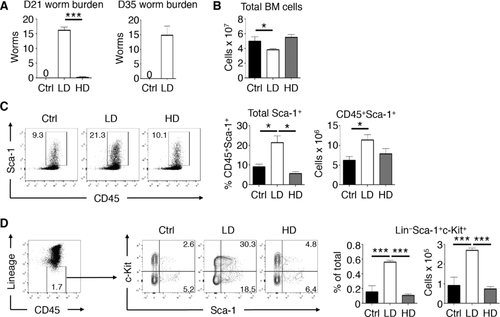
Chronic T. muris infection alters bone marrow MPP populations
To further characterize the infection-induced changes in the BM during LD T. muris infection, we analyzed progenitor and stem cell populations within the LSK population using the signaling lymphocytic activation molecule family markers CD48 and CD150—multipotent progenitor (MPP) cells are enriched within the CD48−CD150− population while HSCs can be identified as being CD48−CD150+ 15. We found that LD T. muris-infected mice had significant expansion in the number of CD48−CD150− MPPs in the BM compared to naive mice (Fig. 2A). No differences were found in terms of the absolute numbers of HSC in BM during infection. Analysis of the BM at various time points of LD T. muris infection indicated that the expansion of MPPs peaked at day 21 and returned to normal levels by day 35 postinfection (Fig. 2B). To test the functional effects of these changes in the BM, we performed in vitro colony forming assays. BM from LD T. muris-infected mice had significantly higher total CFU counts (Fig. 2C). There were no significant differences found in terms of monoblast (CFU-M) or granulocyte (CFU-G) colony counts; however, BM from LD T. muris-infected mice yielded more precursor CFU-GM (granulocyte, monocyte) colonies. Correlating with enhanced CFU-GM formation, analysis of intermediate progenitor populations of the myeloid lineage showed no differences in common myeloid progenitor cells and an increase in the frequency, but not number, of granulocyte-monocyte progenitor (GMP) cells in the BM of infected mice (Fig. 2D). In parallel, analysis of mature cell lineages confirmed a specific increase in the number of neutrophils in the BM of LD T. muris-infected mice (Fig. 2E). This coincided with an elevation of peripheral neutrophils in the blood while other cell populations remained unchanged on day 21 and 35 postinfection (see Supporting Information Fig. 2). Other blood parameters such as red blood cell count and hematocrit were unaffected, suggesting that LD infection does not cause anemia in mice. Therefore, LD T. muris infection alters primitive progenitor populations in the BM that correlate with an increase in myeloid colony forming potential in vitro and an increase in BM and peripheral blood neutrophils.
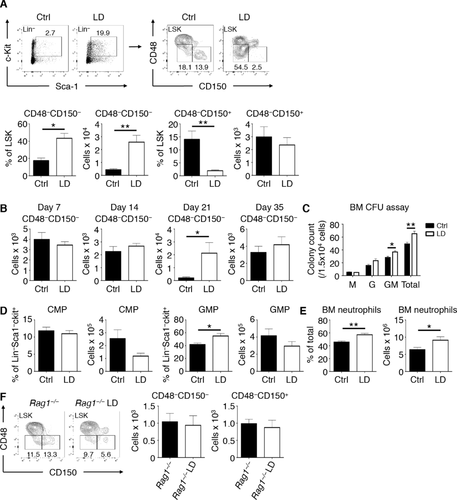
As LD T. muris infection is associated with a Th1 cell response 16, we next tested whether the effects we saw were indeed dependent on adaptive immunity by analyzing the BM of infected Rag1−/− mice that lack T and B cells. In contrast to infected WT mice, LD T. muris-infected Rag1−/− mice did not show an expansion of MPPs in the BM when compared to uninfected controls (Fig. 2F). Similarly, the absolute number of HSCs in infected Rag1−/− mice was unaffected. Thus, LD T. muris infection alters MPP populations in the hematopoietic compartment of the BM and is dependent on the presence of adaptive immune cells.
