Thymocyte death by neglect: Contribution of engulfing macrophages
Abstract
The thymus provides the microenvironment in which thymocytes develop into mature T cells, and interactions with thymic stromal cells are thought to provide the necessary signals for thymocyte maturation. Recognition of self-MHC by T cells is a basic requirement for mature T-cell functions, and those thymocytes that do not recognize the peptide-loaded self-MHC molecules found in the thymus, and therefore lack a TCR signal, undergo a default death pathway named “death by neglect” in the thymic cortex. In the absence of this TCR signaling, it has been suggested that binding of glucocorticoids to — or the ligation of certain cell surface molecules, such as CD8, CD24, CD45, or CD99 on — these neglected thymocytes will induce them to enter the apoptotic program. Apoptotic thymocytes are cleared by the surrounding macrophages and, as a consequence, these macrophages are known to release various molecules, such as adenosine, retinoids, TGF-β, ATP, and carbon monoxide. Interestingly, all these molecules have been described to induce or promote apoptosis in thymocytes in the absence of TCR signaling. Here, we propose that thymic macrophages, because they continually engulf apoptotic cells, might constantly provide these cell death-inducing signals, and thus contribute to the formation of a thymic milieu that ensures the effective induction of “death by neglect”.
T-cell development in the thymus
The thymus is the primary lymphoid organ responsible for the generation of immunocompetent T lymphocytes from bone marrow-derived CD4−CD8− double negative progenitors. This occurs through a series of differentiation and selection steps 1. T-cell differentiation is not a thymocyte autonomous process, but depends on signal combinations provided by dendritic cells, macrophages, endothelial cells, fibroblasts, and thymic epithelial cells (TECs) that surround the developing thymocytes 2. At the start of differentiation, progenitor cells rearrange their TCR-β gene locus to express a pre-TCR complex consisting of the polymorphic β-chain, a nonpolymorphic pre-α chain, and CD3. Following pre-TCR expression, thymocytes divide rapidly, acquire CD4 and CD8 molecules becoming CD4+CD8+ double positive (DP) cells, and undergo TCRα rearrangement.
Those cells that successfully generate a functional α chain replace the pre-TCR with mature TCRαβ and thus become CD4+CD8+ DP TCRαβlow cells 3. These cells can interact with self-peptides presented on major histocompatibility complex (MHC) molecules on thymic nonlymphoid cells. Their destiny will depend on the nature of the TCR-αβ signal. Thymocytes receiving a strong TCR-αβ signal undergo activation-induced apoptosis, a major mechanism for promoting self-tolerance (negative selection), while those which receive a TCR-αβ signal of intermediate strength continue to differentiate (positive selection) into CD4+ or CD8+ TCR-αβhigh single positive cells 4 and complete their maturation in the thymic medulla. In the medulla, they will be further exposed to “self-specific” peripheral tissue antigens presented either by medullary TECs as a consequence of TEC expression of the autoimmune regulator transcription factor Aire 5 or by thymic dendritic cells. Those cells that survive this second wave of negative selection will migrate into the periphery and form the peripheral T-cell repertoire.
However, 90% of the DP thymocytes produced express TCRs that do not recognize peptide-loaded self-MHC molecules present in the thymus and undergo a default death pathway named “death by neglect”. While numerous investigations have addressed the signals and signaling pathways that guide the development of thymocytes, there is still uncertainty as to the mechanisms by which death by neglect is regulated, even though these processes affect the majority of thymocytes produced. It has been proposed that, in the absence of TCR signaling, binding of glucocorticoids (produced by either TECs or the adrenal cortex) to, or the crosslinking of various cell surface receptors (such as CD8, CD24, CD45, or CD99) on, thymocytes will induce the cell death program in the neglected DP cell population (see Signals thought to regulate the death of neglected thymocytes).
