NK cells are strongly activated by Lassa and Mopeia virus-infected human macrophages in vitro but do not mediate virus suppression
Abstract
Lassa virus (LASV) and Mopeia virus (MOPV) are closely related Arenaviruses. LASV causes hemorrhagic fever, whereas MOPV is not pathogenic. Both viruses display tropism for APCs such as DCs and macrophages. During viral infections, NK cells are involved in the clearance of infected cells and promote optimal immune responses by interacting with APCs. We used an in vitro model of human NK and APC coculture to study the role of NK cells and to characterize their interactions with APCs during LASV and MOPV infections. As expected, NK cells alone were neither infected nor activated by LASV and MOPV, and infected DCs did not activate NK cells. By contrast, LASV- and MOPV-infected macrophages activated NK cells, as shown by the upregulation of CD69, NKp30, and NKp44, the downregulation of CXCR3, and an increase in NK-cell proliferation. NK cells acquired enhanced cytotoxicity, as illustrated by the increase in granzyme B (GrzB) expression and killing of K562 targets, but did not produce IFN-γ. Contact between NK cells and infected macrophages and type I IFNs were essential for activation; however, NK cells could not kill infected cells and control infection. Overall, these findings show that MOPV- as well as pathogenic LASV-infected macrophages mediate NK-cell activation.
Introduction
Lassa fever (LF) is a viral hemorrhagic fever caused by Lassa virus (LASV). It is endemic in West Africa and causes 100,000–300,000 cases and 5000–6000 deaths each year 1. The absence of a vaccine and the limited use of ribavirin, the only antiviral drug licensed, in endemic countries, render LF a public health problem. LASV and Mopeia virus (MOPV) are very closely related Old-World Arenaviruses with a common animal reservoir, Mastomys natalensis, a peridomestic rodent 2. Unlike LASV, MOPV is not pathogenic to nonhuman primates (NHPs), in which this virus has even shown to confer protection against challenge with LASV 3. The immune responses to LASV and MOPV are poorly understood. The control of LASV seems to involve the induction of T cells, rather than humoral responses 4. Indeed, cellular immune responses specific for viral glycoproteins appear to protect NHPs against lethal challenge 5. By contrast, severe LASV infections seem to be associated with immunosuppression and structural changes to secondary lymphoid organs.
LASV and MOPV display tropism for APCs, such as DCs and macrophages (MΦs) 6-8. These cells are the first targets of the viruses and they release large numbers of viral particles without cytopathic effects. APCs display only very low levels of activation or maturation after LASV infection 6 and produce only small amounts of type I IFN 9. By contrast, MOPV infection results in type I IFN production by MΦs and, to a lesser extent, by DCs, and triggers the early and strong activation of MΦs 8. The different responses of APCs to LASV and MOPV infections are probably involved in the difference in pathogenicity between the two viruses. It has been shown that CD4+ and CD8+ T cells are strongly and rapidly activated in response to MOPV-infected DCs, resulting in proliferation, differentiation into effector, cytotoxic, and memory cells. By contrast, LASV-infected DCs can induce only weak and delayed T-cell responses in vitro 10.
Like APCs, NK cells are at the crossroads between the innate and adaptive responses. They have effector functions in innate immunity, through their cytotoxic properties, and also produce cytokines involved in the induction of T-cell responses. They directly sense pathogens via TLRs 11. NK cells express a repertoire of activating and inhibitory receptors on their surface, which recognize aberrant cells. Some of these receptors are constitutively expressed by almost all NK cells, whereas the expression of others is tightly regulated by environmental stimuli. NK-cell activation is controlled by the balance between activating and inhibitory signals from target cells. NKp30, NKp44, and NKp46 belong to the natural cytotoxicity receptor family, NKG2D is a c-type lectin molecule and all these receptors are involved in NK-cell-mediated cytolysis 12. The inhibitory receptors include killer cell Ig-like receptors (KIR), such as KIR2DL2/3 (CD158b), which bind to class I MHC molecules 13. MHC-restricted recognition enables NK cells to discriminate between healthy and transformed cells. It is now widely recognized that NK cells are important mediators during viral infections, particularly in terms of their role in mediating the clearance of infected cells 14. Moreover, NK cells interact with DCs and MΦs, thereby potentiating immune mechanisms. These interactions promote cell activation, cytokine production, NK-cell proliferation and cytotoxicity, and DC and MΦ maturation 15. During viral infections, DCs and MΦs can increase IFN-γ production by NK cells, leading to the induction of a Th1-polarized T-cell response and the control of viral replication 16. NK cells have also been shown to mediate the cytolysis of DCs infected with Ebola and Marburg viruses 17. The role of NK cells in LASV infection remains unknown. We have previously shown that the LASV infection of NHPs leads to transient NK-cell depletion 18. Given the important role of NK cells, knowledge of their contribution during infections would improve our understanding of the immune responses induced by LASV and MOPV.
