AP-1 is involved in ICOS gene expression downstream of TCR/CD28 and cytokine receptor signaling
Abstract
It has been proposed that sustained ICOS expression in chronic inflammatory immune conditions, such as autoimmunity and allergy, contributes to symptom exacerbation. Therefore modulation of ICOS gene expression could be a potential therapeutic strategy for such immune diseases. However, the precise molecular mechanisms controlling ICOS gene expression remain poorly understood. In this study, we explored transcription factors involving in ICOS gene expression and examined their roles in a physiological situation. Microarray analysis revealed that one AP-1 molecule, Fos-related antigen-2 (Fra2), was highly correlated with ICOS expression. Ectopic expression of Fra2 and other AP-1 molecules upregulated ICOS expression on T cells. We identified an AP-1-responsive site (AP1-RE) within the ICOS promoter region and demonstrated AP-1 actually binds to AP1-RE upon TCR/CD28 stimulation. Meanwhile, we found several cytokines could upregulate ICOS expression on both naïve and effector T cells in a manner independent of TCR/CD28 stimulation. These cytokine stimuli induced AP-1 binding to AP1-RE. Together, our results indicate AP-1 transcription factors are involved in ICOS gene expression downstream of both TCR/CD28 signaling and cytokine receptor signaling, and suggest AP-1 activation via cytokine receptor signaling may be one of the mechanisms maintaining high level ICOS expression in chronic inflammatory immune responses.
Introduction
Althoughengagement of the T-cell Ag-specific receptor by Ag/MHC products is essential for the initial stages of T-cell activation, a second signal termed a costimulatory signal is necessary for clonal expansion and functional differentiation of Ag-specific T cells 1. ICOS is a third member of CD28 family of costimulatory molecules 2. ICOS has several unique features, which differ from CD28. For instance, ICOS is induced upon T-cell activation and has a minimal role in the induction of IL-2 production 2-5. Until now, a large number of studies have demonstrated that ICOS serves to enhance T-helper cell differentiation and effector function, and plays a critical role in T-dependent B-cell antibody responses 4-6. Furthermore, in humans, ICOS has been identified as one of the genes which when mutated is responsible for common variable immune deficiency (CVID) 7, indicating its critical role in immune responses.
Meanwhile, it has been proposed that sustained ICOS signaling could exert a pathological role for certain chronic immune responses, such as autoimmunity and allergy. For example, Roquin mutant mice, that show deficiency in the degradation pathway of ICOS mRNA, express ICOS at a high level and develop an autoimmune phenotype in a manner dependent on ICOS 8. We previously reported that sustained ICOS expression was crucial in a mouse model of chronic graft versus host reaction (GVHR) but not in acute GVHR 9, 10. In humans, it was reported that a single nucleotide polymorphism (SNP) within the ICOS promoter region, which results in the augmentation of ICOS expression, was associated with susceptibility for allergy sensitization 11. In several autoimmune conditions such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), sustained ICOS expression has been observed and a pathological role of ICOS was suggested 12, 13.
This evidence indicates that regulation of ICOS gene expression is a critical factor in determining the outcome of immune responses. Recently, Tan et al. 14, 15 reported that NFAT1 and MAPK pathways were involved in ICOS gene expression downstream of TCR signaling, and they also reported that NFAT1 co-operated with T-bet and GATA-3 for ICOS gene expression in Th1 and Th2 cells, respectively. However, the precise mechanisms sustaining ICOS expression in chronic inflammatory environments such as autoimmunity and allergy remain largely unclear. In this report, we studied molecular mechanisms for ICOS gene expression and found that the AP-1 transcription factor enhanced ICOS gene expression, acting through the ICOS promoter. Furthermore, we report for the first time that several cytokine stimuli, independent of TCR/CD28 stimulation, are capable of inducing AP-1 binding to the ICOS promoter region and inducing ICOS gene expression. We discuss the role of a cytokine-AP-1 axis for sustaining ICOS expression in chronic inflammatory immune responses such as autoimmunity and allergy.