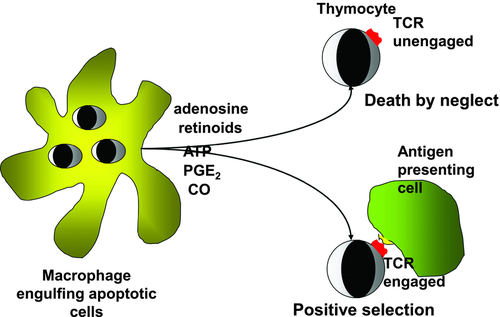
In vivo, the apoptosis program is completed by phagocytosis, with the majority of apoptotic thymocytes in mice being cleared by the thymic F4/80+ macrophages 6. Recently, we observed that macrophages, when engulfing apoptotic thymocytes, release adenosine 7 and retinoids 8 Zsuzsa — two molecules that have been known for many years to induce apoptosis in DP thymocytes in vitro 9, 10. Interestingly, other molecules, such as transforming growth factor (TGF)-β, carbon monoxide, ATP, and prostaglandin (PG)E2, which have previously been reported to either induce or enhance apoptosis of DP thymocytes in vitro in the absence of TCR signaling 11-14, have also been reported to be produced by macrophages engulfing apoptotic cells 15-17. Here, we propose that because apoptosis and the consequent engulfment of apoptotic cells are continuously ongoing processes in the thymus, molecules released continuously by engulfing macrophages might contribute to the formation of a thymic milieu that, in the absence of TCR signaling, ensures the induction of apoptosis of neglected thymocytes especially in the cortex, where the majority of cells die.
Apoptosis regulation in the CD4+, CD8+, DP stage
Apoptosis, the most dominant form of cell death in mammals, is characterized by initiator and subsequent executor caspase activation processes; these drive all the characteristic morphological and molecular events of apoptosis. The initiator caspases are activated by either extrinsic or intrinsic cell death pathways: the extrinsic pathway is triggered by ligation of cell surface death receptors such as Fas and TNF receptor leading to activation of caspase-8 18, while the intrinsic pathway is initiated by formation of mitochondrial membrane channels containing either Bax or Bak, two intracellular proapoptotic multidomain B-cell lymphoma 2 (Bcl-2) protein family members. The appearance of these channels results in the permeabilization of the mitochondrial outer membrane, and the subsequent release of cytochrome c and other proapoptotic factors that trigger activation of another initiator caspase, caspase-9 19.
The expression of Bax and Bak is mandatory for the initiation of the intrinsic cell death pathway, as Bax/Bak double-mutant thymocytes are resistant to all signals that induce apoptosis via the mitochondria 20. The formation of Bax or Bak-containing membrane channels is negatively regulated by the antiapoptotic members of the Bcl-2 protein family, such as Bcl-2, Bcl-xL, and Mcl-1, while promoted by the BH3 only members of this family 21. Signals that regulate apoptosis via the intrinsic pathway control either the level or the availability of these pro and antiapoptotic Bcl-2 family proteins in thymocytes. Simultaneous loss of three BH3 only proteins, Puma, Bid, and Bim in triple knockout mice results in T-cell developmental defects that recapitulate those found in Bax/Bak double-mutant mice 22 indicating that, in thymocytes, these three BH3 only domain proteins mediate the initiation of the cell death program induced by all intrathymic signals that trigger the mitochondrial pathway of apoptosis. Regardless of whether the intrinsic or extrinsic pathway is triggered by the apoptotic signals received by thymocytes, a common executor pathway then follows. This involves activation of the effector caspases, such as caspase-3, -6, and-7, by limited proteolysis catalyzed by the initiator caspases. The activated effector caspases then cleave at least 500 different proteins resulting in all the molecular and morphological changes that characterize apoptosis 23.
The first wave of thymic apoptosis occurs in those thymocytes that are not able to produce the preTCR and such apoptosis is thought to be induced by the BH3 only Bcl-2 family proteins Bim and Bid 24. Those thymocytes that express the pre-TCR transit to the DP stage and enter a period of quiescence that lasts an average of 3 or 4 days, during which they undergo multiple rounds of TCR-α rearrangements to maximize the chances of forming a functional TCR. During this period, expression of Bcl-xL 25 and Mcl-1 26, two antiapoptotic Bcl-2 family members, allows the opportunity to test the newly displayed TCR. While the expression of Mcl-1 is continuous during thymocyte development, Bcl-xL is induced by retinoid-related orphan receptor (ROR)-γ, which is specifically expressed on DP thymocytes 27. Due to a failure in IL-7 signaling 28, DP thymocytes express low levels of the antiapoptotic Bcl-2. However, once the α chain is successfully rearranged and the cells receive a weak TCR-αβ signal (i.e. the signal for positive selection), positively selected DP thymocytes regain IL-7 sensitivity 28 and increase expression of Bcl-2, thus ensuring their survival. It should be noted, however, that the effects of Bcl-2 can, in appropriate circumstances, be overridden, as discussed in the next paragraph.