Chronic T. muris infection stimulates proliferation in hematopoietic progenitors
IFN-γ has previously been shown to activate HSCs during chronic infection 6. Thus, we measured proliferation of hematopoietic progenitor populations during our model of Th1 cell-associated chronic helminth infection by pulsing mice with 5-ethynyl-2-deoxyuridine (EdU) prior to the peak of the observed BM response on day 21 postinfection with T. muris. LSK cells in the BM of T. muris-infected mice had >twofold increased EdU retention compared to LSK cells from naïve mice (Fig. 3). Among the LSK population, there was a trend for an increase in MPP cells that were EdU+ during infection and HSCs had a significant increase in EdU retention compared to naïve controls. Together these data show that chronic T. muris infection broadly stimulates proliferation in hematopoietic progenitor cells with HSCs displaying significantly enhanced activation.
Alterations in hematopoiesis during chronic T. muris infection is IFN-γ-dependent
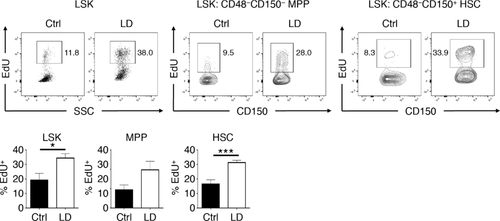
Since we found that changes in the BM in response to LD T. muris infection depended on adaptive immunity, we sought to determine whether the infection-mediated effects on hematopoiesis were indeed driven by the Th1 cell-associated cytokine IFN-γ. To directly test this, we neutralized IFN-γ systemically during the course of the infection using monoclonal antibody treatment. Blockade of IFN-γ did not cause mice to clear a LD T. muris infection, as infected mice treated with control IgG antibodies or anti-IFN-γ had comparable worm burdens (Fig. 4A). Further, the infection-induced increase in LSK cells, in terms of frequencies and absolute numbers, was completely reversed upon blockade of IFN-γ (Fig. 4B). As shown in Fig. 4C, the expansion of CD48−CD150− MPPs in the BM during LD T. muris infection was reversed in infected mice treated with α-IFN-γ that resembled naive MPP numbers. Further, neutralization of IFN-γ reversed the reduction in total BM cellularity caused by chronic T. muris infection (Fig. 4D). However, peripheral blood neutrophilia was still observed in chronically infected mice, independent of IFN-γ signaling (Fig. 4E). Thus, alterations in hematopoietic progenitor populations in the BM, elicited by LD T. muris intestinal infection, is completely dependent on IFN-γ signaling while peripheral blood neutrophilia is not dependent on IFN-γ during infection.
A mixed population of IFN-γ-expressing T cells accumulates in the BM chronic T. muris infection
Given that we found that IFN-γ was playing a major role in influencing hematopoiesis during LD T. muris infection, we next assessed whether IFN-γ-expressing cells are found in the BM. To do this, we infected IFN-γ-YFP reporter (GREAT) mice with LD T. muris and characterized IFN-γ expression in the BM. Compared to naive controls, LD T. muris-infected mice had a small yet significant increase in IFN-γ+ cells in the BM on day 21 post-infection (Fig. 5A). The appearance of these IFN-γ+ cells coincides with the peak of BM response described earlier as we could not detect a significant increase in these cells as late as day 14 post-infection (see Supporting Information Fig. 3). Additionally, the IFN-γ-producing BM cells from LD T. muris-infected mice also had a significantly higher MFI compared to controls. Upon examination of specific cell types in the BM, we found that the majority of cells with increased IFN-γ expression during infection were CD3+ lymphocytes (Fig. 5B). IFN-γ expression was significantly increased in CD4+, CD8+, and CD4−CD8− double negative (DN) T-cell populations in the BM of LD T. muris-infected mice compared to BM from naive mice. These IFN-γ+ T cells in the BM were also significantly increased in terms of absolute numbers during LD T. muris infection. In contrast, non-T cell populations such as NK cells showed no significant differences in IFN-γ expression. Therefore, a mixed population of IFN-γ+ T cells accumulates in the BM during persistent LD T. muris intestinal infection.