NHPs are the only relevant model for studies of the immunological mechanisms occurring during LF, but their use is limited due to BSL4 restrictions. Thus, we used an in vitro model of human NK cell and APC coculture to study NK-cell activation in response to LASV and MOPV alone, or after stimulation with infected DCs and MΦs. This approach provides insight into the immune mechanisms operating during LF and clarifies the importance of NK/APC interactions in the initiation of immune responses.
Results
NK cells are neither infected nor activated by LASV and MOPV
We investigated the potential of LASV and MOPV to infect NK cells. After immunofluorescence staining, no infected NK cells were observed and no infectious viral particles were detected in the super-natants (data not shown). Thus, LASV and MOPV were unable to infect NK cells.
NK cells are known to express functional TLR3, TLR7, and TLR8 and are important sensors during infections, recognizing virus-derived RNAs 11. We investigated the activation of NK cells in the presence of LASV or MOPV by flow cytometry. The expression of CD69 by NK cells was not modified by both viruses whereas it was strongly increased by IL-2/PHA stimulation and, to a lesser extent, by TLR7 stimulation. The NKp30-expressing NK-cell number was lower in the presence of the viruses in each independent experiment (although not significant) as well as after TLR7 stimulation whereas it was increased by IL-2/PHA stimulation (Fig. 1A). The expression of other activating (NKp44, NKp46, and NKG2D) and inhibitory (KIR2DL2/3) NK-cell receptors was not modified by contact with LASV or MOPV and no NK-cell proliferation was observed either (data not shown).

CXCR3 is the receptor for CXC chemokines and is involved in chemotaxis. The presence of replicative or inactivated LASV and, to a lesser extent, MOPV, upregulated CXCR3 expression at the surface of NK cells whereas TLR7 stimulation induced a downregulation of CXCR3 (Fig. 1B). No difference in the CXCR3 mRNA level was observed between mock and infected cultures (data not shown).
Unlike PMA/ionomycin stimulation, LASV and MOPV did not induce IFN-γ gene expression by NK cells (Fig. 1C). The proportion of NK cells expressing the lytic molecule granzyme B (GrzB) was neither modified by LASV and MOPV nor by TLR stimulation, and the cytotoxic effects of NK cells on K562 targets (lacking MHC-I molecules) were also unaffected (Fig. 1D).
Thus, LASV and MOPV can neither infect NK cells nor activate these cells, induce proliferation or modify their effector properties. However, the expression of CXCR3 at the surface of NK cells was increased by LASV and, to a lesser extent, by MOPV, and NKp30 also appeared to be slightly downregulated.
LASV- and MOPV-infected MΦs modify the NK-cell repertoire and induce NK-cell proliferation
Unlike DCs, MΦs have been reported to be activated early in infection with MOPV and, to a lesser extent, with LASV 6, 8. In our model, DCs and MΦs were infected with LASV or MOPV and co-cultured with autologous NK cells. Cells were analyzed 3 days after to study the activation of infected APCs cocultured with NK cells and to determine whether they could mediate NK-cell activation and proliferation. As a positive control, NK cells were activated directly with IL-2/PHA and APC-mediated NK-cell activation was performed with LPS-matured DCs and MΦs.
Infected DCs were not activated in the absence or presence of autologous NK cells (data not shown). Consistent with our previous studies, the expression of CD40 and CD80 at the surface of MΦs was increased by MOPV infection only and CD86 was upregulated in the presence of both viruses (Fig. 2A). The analysis of NK/MΦ cocultures revealed an increase in the proportion of CD40-, CD80-, and CD86-expressing MΦs in the presence of both viruses. Moreover, the activation of infected MΦs was substantially improved in the presence of autologous NK cells.

No change in the expression of CD69, activating (NKp30, NKp44, NKp46, and NKG2D) or inhibitory (KIR2DL2/3) NK-cell receptors and CXCR3 was observed in the presence of LASV- or MOPV-infected DCs (Fig. 2B and data not shown). The percentage of CD69- and NKp30-expressing NK cells was significantly increased by IL-2/PHA stimulation and by the presence of LPS-activated DCs (except for NKp30 expression), whereas CXCR3 surface expression decreased.