In contrast to a weak TCR-αβ signal and positive selection, a strong TCR-αβ signal initiates negative selection and the second wave of apoptosis, which occurs via two molecular elements: (i) Bim 29, a BH3 only protein that can neutralize the activity of each antiapoptotic Bcl-2 family members in a dose-dependent manner 21, and (ii) activation of Nur77, a transcription factor that can upregulate the initiators of the extrinsic cell death pathway 30. In addition, following translocation into the mitochondria, Nur77 can interact with Bcl-2 and convert it into a proapoptotic protein 31. As a result of the Nur77-Bcl-2 interaction, even positively selected thymocytes, which express higher levels of Bcl-2 and have entered the medulla, will subsequently be negatively selected if they carry autoreactive TCR 32.
As well as a weak TCR-αβ signal (positive selection) and a strong TCR-αβ signal (negative selection), there is a third option of no TCR-αβ signal and hence death by neglect as already discussed. Given that (i) Bim and Bid are believed to participate in the apoptosis driven by the failure to generate pre-TCR 24, (ii) Bim alone (of the BH3 only proteins of the Bcl-2 family) participates in negative selection 29, and (iii) simultaneous loss of Puma, Bid, and Bim prevents all forms of thymocyte death 24, it would seem that the signaling pathways that initiate death by neglect must involve Puma. However, loss of Bim also enlarges the size of the DP thymocyte population 29 indicating that Bim might also play a role in the initiation of death by neglect.
Signals thought to regulate the death of neglected thymocytes
Glucocorticoid hormones have been known for a long time to induce apoptosis in immature thymocytes 33. Increasing evidence suggests that the effector phase of glucocorticoid-induced apoptosis is mediated via the mitochondrial pathway involving Bcl-2 family members, as disruption of Bcl-2 in mice enhances dexamethasone-induced thymocyte apoptosis 34, whereas lack of Bax and Bak prevents it 20. Simultaneous loss of Puma and Bim inhibits the process, in line with the observations that glucocorticoids induce the expression of these proapoptotic BH3 only proteins in thymocytes 35, 36.
Since the discovery that adrenalectomy in experimental animals results in thymic hypertrophy that cannot be reversed by adrenalin 37, it has been proposed that glucocorticoids play a central role in mediating thymocyte death by neglect. This concept was further confirmed by the observation that coculturing thymocytes with TECs induced apoptosis in thymocytes that did not receive simultaneous TCR signals 38, and both TECs and thymocytes were found to produce glucocorticoids 39. In addition, at physiological glucocorticoid levels, massive thymocyte apoptosis was found in transgenic rats overexpressing a mutant glucocorticoid receptor with increased ligand sensitivity 40. Nonetheless, loss of the glucocorticoid receptor did not affect thymic homeostasis 41 indicating that other signals must also contribute to driving the apoptosis of neglected thymocytes.
As a result, significant attention has been focused on the possibility of interactions between surface membrane proteins generating an active cell death signal, as opposed to a simple default pathway triggered by lack of signal. Ligation of several cell surface molecules such as CD8 42, CD24 43, and CD45 44 has been reported to induce thymocyte apoptosis in the absence of TCR engagement. However, the involvement of these surface molecules in DP thymocyte apoptosis was never tested in the normal physiological environment of the thymus. Recently, a signaling pathway induced by the interaction between the mouse homologue of CD99 (designated D4) and its ligand, paired immunoglobulin–like type 2 receptor (PILR) found on the surface of TECs, has also been suggested as a mechanism of thymocyte apoptosis by neglect. This form of thymocyte apoptosis is caspase 8 dependent and is not inhibited by Bcl-2 45. However, caspase-8 knock out thymocytes undergo normal thymocyte development 46, contradicting a nonredundant role for D4-induced apoptosis in the neglect pathway.
Apoptosis-inducing signals are released by macrophages engulfing apoptotic cells
The thymocyte apoptosis program in vivo is completed by the clearance of apoptotic cells by professional phagocytes. Studies which examined the spatial distribution of apoptotic thymocytes found that the majority of the immature thymocytes die in the cortical region of the thymus and that all the detectable dead cells are localized within the thymic macrophages 6. In fact, DP thymocytes throughout the cortex form clusters with ED1+ (pan macrophage marker) and ED2+ (cortical) macrophages, indicating a strong interaction between macrophages and immature thymocytes during their differentiation 47. Macrophages do not simply clear apoptotic thymocytes, but release various signaling molecules and cytokines in response to the recognition and engulfment of apoptotic cells. In response to “find me” signals released by apoptotic cells, macrophages produce ATP that contributes to their migration to the apoptotic site 15. Following engulfment, they release TGF-β, PGE2 16, and adenosine 7 to prevent proinflammatory cytokine production in an autocrine manner. While degrading heme-containing proteins, they also produce carbon monoxide as a result of heme oxygenase-I action 17. Macrophages in the thymus also produce retinoids in an engulfment-dependent manner, and macrophage-derived retinoids and TGF-β contribute to the appearance of transglutaminase 2, an apoptosis-related multifunctional protein 48 in apoptotic thymocytes 8.
