Leishmania donovani glycosphingolipid facilitates antigen presentation by inducing relocation of CD1d into lipid rafts in infected macrophages
Abstract
NKT cells respond to presentation of specific glycolipids with release of both Th1- and Th2-type cytokines. Leishmania donovani (LD)-infected splenic macrophages (sMϕ(I)) and bone marrow-derived dendritic cells (BMDC(I)) failed to activate NKT cells in response to α-galactosyl ceramide (α-GalCer). The defective antigen presentation could be corrected by treating the cells with the immunostimulating glycosphingophospholipid (GSPL) of LD parasites. In vitro pulsing of BMDC(I) or sMϕ(I) with GSPL, caused the activation of the Vα14+ CD1d1-specific NKT cell hybridoma DN32.D3. Localization of MHC II and CD1d molecules to membrane lipid rafts has been suggested to play an important role in antigen presentation. Confocal analysis clearly demonstrated that LD infection changed the pattern of CD1d distribution to the non-lipid raft regions and this change could be reversed by GSPL treatment. Isoelectric focusing gel shift assay indicated that GSPL binds to CD1d. GSPL-treated but not untreated BMDC(I) formed immune synapses with NKT cells and this was associated with calcium mobilization. In conclusion, GSPL treatment was associated with modification of BMDC(I)/sMϕ(I) lipid raft structure, which is a site for immune regulation.
Introduction
The disease visceral leishmaniasis or kala-azar, caused by the protozoan parasite Leishmania donovani (LD), is characterized by defective cell-mediated immunity 1, 2. The reduced Ag-presentation and accessory functions of Leishmania-infected Mϕs is not due to altered levels of surface-expressed peptide-MHC complexes 2. Rather, the modified lipid raft composition following LD infection 3, 4 likely accounts for the defective Ag-presentation by these splenic macrophages (sMϕs), since intact lipid raft domains are necessary for Ag-presentation at low Ag concentrations 5-8. Inhibition of Ag-presentation can be reversed on restoration of raft architecture. Cholesterol and sphingolipid-enriched lipid rafts have been thought to act as platforms through which signal transduction events are coordinated and pathogens gain entry to infect host cells 6, 7.
Certain glycolipid Ags for Vα14i NKT cells can modulate the cytokine balance of the immune response 9. Our earlier work with the immunostimulating glycosphingophospholipid (GSPL) Ag from LD established that GSPL is expressed on the surface of infected sMϕs (sMϕ(I)) 10. Though GSPL activates T cells to produce IFN-γ, GSPL-expressing sMϕ(I)s fail to promote T-cell responses. In this study, we demonstrate that disruption of lipid raft structures on LD infection results in partitioning of murine CD1d (mCd1d) to non-raft domains. GSPL treatment localizes mCD1d to rafts and the presentation of the mCD1d-binding glycosphingolipid (GSL) antigen to NKT cells is facilitated by intact membrane rafts. We propose that rafts facilitate the presentation of Ag through enhancing the interaction of mCD1d/GSL complexes on the APC and the NKT Vα14 TCR.
Results
GSPL treatment restores CD1d within lipid rafts of infected BMDCs
Raft disruption impairs lipid Ag-presentation by mCD1d to NKT cells 11, 12. Raft disruption also compromises the Ag-presenting ability of LD-infected Mϕs 5. To ascertain the potential involvement of lipid rafts in controlling iNKT cell activation we analyzed the membrane distribution of mCD1d in untreated and GSPL-treated infected BMDCs (BMDC(I)). Representative images are presented in Fig. 1. BMDC(I) were treated with graded concentrations of GSPL (0.5–100 μg/105 cells, 1 h, 37°C) and CD1d, GM1 and I-Ad expression was studied. In the uninfected BMDCs cell surface CD1d and CTX-B appeared to be co-localized (panel Ai). The changed pattern of CD1d distribution to the non-lipid raft on LD infection (panel Aii) was rectified on GSPL treatment that resulted in the relocation of mCD1d molecules in the lipid rafts (panel Aiii). CD1d expression was also restored within the membrane rafts in control BMDC(I) treated with exogenous cholesterol (data not shown). Since the minimum concentration required for efficient translocation of CD1d within lipid rafts was 10 μg/105 cells, BMDC(I) treated with 10 μg GSPL/105 cells for 1 h at 37°C will henceforth be referred to as GSPL-treated LD-infected BMDC (GSPL-BMDC(I)). Toxicity of GSPL at these concentrations was excluded due to no changes in the mitochondrial activity as determined in a cell proliferation assay, and no gross membrane or surface molecule alterations as measured by PI staining and FACS analysis (data not shown).