Results
Fra2 expression is associated with ICOS expression
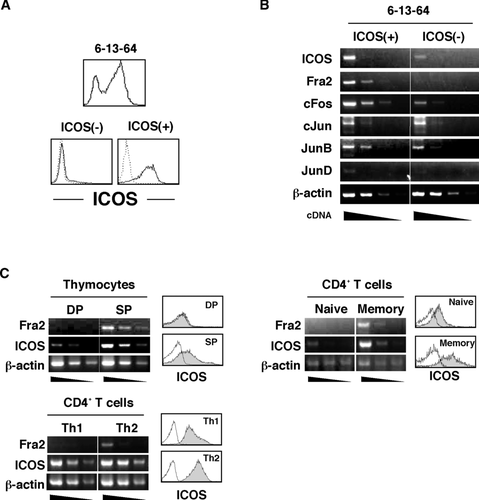
Through a panel analysis of murine T-cell hybridomas for their ICOS expression, we found that although almost all hybridomas we tested did not express ICOS, some hybridomas were ICOS positive. Among them, the 6-13-64 hybridoma was heterogeneous for ICOS expression at the cellular level (Fig. 1A). Interestingly, isolated ICOS+ cells from the 6-13-64 hybridoma gradually lost their ICOS expression during culture while isolated ICOS– cells did not reexpress ICOS (data not shown). From this observation, we speculated that during the course of cell culture, ICOS+ cells might alter their expression of certain molecules that regulate ICOS gene expression. In order to identify these molecules, ICOS+ and ICOS– populations were isolated by flow cytometry and subjected to microarray analysis to compare their gene expression profiles. Among obtained genes that exhibited relatively higher expression in the ICOS+ than in the ICOS– population, one of the AP-1 molecules, Fos-related antigen-2 (Fra2) showed the highest fold-change score (Table 1). RT-PCR analysis confirmed that the Fra2 mRNA level was higher in the ICOS+ population than in the ICOS– population supporting the result of microarray analysis (Fig. 1B). In addition, other AP-1 members such as JunB, JunD, and c-Fos also showed higher expression in ICOS+ than in ICOS– cells (Fig. 1B). Because AP-1 transcription factor is well known to be activated downstream of TCR/CD28 signaling and cytokine receptor signaling 16, we postulated that Fra2/AP-1 molecules play a role in induction and maintenance of ICOS expression and decided to further analyze the role of Fra2/AP-1. In order to generalize the relationship between Fra2 and ICOS expression in physiological situations, expression levels of Fra2 in several mouse primary T-cell populations that exhibited different ICOS expression levels were investigated (Fig. 1C). It had been shown that thymus CD4 single positive but not CD4/CD8 double-positive cells express ICOS on cell surface 17. Among Th subsets in the periphery, Th2 cells showed relatively higher expression levels of ICOS compared with Th1 cells 18. ICOS expression is also reported to be higher in memory (CD44high) CD4+ T cells than naïve (CD44low) CD4+ T cells 19. Using these T-cell populations, we tested the relationship between Fra2 and ICOS mRNA levels and found a clear positive relationship between Fra2 and ICOS in all three cases (Fig. 1C).
| Accession No. | Symbol | Description | Fold Changeb | p-value |
|---|---|---|---|---|
| ICOS+/ICOS– | ||||
| NM_008037 | Fosl2 | Fos-like antigen 2 (Fos-related antigen 2, Fra2) | 13.48 | 0.0017 |
| NM_013498 | Crem | cAMP responsive element modulator | 12.29 | <0.0001 |
| NM_010444 | Nr4a1 | Nuclear receptor subfamily 4, group A, number 1 | 10.16 | 0.0001 |
| NM_015811 | Rgs1 | Regulator of G-protein signaling 1 | 7.68 | 0.0008 |
| NM_013642 | Dusp1 | Dual specificity phosphatase 1 | 5.93 | 0.0004 |
| NM_009061 | Rgs2 | Regulator of G-protein signaling 2 | 4.57 | 0.0017 |
| NM_017480 | Icos | Inducible T-cell costimulator (ICOS) | 4.56 | 0.0093 |
| NM_007904 | Ednrb | Endothelin receptor type B | 4.32 | 0.0002 |
| NM_019511 | Ramp3 | Receptor (calcitonin) activity modifying protein 3 | 3.95 | 0.0208 |
| NM_009400 | Tnfrsf18 | Tumor necrosis factor receptor superfamily, member 18 | 3.91 | 0.0004 |
| NM_007836 | Gadd45a | Growth arrest and DNA-damage-inducible 45 alpha | 3.81 | 0.0022 |
| NM_008357 | Il15 | Interleukin 15 | 3.72 | 0.0047 |
- a Gene expression levels were compared between ICOS+ and ICOS– 6-13-64 cells.
- b Fold change of each gene was calculated as (means of signal intensity of ICOS+ cells )/(means of signal intensity of ICOS– cells).
Fra2 upregulates ICOS expression on activated T cells
Since T cells increase cell surface expression of ICOS upon activation, kinetic analysis was performed for gene expression of both Fra2 and ICOS. We found that the fold increase of Fra2 mRNA was greater than that of ICOS mRNA at early time point after T-cell activation (Fig. 2A). These results suggested possible involvement of Fra2 in ICOS gene expression. We next asked whether Fra2 actually regulates ICOS gene expression in primary T cells. To address this question, Fra2 gene was retrovirally transduced to mouse primary CD4+ T cells that were cultured under Th1 and Th2 differentiation conditions. Fra2 overexpression in Th1 cells resulted in ICOS upregulation to a level comparable with that in mock-transduced Th2 cells, while Fra2 overexpression in Th2 cells further enhanced ICOS expression (Fig. 2B). Because the ICOS expression level on Th2 cells was relatively higher than that on Th1 cells (Fig. 1C), it was feasible that ICOS upregulation by ectopic Fra2 expression resulted from induction of Th2 cells even in Th1 differentiation conditions. This possibility was ruled out because Fra2-transduced T cells cultured in Th1 differentiation conditions produced exclusively IFN-γ but not IL-4 (data not shown). Levels of ICOS mRNA were upregulated, indicating that Fra2-enhanced upregulation of ICOS occurs at the transcriptional level (Fig. 2C).