LASV- and MOPV-infected MΦs induced a significant increase in the percentage of CD69-, NKp30-, NKp44- (only for LASV-infected MΦs) expressing NK cells (Fig. 2C and E). However, the expression of the NKp46 and NKG2D activating and inhibitory KIR2DL2/3 receptor by NK cells was not modified (data not shown). The percentage of NK cells expressing CXCR3 was significantly lower in the presence of LASV- and MOPV-infected MΦs, but analysis of the levels of CXCR3 mRNA revealed no difference between mock and infected cocultures (Fig. 2C, E, and data not shown). The modification of the NK-cell repertoire depends on viral replication, as there is no change in the expression of most NK-cell surface molecules in response to inactivated viruses. Still, the infection of MΦs with inactivated LASV induced a significant decrease in NKp30-expressing NK cells and an increase in CXCR3-expressing NK cells. LPS-activated MΦs induced a significant increase in CD69 and NKp44 expression and a decrease in NKp30 and CXCR3 expression in NK cells. The stimulation of NK cells with IL-2/PHA in the presence of MΦs triggered a significant increase in the expression of CD69 by NK cells, together with a decrease in the number of CXCR3-expressing NK cells.
Unlike DCs, LASV-, and MOPV-infected MΦs induced a significant increase in NK-cell proliferation, as shown by the analysis of Ki67 expression (Fig. 2D and E) and BrdU incorporation (data not shown). IL-2/PHA stimulation induced a significant increase in the number of Ki67-expressing NK cells in NK/DC cocultures.
Our results clearly demonstrate that NK cells are strongly activated and proliferate in the presence of LASV- and MOPV-infected MΦs, but not in the presence of infected DCs.
LASV- and MOPV-infected MΦs, but not DCs, only induce minor IFN-γ production by NK cells
We used PMA/ionomycin and IL-12/IL-18 as positive controls of IFN-γ production by NK cells. The infection of DCs with LASV or MOPV did not induce IFN-γ gene expression, whereas a significant increase in IFN-γ mRNA levels was observed in cocultures of NK cells with LASV- or MOPV-infected MΦs and with LPS-activated APCs or by IL-2/PHA stimulation (Fig. 3A). Low levels of IFN-γ protein production were observed by flow cytometry (Fig. 3B), but IFN-γ was not detected in the supernatant of cocultures by ELISA or in ELISPOT assays (data not shown). We also observed an increase in levels of TNFα and β transcripts but TNF-α was not detected in NK cells by intracellular flow cytometry or ELISA (data not shown). Thus, our results demonstrate that, despite the increase in IFN-γ gene transcription, LASV- and MOPV-infected MΦs do not induce major IFN-γ secretion.
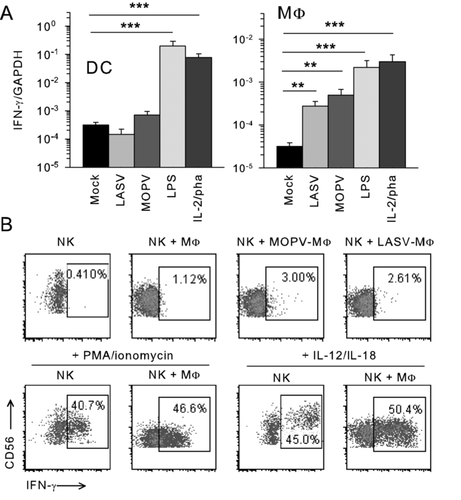
NK cells acquire cytotoxic properties after contact with LASV- or MOPV-infected MΦs but not with infected DCs
NK cells mediate cytotoxicity either via the exocytosis of lytic granules containing perforin and granzymes or through death receptor ligands, such as FasL or TRAIL, transmitting apoptotic signals. We investigated whether the infection of APCs by LASV or MOPV increased NK-cell cytotoxicity, by analyzing GrzB and perforin gene expression by qRT-PCR and quantifying these molecules by intracellular flow cytometry. Neither LASV- nor MOPV-infected DCs induced GrzB production in NK cells (Fig. 4A and B). LPS-activated DCs increased GrzB gene transcription by NK cells, although no change in intracellular GrzB protein levels was observed. IL-2/PHA stimulation induced an increase in GrzB transcript and protein production. By contrast, although the modulation of GrzB mRNA levels was not significant, we observed a significant increase in GrzB protein levels in NK cells in the presence of LASV- and MOPV-infected MΦs, as observed with LPS-activated MΦs or IL-2/PHA treatment (Fig. 4A and B). There was no modification in perforin transcript and protein production in NK cells (data not shown). We also observed a significant increase in FasL and TRAIL mRNA levels in NK/MΦ cocultures in the presence of both viruses (Fig. 4C).