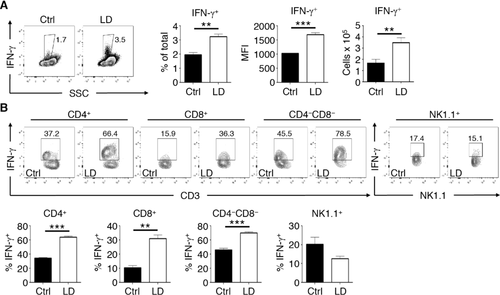
BM IFN-γ+ T cells display activated and central memory phenotypes during chronic T. muris infection
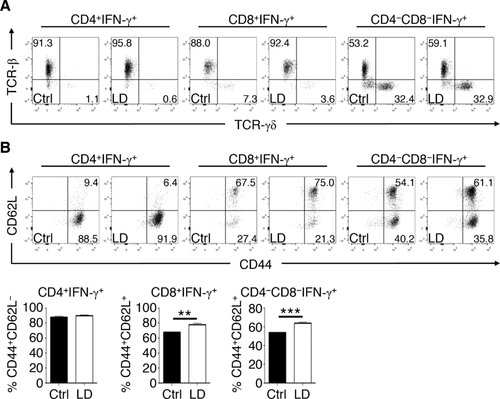
We next phenotyped surface marker expression on IFN-γ+ T cells in the BM of LD T. muris-infected mice. The majority (> 90%) of IFN-γ-expressing CD4+ and CD8+ T cells in the BM were predominately TCR-β+ but were of comparable frequency between LD T. muris-infected and uninfected mice (Fig. 6A). Conversely, IFN-γ+ DN T cells could be split into both TCR-β+ and TCR-γδ+ populations (2:1 ratio, respectively) and were of similar frequencies between LD T. muris-infected and naive controls. Analysis of CD44 and CD62L expression showed that IFN-γ+ CD4+ T cells in the BM from both LD T. muris-infected and naive mice were mainly CD44+CD62L−, consistent with an activated phenotype (Fig. 6B). However, for both IFN-γ+ CD8+ and DN T cells, there was a significant increase in the frequency of CD44+CD62L+ cells in the BM of LD T. muris-infected mice, indicative of an increase in central memory type cells. Thus, LD T. muris infection induces a mixed population of T cells expressing IFN-γ that display activated and central memory phenotypes in the BM.
Discussion
In the present study, we have shown that a persistent, low burden infection with the intestinal helminth T. muris broadly influences hematopoietic progenitor populations in the BM. Although there have not been any previous studies on the direct effects of intestinal helminths on the BM, it has been shown that chronic intestinal inflammation can profoundly influence hematopoiesis; during colitis, proinflammatory cytokines can induce the dysregulated proliferation of HSCs and can drive myelopoiesis that further exacerbate chronic intestinal inflammation 9. This is consistent with our results showing enhanced proliferation of HSCs and an expansion of MPPs in the BM, associated with an increase in myeloid colony formation in vitro that occurs during a persistent intestinal T. muris infection. However, in contrast to colitis, chronic intestinal infection with T. muris is not associated with overt morbidity in mice, due to the induction of regulatory responses such as IL-10 17, 18. Because we found that the infection-induced effects on the BM peaked on day 21 and subsided by day 35, we hypothesize that these regulatory responses eventually dampen this stimulation of hematopoietic compartment. Future studies will aim to characterize the role of cytokines such as IL-10 on localized BM responses during our chronic infection model. Since we concurrently observed a dependence on IFN-γ signaling in our model, it is likely that proinflammatory cytokines induced during both colitis and intestinal helminth infection have a similar overall impact on BM responses during the peak of the Th1-cell response. Additionally, as we saw an increase in the proliferation of hematopoietic progenitors such as HSCs in the BM during chronic infection, it would be informative to assess the long-term self-renewal and repopulation capacity of HSCs in our model through BM transplants and limiting dilution studies.