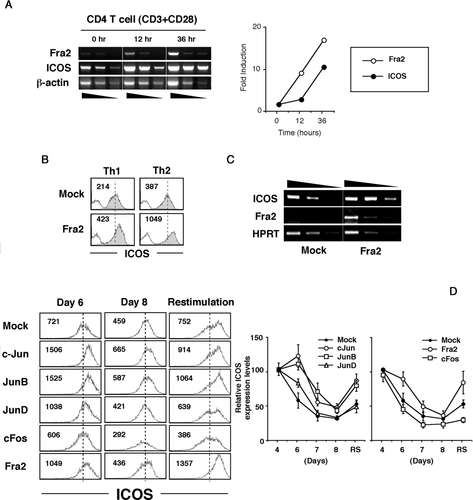
AP-1 molecules enhance ICOS expression on activated T cells
Because Fos family gene products require Jun gene family proteins to form functional AP-1 transcriptional complexes 16, we assessed the role of other AP-1 members, especially Jun family genes, for ICOS expression on activated T cells. Each AP-1 molecule was retrovirally overexpressed in primary T cells, and their ICOS expression was kinetically analyzed (Fig. 2D). At 6 days after first stimulation (4 days after AP-1 introduction), c-Jun, JunB, JunD, and Fra2 each enhanced ICOS expression on activated T cells. By 8 days of culture, ICOS expression by JunD- and Fra2-transduced T cells was almost equal to that of mock-transduced T cells, while ICOS expression was still higher in c-Jun- and JunB-transduced T cells. Upon restimulation at day 7, ICOS expression was drastically enhanced in Fra2-, JunB-, and c-Jun-transduced T cells. These results indicated that Fra2/Jun/AP-1 molecules could enhance and/or sustain ICOS gene expression of activated T cells.
AP-1 molecules enhance ICOS promoter activity through AP1-RE
In order to test whether AP-1 molecules regulate ICOS gene expression via a direct interaction with the transcriptional machinery, we analyzed the role of AP-1 in regulating ICOS promoter activity. Approximately 800 bp of the 5’ upstream region (−780 to +32) of mouse ICOS gene was cloned into the promoter-less luciferase reporter vector (pGL3B-Luc) to generate ICOSp(780)-Luc (Fig. 3A). Consistent with a previous report 14, this region exhibited promoter activity in ICOS expressing thymoma EL-4 cells upon stimulation with PMA/Ionomycin or TCR/CD28 (Fig. 3B and data not shown). Thus we utilized the ICOSp(780)-Luc reporter to evaluate the effect of AP-1 molecules on ICOS promoter activity. Overexpression of Jun family members drastically enhanced ICOS promoter activity in EL-4 cells activated with PMA/ionomycin (Fig. 3C). On the other hand, overexpression of Fos gene family members resulted in limited effects, but we repeatedly observed that c-Fos inhibited and Fra2 enhanced ICOS promoter activity (Fig. 3). These different stimulatory effects observed between Jun family members and Fra2 in transient overexpression experiments in EL-4 cells might result from the fact that Jun family members can homo-dimerize, while Fra2 required Jun family molecules to form a functional AP-1 complex 16. Otherwise this result may represent expression levels of endogenous Fos family molecules that were functionally saturated in EL-4 cells, because we did not see a clear additive effect with Jun and Fos family cotransfection (data not shown). In order to further evaluate the physiological role of AP-1 molecules, knockdown analysis was performed by shRNA. Fra2, c-Jun, JunB, and c-Fos shRNA resulted in attenuation of ICOS promoter activity (data not shown) and decreased surface ICOS expression levels (Fig. 3D). From this result, the observed suppressive effect on ICOS expression by c-Fos overexpression (Fig. 2D and Fig. 3C) might be due to dominant negative effects of overexpressed c-Fos as reported previously 20. We next tried to identify the Jun/Fos-responsive site within the ICOS promoter region. To this end, a series of ICOS promoter deletants was constructed and their responsiveness to overexpressed c-Jun was evaluated. The Jun-responsive site was identified between −198 and −173 regions within the ICOS promoter region (Fig. 3E). Furthermore sequence analysis revealed that this site had a putative AP-1-binding motif (Fig. 3F). To evaluate the importance of this putative AP-1-binding motif for c-Jun-enhanced ICOS promoter activity, two mutant variants of ICOSp(780)-Luc carrying a 3 bp mutation within the AP-1-binding motif were analyzed (Fig. 3F). As expected, c-Jun-dependent augmentation of ICOS promoter activity was completely abrogated by these mutations (Fig. 3G) indicating that the c-Jun-mediated effect was exerted through this site. We designated this site as an AP-1-responsive site (AP1-RE).