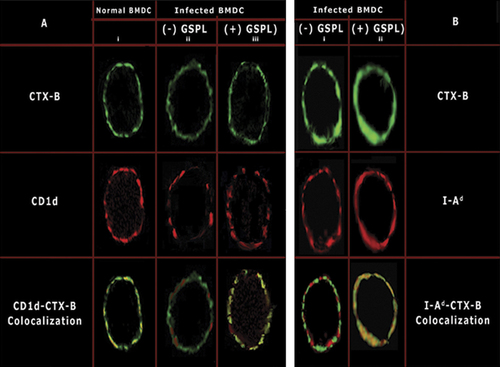
Plasma membrane distribution of CD1d and I-Ad relative to the plasma membrane raft marker GM-1. (A and B) Uninfected BMDCs or BMDC(I) were either mock-treated ((-)GSPL) or treated with GSPL (10 μg/105 cells) for 1 h at 37°C. Excess GSPL was washed off and cells were incubated at 4°C and stained for (A and B) GM1 (CTX-B, green), (A) CD1d and (B) MHC class II (both red). Cells were fixed and analyzed by confocal microscopy. Red/green co-localization is shown in yellow. Images are representatives of four independent experiments.
To determine whether administration of GSPL affects MHC class II expression on the GSPL-BMDC(I) plasma membrane, MHC class II-clustering pattern was studied. Equal numbers of cells (1×106) were used for the MHC class II and GM1-clustering assay. Administration of GSPL led to an induction of MHC class II clustering in the lipid rafts (panel Bii).
Cell surface expression of CD1d on BMDC(I)
While CD1d associates with β2-microglobulin in a similar manner to MHC class I, functionally CD1d is similar to MHC class II 13. Since expression surface MHC class II molecules are comparable in uninfected and sMϕ(I) 2, we analyzed the expression and distribution of mCD1d on BMDC(I) surface. Compared to uninfected BMDC, LD infection did not alter the overall surface (Fig. 2A) or intracellular (Fig. 2B) expression levels of mCD1d, indicating that LD infection does not alter the overall intracellular mCD1d metabolism rate.
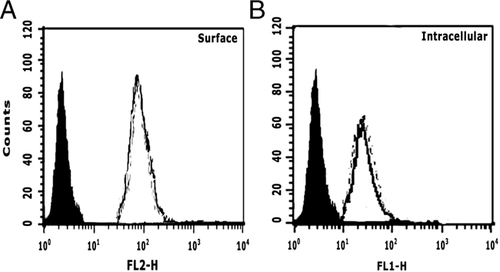
LD infection does not alter expression levels of CD1d molecules. (A) Surface and (B) intracellular mCD1d expression were analyzed in uninfected BMDCs (black line) and BMDC(I) (dotted line) cells using an anti-mCD1d Ab (1B1). Rat IgG2b was used as the isotype control Ab (solid black peak).
GSPL treatment and iNKT cell stimulation
The stimulatory properties of GSLs were studied by using a mouse Vα14+NKT cell hybridoma (DN32.D3). The Ag-presenting ability of GSPL-treated LD-infected Ag processing cell (APC(I)) was compared to that of mock-treated APC(I). iGb3 (iso globotriosyl ceramide) and Gb3 (globotriosyl ceramide) (Alexis-Biochemicals, PA, USA), were taken as the positive and the negative control of NKT cell stimulation respectively 14.