With respect to other mucosal sites, Maltby et al. showed that during acute pneumonia virus infection in the lung, an increase in myelopoiesis occurs in the BM that is IFN-γ-driven 8; however, upon blockade of IFN-γ in their model, viral load increased that may have confounded their findings. In contrast, when we neutralized IFN-γ during LD T. muris infection, we saw no effect on intestinal worm burden, suggesting that the infection-induced effects on the BM were primarily IFN-γ-mediated and not dependent on the presence of worms alone or other factors associated with the chronic infection. Other infection models that induce Th1-cell responses suggest a consistent role for IFN-γ in affecting BM output: IFN-γ negatively regulates granulopoiesis and promotes monopoiesis during mycobacterial infections 19 which also occurs during LCMV infection 20. However, we did not observe a specific skewing toward monopoiesis in the BM of mice chronically infected with T. muris, based on colony forming potential and analysis of intermediate GMPs as well as mature lineages in the BM showing increases in granulocytes such as neutrophils. This enhanced myelopoiesis is consistent with our previous in vivo studies showing an increase in myeloid cells at peripheral sites such as the lungs during chronic T. muris infection 21. While many of the infection-induced effects in the BM we saw were IFN-γ-dependent, peripheral blood neutrophilia elicited by chronic T. muris infection appeared to be IFN-γ-independent. As low burden infections persisted in the intestine even after neutralization of IFN-γ, it is possible that other factors drive blood neutrophilia such as tissue damage, other cytokines, and worm antigens induced by the physical presence of the worms. Together, these findings are consistent with the notion that the role of IFN-γ during hematopoiesis is context dependent 22 that will vary between different infection models. Additionally, IFN-γ can differentially affect BM responses by synergizing with other cytokines 23 that are likely considerably different in our model of intestinal helminth infection compared to these other experimental models. Thus, chronic T. muris infection induces myelopoiesis in the BM similar to other Th1 cell-inducing models but with differences in myeloid lineage-specific skewing. Future studies will aim to further characterize the localized effects of IFN-γ signaling in the BM by performing low dose T. muris infection in mice with deletion of the IFN-γR in nonhematopoietic cells such as stromal, reticular, endothelial, and mesenchymal progenitor cells.
IFN-γ has been previously shown to be elevated both in the mesenteric lymph nodes and systemically in the blood during chronic T. muris infection 24. Our results suggest that in addition to this systemic induction of IFN-γ, IFN-γ+ T cells also accumulate in the BM during the chronic intestinal helminth infection. The accumulation of activated T cells expressing IFN-γ in the BM has been previously described 7, and has been shown to occur in the contexts of T cell-driven colitis 9 and anti-tumor responses 25. Indeed, the BM is considered a preferential site in which memory T cells home 26, consistent with our results showing that both CD8+ and DN IFN-γ+ T cells in the BM display a central memory phenotype during chronic T. muris infection. DN T cells have roles in various Th1 cell-mediated responses including intracellular bacterial and viral infections 27, 28. Further, during intracellular infection with Leishmania major, DN T cells that can rapidly produce IFN-γ are critical for primary and secondary responses and display memory-like cell properties 29. This is in line with our results showing that central memory-like DN T cells had the largest increase in IFN-γ production during a Th1 cell-associated chronic T. muris infection. Future studies will focus on determining whether these BM-residing T cells expressing IFN-γ are key in modulating hematopoietic progenitor populations during chronic T. muris infection.