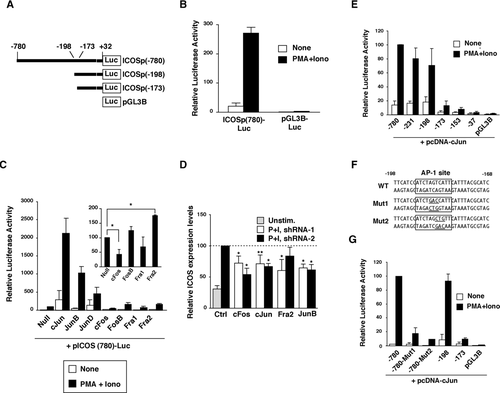
AP-1 binding to the AP1-RE is induced upon TCR/CD28 stimulation
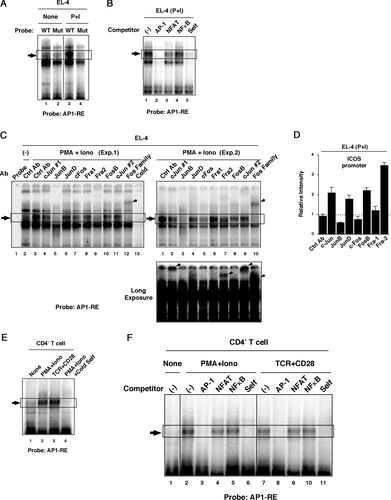
Next, we asked whether T-cell activation signals such as PMA/Ionomycin or TCR/CD28 actually induce AP-1 binding to the AP1-RE. A radio-labeled oligonucleotide probe (ICOSp(198-168)) that includes the AP1-RE was prepared (Fig. 3G), and its molecular-binding property was analyzed by EMSA. Nuclear extract from EL-4 cells yielded a stimulation-inducible molecule that bound to the AP1-RE probe (Fig. 4A). Consistent with promoter activity, mutation of the AP-1-binding motif (Fig. 3G) resulted in disappearance of the stimulation-inducible band (Fig. 4A). To evaluate whether the AP-1 molecule binds to AP1-RE, competition analysis using an excess of cold oligonucleotides that have a consensus-binding motif for AP-1, NFAT, or NFκB was performed. The consensus AP-1 probe clearly competed with the AP1-RE probe while the effect of the NFAT and NFκB probe was limited (Fig. 4B). This result indicated that AP-1 molecules preferentially bound to the AP1-RE. To further analyze AP-1 binding to the AP1-RE, we utilized two different approaches: super-shift/depletion analysis in EMSA, and ChIP analysis using antibodies against each AP-1 molecule. In EMSA, whether a band is super shifted or depleted depends on the nature of each antibody. Anti-JunB antibody depleted the stimulation-inducible band (Fig. 4C). On the other hand, anti-c-Jun, anti-Fra2, and anti-Fos-family Abs yielded super-shifted complexes (Fig. 4C). These results suggested that at least c-Jun, JunB, and Fra2 were contained in the stimulation inducible band, while it was less clear about the involvement of other AP-1 molecules. In ChIP analysis, c-Jun, JunD, Fra2, and FosB binding to the ICOS promoter region were detected upon stimulation (Fig. 4D). Clear detection of JunD and FosB in ChIP analysis, but not by EMSA, might result from different sensitivities of the two assays and/or differences in the nature of AP-1:DNA binding between in vitro (EMSA) and in vivo (ChIP) situations. Nondetection of JunB by ChIP while depletion of a band that was observed in EMSA might reflect the inability of the anti-JunB antibody to recognize fixed AP-1:DNA complexes. These results indicated that several AP-1 molecules actually bind to the AP1-RE in the ICOS promoter region upon stimulation. Stimulation-inducible molecules that bound to the AP1-RE were also observed in mouse primary CD4+ T cells upon TCR/CD28 or PMA/Ionomycin stimulation (Fig. 4E). Similar to EL-4, competition analysis demonstrated that the AP-1 transcription factor was preferentially bound to the AP1-RE in primary CD4+ T cells (Fig. 4F). These results indicated that AP-1 molecules downstream of TCR/CD28 signaling could enhance ICOS gene expression through the AP1-RE site.