Murine GSPL-APC(I) were incubated with graded amounts of GSL and DN32.D3 cells, and supernatant IL-2 levels in 24-h culture were measured. As represented in Fig. 3, GSPL-mediated NKT cell stimulation was dose dependent (panels A and B). GSPL-BMDC(I) (Fig. 3A) consistently produced more IL-2 as compared to GSPL-sMϕ(I) (Fig. 3B). While untreated BMDC(I) failed to present iGb3 to DN32.D3, iGb3-loaded GSPL-BMDC(I) strongly stimulated DN32.D3 (Fig. 3C). No IL-2 was detected in the absence of Ags (GSLs), APCs or effector cells (DN32.D3 cells) (representative data for iGb3 presentation by GSPL-BMDC(I) are shown in Fig. 3C, inset). GSPL (Fig. 3A) was nearly as potent as iGb3 (Fig. 3C). However, both BMDC(I) and GSPL-BMDC(I) failed to present Gb3 (data not shown). Notably, Gb3, an isomer of iGb3, is not an Ag for NKT cells 14. IL-2 production was blocked in the presence of anti-CD1d antibody, but not anti-CD1b antibody or isotype control, confirming that IL-2 production is CD1d dependent. The Ag-presenting ability of the GSPL-sMϕ(I) was comparable to that of the untreated uninfected macrophages (data not shown). To determine if BMDC(I) can present a glycolipid Ag that does not require endosomal trafficking to be recognized by T cells 15, we analyzed the response to α-galactosyl ceramide (α-GalCer). LD infection inhibited CD1d-mediated presentation at low α-GalCer concentration, unlike untreated uninfected BMDC (Fig. 3D, inset). This inability could be overcome at higher Ag concentration (main Fig. 3D) or on GSPL treatment (Fig. 3D).
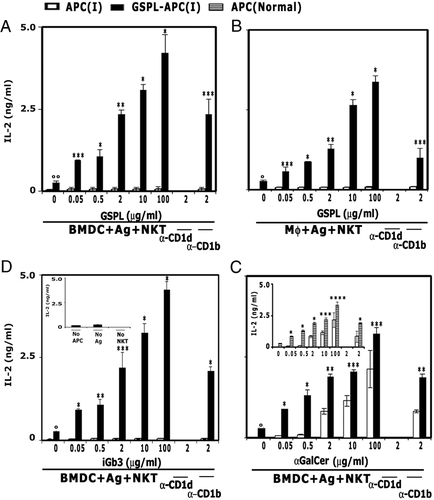
Presentation of GSLs to NKT cells is facilitated by membrane rafts. NKT cells were stimulated with GSPL-treated or untreated (A, C and D) BMDC(I) or (B) Mϕ(I)) pulsed with the indicated concentrations of (A, B) GSPL, (C) iGb3 and (D) α-GalCer in the presence/absence of α-CD1d. The amount of IL-2 produced was quantitated by ELISA. The inset in (C) shows GSPL-BMDC(I) pulsed with iGb3 (2 μg/mL) in the absence of the indicated components. The inset in (D) shows GSPL-treated uninfected BMDCs pulsed with the indicated concentrations of α-GalCer in the presence/absence of α-CD1d. Data are mean±SD for triplicate samples and are representatives of at least three experiments. *p<0.0001; **p<0.001; ***p<0.01; ****p<0.05 compared with IL-2 production by the GSPL-treated APC (I) without added antigens; °p<0.001; °°p<0.01 compared with IL-2 production by the untreated APC(I); paired two-tailed Student's t-test.
Presentation of peptide antigen
Previous studies have established that due to disrupted raft architecture, sMϕ(I) fail to activate T cells in response to low dose of exogenous peptide 3, 4, 8, 9. Since we observed that in vitro GSPL treatment restores the defective raft architecture associated with LD infection, including the altered expression and distribution of MHC class II, we wanted to see if GSPL treatment restored the peptide presenting ability of these cells. The Ag-presenting ability of sMϕ(I) and GSPL-sMϕ(I) was investigated with 2 μM λR12–26. As shown in Fig. 4, GSPL-sMϕ(I) activated the T-cell hybridoma 9H3.5 at 2 μM peptide concentration, while untreated sMϕ(I) failed to activate the T cells at a similar peptide concentration.