After 2 days of NK-cell coculture with LASV- or MOPV-infected APCs, K562 targets were added to confirm the cytolytic potential of NK cells. The surface exposure of CD107a commonly reflects NK-cell degranulation and, thus, cell lysis 19. LASV- or MOPV-infected DCs did not increase the ability of NK cells to lyse K562 cells, whereas we observed a significant increase in NK-cell degranulation in response to K562 cells after stimulation with LASV- or MOPV-infected MΦs (Fig. 4D). No lysis of K562 cells was observed when MΦs were infected with inactivated viruses, confirming the need for viral replication in MΦs for the stimulation of NK cells and enhanced killing of K562 targets. NK cells also acquired an enhanced cytotoxic potential after IL-2/PHA stimulation (Fig. 4D).
We then investigated whether NK cells killed infected APCs in cocultures. We observed no difference in CD107a exposure on the surface of NK cells between mock- and LASV- or MOPV-infected cultures, demonstrating that NK cells were not able to kill LASV- and MOPV-infected APCs (Fig. 4D). We compared infectious viral particle release by APCs in the presence and absence of NK cells. DCs from each donor produced more infectious LASV or MOPV in the presence of NK cells, but these differences were not significant overall due to the variability of human donors (Fig. 4E). We obtained similar results for MΦ infection. LASV production by MΦs seemed to be reduced, from 3 days postinfection, in the presence of NK cells, but these differences do not remain significant either (Fig. 4E). After IL-2/PHA stimulation, NK cells did not kill infected APCs as the infectious viral particle release was not modified (data not shown).
Our results demonstrate that, unlike DCs, LASV- and MOPV-infected MΦs enhance the cytotoxicity of NK cells. However, NK cells neither killed infected APCs nor participate to viral clearance.
Cell contact is necessary for NK-cell activation mediated by LASV- or MOPV-infected MΦs
We investigated the importance of cell contacts between NK cells and infected APCs by culturing cells in a Transwell chamber, separated by a semipermeable membrane allowing the passage of soluble molecules. The increase in expression of CD69, NKp30, and other activating NK-cell receptors was abolished during the infection of MΦs with LASV or MOPV (Fig. 5A and data not shown). However, a decrease in CXCR3 surface expression was observed. NK cells did not proliferate, displayed no change in GrzB levels and were unable to lyse K562 cells in response to LASV- and MOPV-infected MΦs (data not shown).

NK-cell activation is triggered by some NK-cell surface molecules and receptors. The blockade of CD40L, NKG2D, NKp30, NKp44, or NKp46 with neutralizing Ab had no effect on the expression of NK-cell surface molecules (data not shown).
We show here that cell contacts between NK cells and infected MΦs are essential for activation of NK cells and increase cytotoxicity while they do not seem to be involved in the modulation of CXCR3 expression.
Type I IFN, but not CXC chemokines, participates in NK-cell activation by LASV- and MOPV-infected MΦs
We previously showed that MΦs secrete type I IFNs in response to MOPV infection, but that only low levels of these compounds are produced during LASV infection. CXCL9, CXCL10, and CXCL11 are secreted in response to type I and II IFNs and bind CXCR3. The presence of type I IFN and CXC chemokines was analyzed in the supernatants of NK/MΦ cocultures. In cocultures with NK cells, MOPV-, and to a lesser extent LASV-, infected MΦs secreted significant amounts of type I IFN and CXCL11 (Fig. 5B). Neutralizing mAbs directed against IFN-R and IFNα were used to inhibit type I IFN, and NK-cell stimulation by CXCL9, CXCL10, and CXCL11 was prevented with neutralizing mAbs directed against CXCR3 or CXC chemokines themselves. Our experiments with an irrelevant Ab gave results similar to those reported in Fig. 2. The inhibition of type I IFN reduced the increase in CD69 and NKp30 expression (Fig. 5C). However, neutralizing mAbs against type I IFN induced a decrease in CXCR3 surface expression, although this decrease was smaller than that obtained with the irrelevant Ab. Moreover, we observed a global increase in CXCR3 expression (Fig. 5C). NK-cell proliferation and the intracellular GrzB expression induced by LASV- and MOPV-infected MΦs were also abolished by the blockade of type I IFN (data not shown). After CXCR3 neutralization, NK cells remained activated in terms of the upregulation of CD69 and NKp30, proliferation and enhanced GrzB expression (data not shown). Neutralizing mAbs against CXC chemokines gave similar results. In addition, they induced a decrease in CXCR3 surface expression, but smaller than that obtained with the irrelevant Ab. Thus, our findings demonstrate that the type I IFN secreted by MΦs are necessary for NK-cell activation during LASV and MOPV infection but CXC chemokines have minor effects.