Cytokine signaling induces ICOS upregulation independent of TCR/CD28 stimulation
It is known that certain cytokine receptor signaling activates AP-1 transcription factor in T cells 21. Thus we postulated that cytokine signaling also could induce ICOS gene expression as a part of cytokine-induced T-cell activation program through AP-1 activation. To evaluate this hypothesis a panel of cytokines was tested for their ability to induce ICOS upregulation. Preactivated CD4+ T cells were stimulated with a panel of cytokines, and we found that indeed several cytokines upregulated ICOS expression in the absence of additional TCR/CD28 stimulation (Fig. 5A and 5B). IL-2, IL-4, and IL-7 exhibited apparent upregulatory effects on ICOS expression levels. In addition, some cytokines, such as IL-12 and IL-21, showed relatively small but statistically significant upregulatory effects. The physiological significance of these relatively small effects will require further study. Interestingly, cytokine-dependent ICOS upregulation was not limited to preactivated T cells. When freshly isolated CD4+ T cells were stimulated with a panel of cytokines, we found that several cytokines such as IL-2, IL-4, IL-7, IL-6, or IL-21 upregulated ICOS expression in the absence of TCR/CD28 stimulation (Fig. 5C). As shown in Fig. 1C, the ICOSlow population in freshly isolated CD4+ T cells corresponds to CD44low naïve CD4+ T cells, whereas the ICOShigh population corresponds to CD44high memory CD4+ T cells. IL-7 showed upregulatory effect on both naïve and memory CD4+ T cells (Fig. 5C and 5D). Of interest, while IL-2 and IL-4 preferentially upregulated ICOS expression levels on the ICOShigh population, IL-6, and IL-21 preferentially upregulated ICOS expression levels on the ICOSlow population (Fig. 5C and 5D). The effect of IL-21 and IL-6 on ICOS upregulation was not due to induction of phenotypical change from naïve (CD44low) to effector/memory (CD44high) CD4+ T cells, because CD44 distribution pattern was not altered by IL-21 or IL-6 stimulation (data not shown).

AP-1 binding to AP1-RE is induced upon cytokine stimulation
We finally asked whether AP-1 binding to the AP1-RE was induced upon cytokine stimulation. Preactivated CD4+ T cells were stimulated with the indicated cytokine for 12 h and their nuclear extracts were analyzed by EMSA. Inducible AP-1 binding to the AP1-RE was observed in response to stimulation by several cyto-kines (Fig. 6A). For example, IL-2, IL-4, IL-7, and IL-15 induced strong intensity bands while IL-6, IL-12, and IL-21 yielded modest intensity bands (Fig. 6A). Band intensity induced by each cyto-kine was generally correlated with their upregulatory effect on cell surface ICOS expression (Fig. 5B) except for IL-15, which did not induce a significant effect on cell surface ICOS. In addition, consistent with the ability of IL-21 to induce ICOS upregulation on freshly isolated CD4+ T cells (Fig. 5E), we also observed that AP-1 binding to the AP1-RE was induced in freshly isolated CD4+ T cells by IL-21 stimulation (data not shown). These results indicated that, in addition to signaling downstream of TCR/CD28, AP-1 was involved in ICOS gene expression downstream of cyto-kine receptor signaling.

Discussion
In this study, we identified Fra2, a Fos family gene, as an ICOS expression-associated molecule by microarray analysis utilizing the 6-13-64 T-cell hybridoma and demonstrated the role of Fra2/AP-1 molecules for ICOS gene expression in physiological setting. These results indicated that our strategy of utilizing the 6-13-64 T-cell hybridoma was a useful way to screen candidate genes that regulated ICOS expression.
It is well known that the AP-1 molecule is efficiently activated by CD28 costimulation in the presence of TCR signaling and plays an important role for IL-2 gene expression 22, 23. Similar to IL-2 gene expression, maximum ICOS expression requires CD28 costimulation in addition to TCR signaling 24. Thus it is possible that the CD28 costimulatory effect for ICOS expression is exerted via augmentation of AP-1 activation. Indeed, we observed enhanced AP-1 binding to the AP1-RE site by CD28 costimulation in T cells (data not shown). Recently, Tan et al. reported that NFAT1 was involved in ICOS gene expression 14, 15. The relationship between AP-1 and NFAT in the context of ICOS gene expression is very interesting, because a cooperative role of AP-1 and NFAT for gene expression of several inducible genes such as IL-2 has been well characterized 25. If binding sites for AP-1 and NFAT are located proximally, the molecular interaction between AP-1 and NFAT enhances their binding ability to this AP-1:NFAT composite site resulting in enhancement of target gene expression 25. Actually, cold-NFAT probe repeatedly showed a partial inhibitory effect for molecular binding to the AP1-RE probe in EMSA (Fig. 4B and 4F) suggesting the possibility of AP1:NFAT complex binding to the AP-1RE. Another interesting point is the putative positive feedback loop between ICOS and AP-1:NFAT molecules since we had previously reported ICOS cross-linking induced AP-1:NFAT-dependent promoter activity 26. Further studies are ongoing regarding these possibilities.