Overall, our data show for the first time that a chronic intestinal infection with T. muris influences hematopoiesis in the BM via the induction of IFN-γ and is associated with activated and memory-like T cells expressing IFN-γ accumulating in the BM. Additionally, our results highlight that intestinal worm infection-induced effects on the BM are mechanistically consistent with a variety of distinct Th1 cell-associated responses.
Materials and methods
Ethics statement
Experiments were approved by the UBC ACC (Protocol number A13-0010) and were in accordance with the Canadian Guidelines for Animal Research.
Mice and intestinal worm infections
Propagation of T. muris eggs was performed as previously described 30. C57BL/6 GREAT (IFN-γ YFP reporter), and Rag1−/− mice were originally purchased from Jackson Laboratories (Bar Harbor, ME). Mice were either uninfected or infected with a low dose (30 eggs) or high dose (200 eggs) of T. muris by oral gavage. Infections were carried out for either 7, 14, 21, or 35 days before sacrifice of mice. All mice were bred and housed in specific pathogen-free animal facilities at the UBC Biomedical Research Centre (Vancouver, BC).
Serum ELISA
Serum was collected from mice 21 days post-infection with T. muris. Immulon plates (Thermo Fischer Scientific, NY) were coated with 5 μg/mL of dialyzed T. muris antigen overnight at 4°C. Wash buffer was PBS containing 0.05% Tween 20. Plates were blocked and serum samples were diluted in 3% bovine serum albumin in PBS/0.05% Tween 20. Serum samples were incubated on plates for 1 hour at room temperature. Plates were then incubated with rat anti-mouse IgG1 or IgG2a conjugated to horseradish peroxidase (BD Pharmingen, CA) for 1 hour at room temperature. Plates were developed using 3,3',5,5'-tetramethylbenzidine (TMB) substrate (MandelScientific, ON) and stopped with 1N HCl. Plates were read at 450 nm on a Spectramax 384 (Molecular Devices, CA).
Quantitative real-time PCR
Colon tissue was mechanically homogenized using a TissueLyser (Qiagen, Germany) and RNA was extracted using the TRIzol method according to the manufacturer's instructions (Ambion, TX). Complementary DNA (cDNA) was generated using High Capacity cDNA reverse transcription kits according to the manufacturer's instructions (Applied Biosystems, CA). Quantitative real-time PCR was performed using the SYBR FAST master mix (Kapa Biosystems, MA) and SYBR green-optimized primer sets run on an ABI 7900 real-time PCR machine (Applied Biosystems, CA). Ct values were normalized relative to beta-actin (Actb) gene expression. Mouse primer sequences (5’-3’): Actb forward ACTAATGGCAACGAGCGGTTC; Actb reverse GGATGCCACAGGATTCCATACC; Il13 forward GGTCTTGTGTGATGTTGCTCA; Il13 reverse CCTGGCTCTTGCTTGCCTT; Ifng forward GGATGCATTCATGAGTATTGCC; Ifng reverse CCTTTTCCGCTTCCTGAGG.
Isolation of BM cells
Following sacrifice of mice, hip joints were dislocated and the right hind limbs of each mouse were dissected to isolate single long bones, clean of any muscle and other tissues. The ends of each bone were sliced by a razor blade and a 27 gauge needle was used to flush out the BM with media (DMEM supplemented with 10% FBS, 25 mM HEPES, penicillin/streptomycin, 2 mM l-glutamine, and 50 μM β-mercaptoethanol). The BM was then passed through a 70 μm cell strainer to obtain single-cell suspensions. BM samples were red cell-lysed using ammonium chloride buffer, and suspended to a known volume before being enumerated by a hemocytometer and stained for flow cytometry.
Blood parameter analysis
Whole blood was collected into EDTA-coated Microvette collection tubes (Sarstedt, Germany) by saphenous vein bleed of mice prior to sacrifice. Blood samples were analyzed using an Advia 120 hematology analyzer (Siemens, ON).