Discussion
We developed a model of NK cells cocultured with infected APCs, for studies of the role of NK cells and the importance of interactions during LASV and MOPV infections. We used LPS-activated APCs as a positive control for the APC-mediated activation of NK cells. We confirmed that LPS did not activate NK cells directly (data not shown). In the presence of LPS-activated APCs and/or IL-2/PHA, NK cells acquired an activation phenotype including the modulation of CD69, NKp30, NKp44, and CXCR3 expression, the production of IFN-γ and enhanced cytotoxicity. The activation of NK cells observed in response to LASV- and MOPV-infected MΦs is particularly interesting in that it is almost as robust as for positive controls, regarding the expression of NKp30 for instance, but nevertheless presents a different phenotype.
We show here that LASV and MOPV do not infect NK cells. This result was expected and consistent with previous studies showing that α-dystroglycan, the LASV and MOPV entry receptor, is expressed preferentially on DCs and poorly on lymphocytes and that lymphocytic choriomeningitis virus, the prototypic Arenavirus that is closely related to LASV and MOPV, can infect only a few types of lymphocyte. Moreover, after direct contact with the viruses, NK cells were not activated and displayed no change in their effector functions. A slight downregulation of NKp30 expression and an increase in the expression of CXCR3 on the cell surface was even observed in the presence of LASV or MOPV. Interestingly, TLR7 stimulation induced NKp30 downregulation as well. These results suggested that NK cells can detect both viruses, possibly through TLR7 stimulation requiring further investigation.
NK cells display a rapid decrease in surface CXCR3 when cocultured with LASV- or MOPV-infected MΦs. However, the significance of this downregulation is unclear. It is unlikely to be accounted for by the modulation of CXCR3 mRNA synthesis, as analysis of the mRNAs revealed no change during LASV or MOPV infection (data not shown). CXCR3 is the receptor for the inflammatory chemokines CXCL9, 10, and 11. These chemokines, initially described as attracting activated T lymphocytes, are secreted in large amounts during the infection of MΦs with LASV and MOPV in vitro (Pannetier et al., manuscript in preparation). Moreover, the transcripts for CXCL10 and CXCL11 are found in PBMCs and lymph nodes from infected Cynomolgus monkeys 18 and we show here that CXCL11 is detected in LASV- and MOPV-infected NK/MΦ cocultures. CXC chemokines, such as CXCL11, have been reported to induce the rapid desensitization and internalization of their receptor, CXCR3 20. Thus, the downregulation of surface CXCR3 expression could partly be accounted for by receptor internalization. This hypothesis is consistent with our observations, showing that CXCR3 surface expression is also downregulated when cell contact is prevented, implying that soluble factors are involved. Moreover, it is also consistent with our results with neutralizing Ab directed against CXC chemokines that abolish or reduce the downregulation of CXCR3 at the surface on NK cells in the presence of LASV- or MOPV-infected MΦs respectively. Higher concentrations of neutralizing Ab against CXC chemokines may be necessary to block the entire MΦ-mediated secretion of CXC chemokines and fully abrogate CXCR3 downregulation. Moreover, type I IFNs are involved in the induction of CXCR3 ligands, such as CXCL10 and CXCL11 21. We can thus hypothesize that the neutralization of MΦ-secreted type I IFN would decrease the production of CXC chemokines, accounting for the increase in basal levels of CXCR3 expression and the weaker downregulation of CXCR3 at the surface of NK cells. Other factors may account for CXCR3 downregulation. For instance, the soluble form of nonclassical class I MHC HLA-G has recently been reported to be upregulated in some viral infections and to induce the downregulation of CXCR3 at the surface of NK cells 22. The presence of soluble HLA-G could be investigated in our model after LASV and MOPV infection. Furthermore, activated NK cells are known to migrate in response to CXC chemokines. CXCR3 signaling has been shown to be important for the rapid recruitment of murine NK cells to lymph nodes after stimulation with mature DCs 23. We can therefore hypothesize that, after coming into contact with LASV- or MOPV-infected MΦs, activated NK cells reach the secondary lymphoid organs, where they initiate the adaptive immune response. Consistent with our previous in vivo studies 18, the disappearance of NK cells from the blood of monkeys infected with LASV may be accounted for the relocalization of NK cells via the modulation of CXCR3 surface expression. The causes and consequences of the modulation of CXCR3 expression for NK cells with or without APCs remain unclear and further investigations are required.
NK cells play a major role in regulation, initiation of adaptive immunity, and Th1 polarization through the production of IFN-γ 23. IFN-γ is produced during many viral infections, but seems to have little effect on LASV replication in APCs 9, 24. In our in vitro model, we show that only low levels of IFN-γ production by NK cells are induced by LASV- and MOPV-infected DCs and MΦs. This is consistent with our previous study indicating that IFN-γ was not detected in LASV-infected Cynomolgus monkeys 18. We also investigated the role of NK cells in APC maturation and activation in our in vitro model and found that the presence of NK cells neither enhanced the production of type I IFN nor induced the production of IL-12, IL-15, and IL-18 by DCs and MΦs (data not shown). NK cells seem to enhance DC and MΦ maturation, in terms of the expression of class II MHC molecules or costimulatory molecules, such as CD40, CD80, and CD86. Moreover, we show that cell contacts are essential for optimal NK-cell activation. The role of NK cells on APC activation also requires confirmation in vivo.