Cytokine stimulation upregulated ICOS expression both in freshly isolated CD4+ T cells and preactivated CD4+ T cells (Fig. 5). Although it had been reported that several cytokines augment the expression level of ICOS upon TCR/CD28 stimulation 27, 28, we for the first time demonstrated that cytokine stimulation alone was sufficient to upregulate ICOS expression in the absence of TCR/CD28 stimulation. This might be explained by our finding that the ICOS promoter region had been already histone acetylated even in freshly isolated CD4+ T cells (Supporting Information Fig. 1). Furthermore the fact that cytokine stimulation induced AP-1 binding to the AP1-RE site (Fig. 6) indicates involvement of AP-1/AP1-RE in cytokine-induced ICOS gene expression. Although IL-2, IL-4, IL-15, or IL-21 stimulation yielded a similar intensity of AP-1 binding to AP1-RE (Fig. 6A), there was a difference in their actual stimulatory effects on cell surface ICOS expression (Fig. 5A and 5B). These differences might be the result of differences in involved molecules other than AP-1 in each cyto-kine receptor signaling. In addition, the ability of each cytokine to induce ICOS upregulation was different among T-cell subsets (naïve, effector or memory) (Fig. 5C and 5D). Therefore, the nature of each T-cell subset, such as expression level of each cyto-kine receptor and/or signaling molecules may affect the specificity and magnitude of cytokine-induced ICOS gene expression.
What is the physiological implication of cytokine-mediated ICOS upregulation for the immune response? Given that cytokine signaling and AP-1 transcription factor have many target genes, cytokine-mediated ICOS upregulation is a part of activation program induced by cytokine. However, since one role of ICOS is to enhance effector cytokine production by effector and memory T cells, it is possible that a positive feedback loop between ICOS and cytokine gene expression exists. Indeed, ICOS expression was reduced on effector T cells in common gamma (γc) deficient mice 29 indicating that γc cytokine has a certain role for maintaining ICOS expression during effector immune responses. In this context, IL-2, IL-4, and IL-21 could be involved in ICOS upregulation on effector T cells in an autocrine fashion, and this might play a role for further enhancement of ICOS-dependent immune response. Regarding IL-2, recent accumulated evidence reveals that IL-2 has a more complex role than was previously thought for the regulation of Th-cell differentiation and functions 30. Therefore it is possible that IL-2 might regulate Th-cell differentiation and function partly by regulating ICOS expression levels on activated T cells, since it has been well accepted that ICOS plays an important role in regulating Th-cell differentiation and functions. On the other hand, homeostatic cytokine IL-7 might play a role in maintaining basal levels of ICOS expression on peripheral memory (CD44high) T cells that could augment memory T-cell functions upon recall response.
The fact that cytokine induces ICOS upregulation independent of TCR/CD28 stimulation could pose a risk for harmful immune responses if cytokine receptor signaling was unusually augmented. We propose that this cytokine-induced ICOS upregulation is one of the mechanisms sustaining ICOS expression in chronic inflammatory immune response such as autoimmunity and allergy because of their abundant cytokine environment. Th17 and follicular helper T (Tfh) cells are highlighted for their pathological roles in autoimmunity and allergy in addition to their physiological roles 31, 32. IL-6 and IL-21 are involved in differentiation of Th17 and Tfh and high levels of IL-21 are produced from both subsets 33, 34 ICOS has critical roles for development and/or effector functions of Th17 and Tfh 33, 34. Therefore it is possible that IL-21-induced ICOS upregulation and ICOS-dependent IL-21 production form a positive feedback loop to augment Th17 and Tfh-mediated immune responses. Furthermore, in an inflammatory environment, bystander effects of IL-6 and IL-21 for ICOS upregulation on naive T cells may enhance their differentiation toward Th17 and Tfh cells.
An important question that was not addressed in this study is the physiological relationship between AP-1 activity and ICOS expression in chronic immune responses. We had previously demonstrated that ICOS expression was sustained at a high level on polyclonal T cells for several months after chronic GVHR induction, one experimental model of SLE, and that ICOS played a critical role for development of pathology in this model 9, 10. To address this issue, in vivo studies to evaluate AP-1 activity in chronic GVHR are currently underway.
In summary, our study demonstrates the involvement of AP-1 in ICOS gene expression through the AP1-RE site within the ICOS promoter. Moreover, we identify a cytokine-dependent ICOS expression pathway other than the TCR/CD28-dependent pathway. Our results should give new insight into the regulation of ICOS gene expression not only in normal immune responses but also in chronic inflammatory immune responses.
Materials and methods
Mice and cells
BALB/c mice were obtained from Sankyo Laboratory (Hamamatsu, Japan). Peripheral CD4+ T cells were prepared from mouse splenocytes as described previously 35. T-cell hybridomas were generously provided by Dr. S. Macphail (New York University, USA). All mice were maintained in a mouse facility under specific pathogen-free conditions. Mouse procedures were performed under the protocols approved by the Animal Care and Use Committee of the Tokyo University of Science.