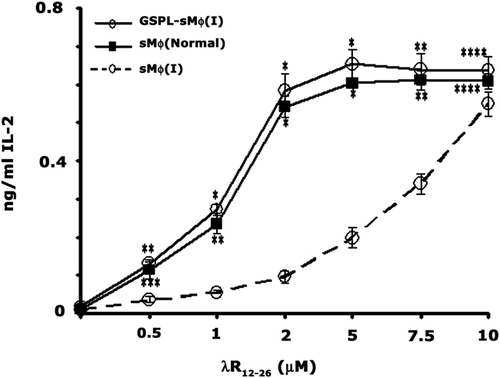
GSPL treatment restores peptide Ag-presentation by sMϕ(I). Untreated sMϕ, untreated sMϕ(I) and GSPL-sMϕ(I) were tested for their ability to activate T-cell hybridoma 9H3.5 cells as determined by IL-2 production in the presence of increasing concentrations of the peptide λR12–26. Data are mean±SD of triplicate samples and are representatives of at least three experiments. *p<0.0001; **p<0.001; ***p<0.01; ****p<0.05; paired two-tailed Student's t-test.
Loading requirements for GSPL
Glutaraldehyde fixed CD1d+ APCs block Ag-uptake and recycling of CD1d between endosomes and the plasma membrane. GSPL-BMDC(I) cells were pulsed with GSPL (1–100 μg/mL) for 12 h before washing and fixation, or fixed and washed before incubation of cells with antigen for 12 h. Cells were then co-cultured with NKT cells before measurement of IL-2 titers. NKT cell recognition of GSPL was markedly reduced if lipid loading was done after fixation of GSPL-BMDC(I) (Fig. 5A). Since this result indicated that GSPL probably had substantial dependence on endosomal loading for presentation, we examined the kinetics of CD1d presentation of GSPL. IL-2 production by NKT cells was triggered by GSPL-BMDC(I) cells following a 6- to 7-h pulse with GSPL Ag (Fig. 5B), while, in cells pulsed with the processing independent antigen α-GalCer maximal IL-2 production was detected within 1 h (Fig. 5C). Kinetics of CD1d presentation of NKT-targeted-α-GalCer and GSPL indicated that α-GalCer preferentially loaded surface CD1d, while GSPL probably required endosomal trafficking.
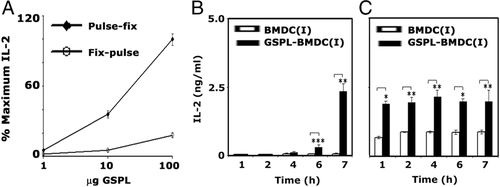
Kinetics of CD1d Ag-presentation. (A) GSPL-treated BMDC(I) were pulsed further with GSPL at the indicated concentrations for 12 h before washing and fixation, or were fixed first and then pulsed with GSPL for 12 h. Cells were then co-cultured with NKT cells before measurement of IL-2 titers. (B and C) Untreated or GSPL-treated BMDC(I) were pulsed for the indicated times with 10 μg/mL (B) GSPL or (C) α-GalCer before washing and incubation for 24 h with NKT cells. Resultant IL-2 concentrations in the media were measured by ELISA. Data are the mean±SD of triplicate samples and are representatives of two independent experiments. *p<0.0001; **p<0.001; ***p<0.05; paired two-tailed Student's t-test.
GSPL accumulation leads to mCD1d/MHC class II translocation into the lipid raft fraction
In an attempt to define its mode of action, we investigated where the exogenously applied GSPL accumulates in the cells. Its distribution was monitored using [3H]GSPL as a tracer, before fractionating cells using a sucrose gradient (5 min application of GSPL; Fig. 6A; solid line, filled square). Distribution of GSPL corresponded with that of the raft marker GM1 (broken line, filled triangle). The specificity of the observed finding is validated by the observation that soluble GSPL (s-GSPL) micelle was predominantly found in the 40–60% interface, as determined in a mock gradient omitting cells (Fig. 6A; broken line, filled circle). Following the deposition of GSPL into the detergent-resistant membranes (DRMs), both mCD1d and MHC class II translocate into the 20% fraction (DRMs) as determined by Western blot analysis (Fig. 6B).