We studied NK-cell cytotoxicity, by investigating CD107a surface expression, which is widely accepted to reflect NK-cell degranulation and cell lysis 19. We show here that the ability of NK cells to lyse K562 targets increased after contact with infected MΦs. NK cells display an increase in the expression of the NKp30 and NKp44 receptors, which are involved in natural cytotoxicity, as well as an increase in the expression of the intracellular lytic molecule GrzB and in the transcription of FasL and TRAIL genes. Based on these findings, the infection of MΦs would be expected to lead to the killing of infected cells by NK cells. It has been shown that NK cells kill filovirus-infected human DCs and that lysis is directly linked to NKp30 upregulation 17. Several checkpoints control the balance between activating and inhibitory signals and NK-cell-mediated lysis. They include the modulation of class I MHC molecules, which may bind to KIRs and contribute to the inhibitory signal, and the modulation of activating receptors and associated ligands. In our model, NK cells stimulated by infected MΦs neither kill infected cells nor participate to viral clearance. This observation is consistent with the constant expression of class I MHC molecules by infected APCs 6, 8 and the absence of NK-cell-activating ligands, such as MIC A/B (data not shown). Our results show that NK cells have a greater cytotoxic potential during the infection of MΦs and that they seem to be able to kill MHC-lacking targets but we observed no lysis of LASV- or MOPV-infected APCs. After stimulation by IL-2/PHA, NK cells did not kill infected APCs either despite an increased cytotoxic potential. This result suggests that the lack of killing of infected APCs was not due to a defect in NK-cell activation. LASV- and MOPV-infected cells rather seem to resist to NK-cell-mediated lysis and apoptosis, as reported for several other viruses 25. This mechanism, consistent with the noncytopathic nature of Arenavirus infections, would enable the virus to persist and disseminate. There is evidence to suggest that the Z protein of LASV can dysregulate apoptosis signaling by binding to promyelocytic leukemia protein (PML), a component of PML nuclear bodies 26. PML has been shown to play a role in apoptosis regulation via the death-receptor pathway and to control class I MHC gene expression 27. Thus, Arenaviruses may potentially interfere with the normal function of PML in nuclear bodies, leading to cell death resistance in infected cells, through inhibition of the apoptosis pathway and class I MHC downregulation in infected cells.
We found no dramatic difference in NK-cell responses between LASV and MOPV infections, despite the striking differences in pathogenesis and APC activation induced by these two viruses. The lack of NK-cell response in the presence of LASV- or MOPV-infected DCs is probably due to the lack of DC activation induced by these two viruses 6, 8. Indeed, the activation of NK cells seems to be correlated to the status of activation of DCs, as observed for LPS-stimulated DCs. NK-cell activation during the MOPV-infection of MΦs is consistent with only MΦs being rapidly and strongly activated by MOPV. More surprisingly, we also found that LASV-infected MΦs mediated NK-cell activation and enhanced the cyto-lytic potential of NK cells. This observation is consistent with our results showing a better MΦ activation in the presence of NK cells in response to LASV, reaching the levels observed after MOPV infection, regarding the expression of CD40, CD80, and CD86. LASV induced a limited activation in isolated MΦs with moderate levels of type I IFN mRNA 9. However, this modest basal activation may initiate a positive loop of activation between MΦs and NK cells, leading finally to a robust NK-cell activation. It would be interesting to determine if this mutual activation of MΦs and NK cells occurs in LASV-infected patients or NHP. Indeed, as MΦ activation seems to be crucial to control Arenavirus infection, such a mechanism could play an important role in the control of LF in survivors. Type I IFNs are well-known mediators of antiviral responses and are crucial for the activation of NK cells 14. Our results suggest that, in addition to cell contact, low levels of type I IFN are sufficient to mediate NK-cell activation, without triggering IFN-γ production or killing infected cells. Finally, we show here for the first time that, in our in vitro model, the pathogenicity of Arenaviruses does not seem to affect NK-cell activation. Further studies are required, to determine the role of NK cells in viral replication and T-cell responses in vivo in an animal model. Unlike NK/DC cross-talk, the interactions between NK cells and MΦs have not been studied in detail although the activation of NK cells in response to MΦs infected with many pathogens or stimulated by exogenous stimuli has already been reported 28, 29. We show here that MΦs are involved in NK-cell activation, whereas DCs are not. This approach confirms the important role of MΦs in mediating NK-cell activation and, more generally, provides new insights and hypotheses into the immune mechanism operating during LF.