Antibodies and reagents
Anti-mouse-ICOS mAb (B10.5) was described previously 19. Purified anti-TCR (H57) and anti-CD28 (PV-1) mAbs were prepared in our laboratory from hybridoma culture media. FITC-labeled anti-CD4 and biotinylated anti-ICOS Abs were prepared in our laboratory. Anti-c-Jun (H-79 and G-4), JunB (C-11), JunD (#329), c-Fos (#4), FosB (#102), Fra1 (R-20), Fra2 (L-15), and Fos family (K-25) Abs were purchased from Santa Cruz. Recombinant mouse cytokines were purchased from Peprotech.
Flow cytometry
Cells were washed with wash buffer (PBS containing 1% BSA and 0.05% NaN3), treated with anti-FcR (2.4G2) and stained with fluorescent-conjugated antibodies. Electronic data were acquired on a FACS Calibur™ and analyzed by CELLQuest™ software (BD Pharmingen). The ICOS+ and ICOS– 6-13-64 populations were isolated from 6-13-64 hybridoma by flow cytometric sorting using FACS Vantage SE™ (BD Pharmingen).
RT-PCR analysis
mRNA was isolated by using TriReagent (Sigma) and was converted to cDNA with SuperScript II (Roche). The following are primers utilized for RT-PCR. c-Fos; F:GATGTTCTCGGGTTTCAACG, R:GTCTCCGCTTGGAGTGTATC, Fra2; F: CGGGAACTTTGACACCTCGT, R: TGCAGCTCAGCAATCTCTTT, c-Jun; F: ACCTTCTACGACGATGCCCTC, R: GTGACACTGGGAAGCGTGTT, JunB; F: ATGGAACAGCCTTTCTATCAC, R: GGGGGGCGTCACGTGGTTCAT, JunD; F: TGCTGGCTTCGCCGGATCTT, R: TGCGTGTCCATGTCGATGGG, ICOS; F: GCTCGGCCGATCATAGGATGT, R: CCTCCACTAAGGTTCCTTTCTTG, b-actin; F: GCGCTCGTCGTCGACAACGG, R: GATAGACAACGTACATGGCTG. PCR reaction was 95°C 5 min, (94°C 60 s, 60°C 30 s, 72°C 60 min) × 30 cycle, 72°C 10 min. Signal intensity of RT-PCR result was calculated by densitometry software (ATTO DensitoGraph 4.0).
Microarray analysis
ICOS+ and ICOS– 6-13-64 cells were isolated by cell sorting and subjected to microarray analysis to compare their gene expression profiles. cDNA prepared from each cell population was labeled with Cy3 or Cy5. The labeled cDNA was hybridized with Mus musculus AROS version 3.0 chip (Operon Biotechnology). The chip was scanned with ScanArray Express (Perkin Elmer) and quantified with QuantArray 3.0 software (Perkin Elmer). Data mining was performed with GeneSpring 6.2.1 software (Silicon Genetics). The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO) 36 and are accessible through GEO Series accession number GSE20922 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE20922).
Vectors
pcDNA-AP-1 vectors were generously provided by Dr. T. Tokuhisa and Dr. M. Hatano (Chiba University, Japan). pMX-IRES-GFP vector was obtained from Dr. T. Kitamura (Tokyo University, Japan). Each Fos/Jun family gene was subcloned from the pcDNA vectors into pMX-IRES-GFP vector. ICOS promoter fragment (position from −780 to +32 relative to transcription start site) was amplified from BALB/c mouse genomic DNA by PCR gene using specific primers (F: GGGCTAGCTCAGATGTTTTAAAATGTCATTTTC and R: GGAAGCTTGTCTGCCAGGAGCAGTTG) carrying BamH I and Hind III restriction enzyme site, respectively. The PCR product was cloned into pGL3-basic (pGL3B) firefly-luciferase reporter vector (Promega) to generate mICOSp(-780)-Luc. Each ICOS promoter 5′-deletant was generated by PCR using mICOSp(-780)-Luc as a template.
Retroviral gene transfection
To prepare virus particles, pMX-IRES-GFP vectors carrying each Fos/Jun family member were transfected to PLAT-E packaging cells 37 by FuGene transfection reagent (Roche). Virus particle in culture supernatants at 48 and 72 h were concentrated by centrifugation (7000 rpm for 12 h). CD4+ T cells were stimulated with anti-TCR (3 μg/mL) plus anti-CD28 (5 μg/mL), and the virus-particle was added to the culture with DOTAP transfection reagent (Roche) at 24 and 48 h. At 72 h, T cells were washed and cultured with recombinant human IL-2 (30 units/mL).