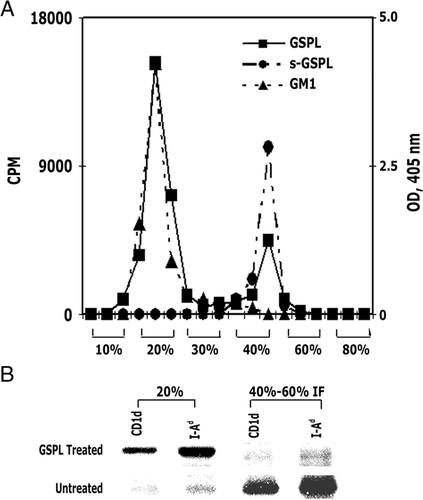
GSPL accumulation in the DRM fraction mediates the translocation of CD1d and MHC class II to the lipid raft. (A) Exogenously added [3H]-labeled GSPL was added to BMDC(I) for 5 min before fractionating the cells on a sucrose gradient. Soluble [3H]GSPL in the absence of BMDC(I) cells and lysed BMDC(I) were also fractionated to determine sGSPL micelle and GM1 cellular locations respectively. The corresponding percentage of sucrose of each fraction is indicated on the x-axis; cpm is indicated on the y-axis to the left and OD (405 nm) to the right. (B) Western blot analysis using antibodies against the indicated proteins of pooled fractions corresponding to 20% (DRMs) or 40–60% interface (IF) of GSPL-treated or untreated BMDC(I) as indicated. The data are representatives of three independent experiments
GSPL treatment and macrophage membrane fluidity
The defective Ag-presenting ability of sMϕ(I) 2, 5 has been correlated to the increased membrane fluidity of the sMϕ(I) cell membranes. An exogenous supply of cholesterol to host cells restores the membrane fluidity of sMϕ(I) 5. Glycolipids have also been shown to be membrane fluidity modulators 16. The membrane fluidity of the GSPL-treated and untreated sMϕ(I) was studied in terms of fluorescence anisotropy (FA) using DPH as a probe. The observed decrease in FA due to infection (0.35±0.017 versus 0.25±0.019) was reversed on GSPL treatment (0.25±0.019 versus 0.34±0.008) (Fig. 7). The anisotropy of the polarization DPH is the indicator of membrane fluidity. These data indicated that the increase and decrease in membrane fluidity was correlated to LD infection and subsequent GSPL treatment.
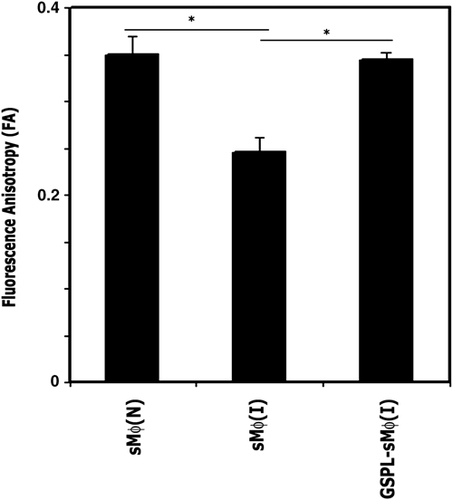
Measurement of FA of untreated, uninfected sMϕ (sMϕ(N)), sMϕ(I) and GSPL-treated sMϕ (I) cells. The FA value was measured using DPH as a probe. The fluorophore was excited at 365 nm, emission intensity was recorded at 430 nm, and FA was calculated. The data shown are representatives of three independent experiments. *p=0.0022; paired two-tailed Student's t-test.