Materials and methods
Cell and virus strains
The VeroE6 and K562 cells were grown in DMEM supplemented with 1% penicillin-streptomycin and 5% and 10% FCS respectively (all from Invitrogen).
Mopeia (AN21366 strain 2) and Lassa (AV strain 30) viruses were grown in VeroE6 cells at 37°C, with 5% CO2. Viral supernatants were harvested and used as the virus stock and the absence of mycoplasma was confirmed. LASV and MOPV titers were determined as described previously 6, 8. Inactivated LASV and MOPV were obtained after 2-h heating at 60°C and at least two freeze/thaw cycles. Virus-free supernatants of VeroE6 cells were used for mock experiments.
All experiments with LASV were carried out in biosafety level 4 facilities (Laboratoire P4 Jean Mérieux-Inserm, Lyon).
Preparation of DCs, MΦs, and NK cells
Monocytes and peripheral lymphocytes were isolated from the blood of consenting healthy donors provided by the Etablissement Français du Sang (Lyon, France), as previously described 6. Monocytes were further purified by immunomagnetic depletion and negative selection with a Monocyte Isolation Kit® and NK cells were isolated from lymphocytes by negative selection with an NK Cell Isolation Kit® (both from Miltenyi Biotech, Auburn, USA). The purity of cells was verified by flow cytometry and ranged from 97 to 99.5% for monocytes, with less than 1% CD3-positive cell contaminants in NK cells (data not shown).
Monocytes were then induced to differentiate into MΦs and DCs by culture for 6 days in RPMI 1640 Glutamax I, 1% penicillin-streptomycin, 10 mM HEPES, 1% nonessential amino acids and 10% FCS (all from Invitrogen), supplemented with 50 ng/mL M-CSF and 10% autologous decomplemented plasma for MΦs, or with 1000 IU/mL GM-CSF and 500 IU/mL IL-4 (all from PeproTech, London, UK) for DCs. We replaced 40% of the medium, and the cytokines, every 48 h.
NK cells were frozen in 90% FCS, 10% DMSO (Sigma, Saint-Quentin Fallavier, France) and stored in liquid nitrogen until coculture with DCs or MΦs.
Infection of DCs and MΦs and coculture with NK cells
DCs and MΦs were harvested and incubated for 1 h at 37°C with virus-free VeroE6 cell supernatant (mock), LASV or MOPV at a MOI of 2, unless otherwise specified. NK cells were then thawed and cocultured with mock-, LASV-, or MOPV-infected APCs (106 cells/mL), at an NK-cell:APC ratio of 1:5. In some conditions, DCs and MΦs were stimulated with 1 μg/mL LPS (Sigma), NK cells were activated by incubation with 200 IU/mL IL-2 (PeproTech) and 1 μg/mL PHA (Sigma) or were stimulated with 10 μg/mL polyI:C, 15 μg/mL imiquimod or 1 μg/mL ssRNA40 (InvivoGen, Toulouse, France). We used 20 pg/mL PMA (Sigma) and 720 ng/mL ionomycin (Sigma) or 50 ng/mL IL-12 (PeproTech) and 50 ng/mL IL-18 (MBL, Naka-ku Nagoya, Japan) to stimulate NK cells. In some experiments, contact between NK cells and APCs was prevented by a polycarbonate membrane with 0.4-μm pores (Corning Life Sciences, Schiphol-Rijk, The Netherlands). In some conditions, CXCR3 was blocked with 5 μg/mL anti-CXCR3 mAb (R&D Systems, Lille, France). Cell contacts were blocked with 5 μg/mL anti-CD40L, 10 μg/mL anti-NKG2D (R&D Systems), 2 μg/mL anti-NKp30, anti-NKp44, or anti-NKp46 Ab (Miltenyi Biotech). The effect of type I IFN was prevented with 2.5 μg/mL anti-IFN-α mAb (PBL Biomedical Laboratories, Piscataway, NJ) and 5 μg/mL anti-CD118 Ab (IFNα/β-R chain 2) (PBL) and a combination of anti-CXCL9, anti-CXCL10, and anti-CXCL9 mAbs (8 μg/mL each, R&D Systems) was used to neutralize CXC chemokines. We used irrelevant IgG2a Ab (R&D Systems) for control experiments.