ICOS promoter assay
Each mICOSp-Luc reporter vector (1 μg) and renilla-luciferase expression vector (20 ng) (pRL-TK, Promega) were transfected to EL-4 cells with Lipofectamine LTX reagent (Invitrogen) by following the manufactures’ instructions. After 24 h, the cells were stimulated with PMA/Ionomycin or left unstimulated for additional 24 h. The firefly-luciferase activity and renilla-luciferase activity were measured with Dual Luciferase Assay System (Invitrogen) using Luminometer, Lumat LB9501 (Berthold). Relative luciferase activity was calculated by normalizing the firefly-luciferase activity to the renilla-luciferase activity.
shRNA knockdown experiments
For shRNA analysis, pSIREN-RetroQ-ZsGreen vector (Clontech) was utilized. Each AP-1 shRNA sequence was reported previously 38-42 and described below. Control (scrambled sequence) pSIREN vector or each AP-1-targetting pSIREN vector (2 μg) was transfected to EL-4 cells using Lipofectamine LTX. At 24 h, the cells were stimulated with PMA/Ionomycin for additional 24 h and cell surface ICOS expression levels on GFP+ population were analyzed by flow cytometry. shRNA sequences. cFos(1): GCAGATCTGTCCGTCTCTA, cFos(2): GCGGAGACAGATCAACTTG, cJun(1): CAGCTTCCTGCCTTTGTA, cJun(2): GCGCATGAGGAACCGCATT, Fra2(1): GGAGAGATGAGCAGCTGTC, Fra2(2): TCAACGCCATCACCACCAG, JunB(1): GCAACGGCGTGATCACGAC, JunB(2): GACCAGGAGCGCATCAAAG.
Electrophoretic mobility shift assay (EMSA)
Cells were washed with PBS and lysed with low salt buffer (10 mM HEPES pH7.9, 10 mM KCl, 0.1 mM EDTA, 0.1% Nonidet P-40) and nuclear proteins were extracted with high salt buffer (20 mM HEPES pH7.9, 0.4 M NaCl, 1 mM EDTA). The nuclear extracts (1 μg) were incubated with end-labeled (32P)-double-strand oligonucleotides probes in reaction mixture (5 mM Tris HCl, 1 mM EDTA, 50 μg/mL Poly dI:dC, 1 mM DTT) for 30 min at RT. For super-shift/depletion assay, the reaction mixture was incubated with each Abs for additional 15 min at RT. The reaction mixture was resolved by 5% polyacrylamide TBE gel. The imaging plate was analyzed with the image analyzer FLA-7000 (FUJIFILM). Signal intensity of the result was calculated by densitometry software (ATTO DensitoGraph 4.0). The following are utilized oligonucleotides sequences. ICOSp-AP1-RE; 5′-TTCATCCATCTAGTCATTCATTTACGCATC-3′, ICOSp-AP1-RE-Mut; 5′-TTCATCCATCTAGCTGTTCATTTACGCATC-3′, Consensus AP-1; 5′-CGCTTGATGACTCAGCCGGAA-3′, NFAT; 5′-GCCCAAAGAGGAAAATTTGTTTCATACAG-3′, NFκB; 5′-ACCAAGAGGGATTTCACCTAAATC-3′.
In vitro culture and assay
Cells were cultured in RPMI1640 medium containing 10% FCS, 2ME, HEPES, L-glutamine, penicillin/streptomycin. For cytokine stimulation assay, CD4+ T cells were cultured with either IL-2 (30 units/mL), IL-4 (100 units/mL), IL-6 (20 ng/mL), IL-7 (50 ng/mL), IL-10 (3 ng/mL), IL-13 (10 units/mL), IL-12 (20 ng/mL), IL-15 (50 ng/mL), IL-17A (50 ng/mL), IL-21 (20 ng/mL), IFN-γ (1000 units/mL), or TNFα (100 ng/mL) for 12 and 24 h for EMSA and flow cytometry, respectively.
Chromatin immunoprecipitation (ChIP) assay
ChIP analysis was performed as described previously 43. EL-4 cells (5 × 105) were stimulated with PMA/Ionomycin for 6 h and then fixed. The fixed cells were sedimented, washed, and lysed with SDS lysis buffer. The lysates were sonicated to reduce DNA lengths to between 200 and 1000 bp. The soluble fraction was diluted, precleared with salmon sperm DNA/protein A-agarose, and then incubated with anti-AP-1 Abs. Immune complexes were precipitated with protein A-agarose and DNA was isolated. Semi-quantitative PCR was performed with the DNA samples with each gene-specific primers.
Statistical analysis
Statistical comparisons were performed by two-tailed Student's t-test. Differences were considered significant for p < 0.05. Results are displayed as mean ± SD.
Acknowledgments
We thank Dr. Richard Hodes for critical reading of the manuscript and helpful comments and Dr. Hidehiro Kishimoto for discussion and suggestion. We thank a member of Science Service, Inc. for their care of experimental animals. We also gratefully acknowledge JT Pharmaceutical Frontier Research Laboratories for providing the anti-ICOS mAb. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan to R.A.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- ChIP
-
- Chromatin immunoprecipitation
-
- Fra2
-
- Fos-related antigen 2
-
- Tfh
-
- T follicular helper