DP thymocytes express receptors for all these macrophage-released molecules 10, 14, 49-51. Interestingly, triggering of these receptors has been known for a long time to affect the apoptosis of DP thymocytes in the absence of TCR signaling. Retinoids induce apoptosis by activating retinoic acid receptor (RAR) γ 10, and they also promote glucocorticoid-induced apoptosis by facilitating the transcriptional activity of the glucocorticoid receptor acting on RAR-α/retinoid X receptors 52. Adenosine induces apoptosis in mouse thymocytes by activating the adenosine A2A receptor/adenylate cyclase pathway leading to the upregulation of Bim 9. PGE2 also induces adenylate cyclase activation and in vivo cell death 11. Although TGF-β alone does not induce apoptosis, it can support both glucocorticoid- and TCR-driven cell death 12. Its mechanism in thymocytes has not so far been investigated, but in B cells, TGF-β has been shown to regulate Bim 53. In addition, both carbon monoxide and ATP have been recognized as thymocyte apoptosis-inducing signals, with ATP activating the mitochondrial intrinsic pathway of apoptosis 13, 14. Based on these observations, we propose that macrophages engulfing apoptotic cells continuously release engulfment-dependent molecules into the microenvironment of DP thymocytes that might drive the death of neglected cells. Since the majority of thymocytes die and are engulfed in the cortex 6, the cortex must contain higher concentrations of these molecules than the medulla. This assumption is supported by the observation that the enzymes related to retinoid synthesis are expressed at higher levels in the thymic cortex 54. In addition, alterations in thymocyte transglutaminase 2 expression following inhibition of the action of retinoids or TGF-β in vivo indicate that at least these two compounds are produced by macrophages in sufficient amounts to affect thymocyte gene expression 8, 12.
Concluding remarks
Although it is generally accepted that glucocorticoids drive the death of neglected thymocytes, based on the observations described in this article, we propose that, in addition to glucocorticoids, thymocytes in the cortex are exposed to many other apoptosis-inducing signals (including cell surface proteins and molecules derived from macrophages engulfing apoptotic cells) that by acting together ensure the induction of death of neglected cells. Because of the multiplicity of the apoptosis-inducing signals, the loss of the receptor for just one such a signal is not expected to perturb thymic homeostasis. Thus, it is not surprising that loss of either the glucocorticoid receptor 41, the retinoic acid receptor γ 49, the adenosine A2A receptor 9, or the two PGE2 receptors 55 did not alter thymic homeostasis.
Previous studies have shown that while both glucocorticoid and TCR signaling individually induce apoptosis in thymocytes, when these signals act together, they result in cell survival 39. The combined effect is, however, dose dependent and stronger TCR signals are opposed only by higher concentrations of steroids. Similarly, thymocytes or T-cell hybridomas receiving TCR signals are protected against death if they are exposed simultaneously to retinoids 56, 57 or to compounds that elevate intracellular cAMP levels such as adenosine 58, but again in a dose-dependent manner. These observations indicate that higher concentrations of retinoids or adenosine in the cortex might not only drive the death of neglected thymocytes, but might also provide protection against TCR-induced cell death, thereby promoting positive selection (Fig. 1). By doing so, as was suggested for the glucocorticoids 39, retinoids, or adenosine might modulate the threshold of negative selection in a dose-dependent manner. Thus macrophage-derived retinoids, or adenosine, produced in an engulfment-dependent manner might also be involved in the fine tuning of TCR-driven positive selection. Whether the same conclusion might apply to other apoptosis-inducing molecules, such as ATP or carbon monoxide, and whether evidence for such a fine tuning role for retinoids or adenosine in vivo indeed exists, has yet to be determined.
Acknowledgments
The studies on which this article is based were supported by Hungarian grants from the National Research Fund (K77587), the TÁMOP 4.2.1./B-09/1/KONV-2010-0007 project (implemented through the New Hungary Development Plan, cofinanced by the European Social Fund), and a Sanofi Aventis Scholarship.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- Bcl-2
-
- B-cell lymphoma 2
-
- DP
-
- double positive
-
- PGE2
-
- prostaglandin E2
-
- TEC
-
- thymic epithelial cell