Flow cytometry
Seventy-two hours after seeding, cells were harvested, washed, and the final pellets were resuspended in 5% human serum in PBS. The expression of cell surface molecules was analyzed by incubating cells for 30 min at 4°C with various Ab. NK cells were gated as CD3− and CD56+ cells, using FITC- or PE-Cy7-conjugated CD3 Ab (Beckman Coulter, Marseille, France) and Alexa Fluor 488-, Alexa Fluor 647-, or PE-Cy5-conjugated CD56 (BD Pharmingen, San Diego, USA). Other NK-cell and MΦ surface molecules were stained with Ab conjugated to FITC (CD80, CD86), PE (CD80 (BD Pharmingen), NKp30 and NKp44 (Beckman Coulter)), PE-Cy5 (CD69 [Beckman Coulter], CD40, CXCR3 (BD Pharmingen)) or Alexa Fluor 647 (NKp30 (BD Pharmingen)). For analysis of the expression of intracellular proteins, cells were permeabilized with the Cytofix/Cytoperm kit (BD Pharmingen), according to the manufacturers’ instructions, and incubated with Ab specific for GrzB (FITC-conjugated, BD Pharmingen), IFN-γ and Ki67 (PE conjugated, BD Pharmingen). Finally, paraformaldehyde-fixed cells were studied by flow cytometry (FacsCanto; BD Biosciences or EPICS-XL, Beckman Coulter). Data were analyzed with FlowJo software.
CD107a assay
K562 target cells were added to the NK/APC cocultures 48 h after seeding, at an E:T ratio of 10:1. FITC-CD107a Ab (BD Pharmingen) was then added and cells were incubated for 5 h at 37°C. Monensin (Golgi-Stop, BD Pharmingen) was added for the last 4 h to prevent CD107a degradation. NK cells were then labeled with PE-Cy5-CD56 Ab (BD Pharmingen) and MΦs were excluded on the basis of CD14 staining (Beckman Coulter). Finally, the expression of CD107a by K562-stimulated NK cells was analyzed by flow cytometry.
ELISA
Supernatants of NK/MΦ cocultures were harvested 72-h postinfection and stored at −80°C. Commercial ELISA kits were used for IFN-α (Bender MedSystems, Vienna, Austria) and CXCL11 (R&D Systems) detection, following the manufacturers’ instructions.
Titration assays
NK, DCs, and MΦs were infected with LASV or MOPV at a MOI of 0.1. In coculture experiments, noninfected NK were added to LASV- or MOPV-infected APCs, at an NK-cell:APC ratio of 5:1. The culture supernatants were harvested and viral titers were determined and expressed in focus-forming units per mL (FFU/mL) as described previously 6, 8.
Analysis of mRNA levels by qRT-PCR
Twenty-four hours after infection, total RNAs was obtained from a coculture of 6 × 105 cells, using RNeasy kit® and DNA I digestion (both from Qiagen, Hilden, Germany). Reverse transcription was then carried out using SuperScript III® reverse transcriptase, RNaseOUT, first-strand buffer, DTT, oligodT, and dNTP mix (all from Invitrogen). The resulting cDNA was analyzed by real-time PCR (Taqman, Applied Biosystems, Foster Coty, USA) with Taqman Universal master mix and Taqman commercial primers and probes for IFN-γ, GrzB, FasL, and TRAIL (Applied Biosystems). The GAPDH gene was amplified in duplex, with commercial primers and probes (Applied Biosystems) for normalization of the results. Relative mRNA levels were then calculated as 2−ΔCt, Δ cycle threshold (Ct) = gene Ct − GAPDH Ct.
Statistical analysis
Statistical analyses were performed with SigmaStat® software. Student's t-tests and Mann-Whitney U-tests were carried out to analyze data from flow cytometry experiments, ELISA assays, and qRT-PCR.
Acknowledgments
M. Russier held a fellowship from the Délégation Générale pour l'Armement (G. Vergnaud, the French Army). We thank C. Clegg and G. Lloyd for providing MOPV, and S. Becker for the AV strain of LASV. We also thank T. G. Ksiazek, P. E. Rollin, and P. Jahrling, for providing us with mAbs specific for MOPV and LASV, the Etablissement Français du Sang for providing human blood, the cytometry platform (S. Dussurgey and T. Andrieu) of the SFR Biosciences Gerland-Lyon Sud (UMS3444/US8), the Laboratoire P4-Jean Mérieux team for access to BSL4 facilities, and T. Walzer for helpful discussions.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- GrzB
-
- granzyme B
-
- KIR
-
- killer cell Ig-like receptor
-
- LASV
-
- Lassa virus
-
- LF
-
- Lassa fever
-
- MOPV
-
- Mopeia virus
-
- NHP
-
- nonhuman primate;
-
- PML
-
- promyelocytic leukemia protein
-
- qRT-PCR
-
- quantitative RT-PCR