EdU labeling and proliferation assay
Mice were infected with T. muris as described above. On days 18 and 20 post-infection, mice were injected i.p. with 3 mg 5-ethynyl-2-deoxyuridine (EdU) dissolved in PBS on each day, prior to sacrifice on day 21. BM cells were harvested as described above and EdU was stained using Click-iT Assay kits for Flow Cytometry (ThermoFisher Scientific, MA) following the manufacturer's protocol. Cells were then surface stained for flow cytometric analysis.
In vivo neutralization of IFN-γ
Mice were infected with T. muris as described above. On day 4 postinfection, mice were injected i.p. with 500 μg of either control IgG1 or anti-IFN-γ (XMG1.2) (produced in-house by AbLab Biologics, UBC (Vancouver, BC)), constituted in sterile PBS. Mice were repeatedly injected thereafter on days 8, 12, and 16 prior to sacrifice on day 21.
Antibodies and flow cytometry
All antibody dilutions and cell staining were done with PBS containing 2% FCS, 1 mM EDTA, and 0.05% sodium azide. Fixable Viability Dye eFluor 506 was purchased from eBioscience to exclude dead cells from analyses. Prior to staining, samples were Fc-blocked with buffer containing anti-CD16/32 (93, eBioscience) and 1% rat serum to prevent nonspecific antibody binding. PE-conjugated anti-CD34 (RAM34) was purchased from BD Biosciences. APC-conjugated anti-CD150 (TC15-12F12.2) was purchased from BioLegend. PE-conjugated anti-CD3e (2C11); PE-Cy7-conjugated anti-Sca1 (D7); eFluor 450-conjugated anti-CD8a (53-6.7), anti-CD48 (HM48-1); APC-conjugated anti-CD4 (GK1.5), anti-CD16/32 (93); and APC-eFluor 780-conjugated anti-c-kit (2B8) were purchased from eBioscience. FITC-conjugated anti-CD19 (1D3), anti-CD11b (M1/70), anti-CD11c (N418), anti-Gr1 (RB6-8C5), anti-NK1.1 (PK136), anti-Ter119 (Ter119); Pacific Blue-conjugated anti-CD45 (I3/2), anti-CD11b (M1/70); and Alexa Fluor 647-conjugated anti-CD11c (N418) were produced in-house (AbLab Biologics, UBC). Data were acquired on an LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar).
CFU assay
BM cells were isolated as above using proper aseptic technique. Following manufacturer instructions, 1.5 × 104 cells from each sample were plated in duplicate in Methocult Media (STEMCELL M3234, Vancouver, BC) supplemented with 50 ng/mL SCF, 10 ng/mL IL-3, and 10 ng/mL IL-6. Plates were incubated for approximately 10 days prior to morphological analysis and differential counts of CFUs.
Statistics
Data are presented as means ± SEM. Statistical significance between two groups was determined by the Student's t-test while comparisons between 3 or more groups were made by ANOVA with a Bonferroni post-hoc test using GraphPad Prism software. Results were considered statistically significant with a *p < 0.05, **p < 0.01, or ***p < 0.001.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research, a Canada Foundation for Innovation grant and the Australian National Health and Medical Research Council to C.Z. A.L.C. was supported by the UBC four-year doctoral fellowship and F.A. is a recipient of CIHR/Canadian Association of Gastroenterology/Crohn's and Colitis Foundation of Canada postdoctoral fellowship. C.Z. is a MSFHR Career Investigator and is a veski innovation fellow. We thank the BRC Animal Care facility; Taka Murakami of the BRC Genotyping facility; Andy Johnson and Justin Wong of the UBCflow facility; Les Rollins, Rupinder Dhesi, Michael Williams, and Helen Merkens of the BRC core staff.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
References
Abbreviations
-
- GMP
-
- granulocyte-monocyte progenitor
-
- HD
-
- high dose
-
- LD
-
- low dose
-
- MPP
-
- multipotent progenitor