Isoelectric focusing analysis of CD1d lipid binding
Empty CD1 molecules band at neutral pH on native isoelectric focusing (IEF) gels. This migration pattern can be altered by the binding of charged phospho- or glycolipids. Shifts were seen when CD1d was loaded with the trisialo ganglioside GT1b (net negative charge) used as positive control 14. GSPL is an anionic glycolipid. Loading of CD1d with GSPL resulted in a shift in electrophoretic mobility (Fig. 8) indicating that GSPL was bound by CD1d.
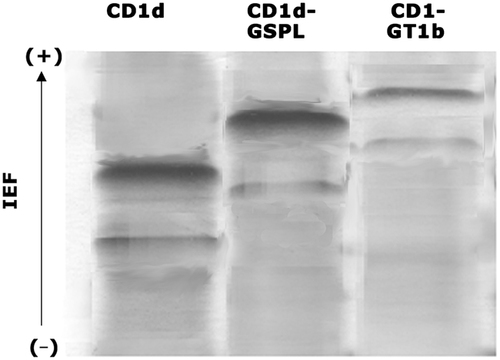
IEF analysis of CD1d-GSPL binding. IEF of empty, GT1b- or GSPL-loaded CD1d as indicated. The data are representatives of three independent experiments.
GSPL treatment, lipid raft polarization and intracellular calcium mobilization during BMDC-iNKT cell interactions
Successful glycolipid antigen presentation is initiated by the interaction of the TCR with a membrane-associated complex in the APC composed of glycolipid-bound CD1d in the nanometer scale gap between a T cell and an Ag-presenting cell, referred to as an immunological synapse (IS) 17, 18. To investigate whether conjugates between BMDC and NKT cells were active IS, confocal microscopy was performed with GSPL-treated or untreated BMDC(I) presenting antigen to DN32.D3 (Fig. 9). Lipid rafts (GM1) of the BMDCs or of the iNKT cells were stained using FITC-CTX-B. The GM1 molecules of BMDC(I) appeared as small fluorescent patches scattered on the plasma membrane in the absence of GSPL (Fig. 9, panel vii). In contrast, GSPL-BMDC(I) lipid rafts became clustered at the BMDC/NKT cell interface after conjugate formation (Fig. 9, panel ii). Since GSPL treatment resulted in the relocation of mCD1d molecules in the lipid rafts, we determined the distribution of CD1d (red) in the IS. mCd1d demonstrated strong co-localization with GM1 at the IS in the GSPL-BMDC(I) (Fig. 9, panel iv). Most (60–80%) cells showed strong co-localization of mCD1d and GM1 at the synapse. In the absence of GSPL treatment, GM1 and CD1d appeared as patches on the surface of BMDC(I) (Fig. 9, panel ix).
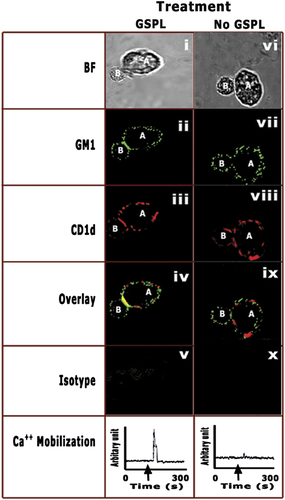
Analysis of conjugate formation and intracellular Ca2+ mobilization in NKT cells. GSPL-treated (i–v) or untreated BMDC(I) (vi–x) were pulsed with GSPL or iGb3. GSL-pulsed cells were incubated with NKT cells for 45 min and then fixed and stained. FITC-CTB-X was used to stain GM1. CD1d was labeled with biotinylated anti-CD1d and then streptavidin-PE. Fixed conjugates were imaged by both phase contrast (BF) and confocal microscopy. A representative image of GSPL-BMDC(I) pulsed with iGb3 is shown. To study intracellular Ca2+ mobilization, T cells (DN32.D3) were loaded with fura 2-AM and then mixed with GSPL-treated or untreated BMDC(I). A, BMDC; B, iNKT. The arrow in the graphs indicates the addition of BMDCs to the fura 2-AM-loaded DN32.D3 cells. Images of representative cells in four independent experiments are shown.
Ag-engagement of the TCR initiates an influx of Ca2+ entry across the plasma membrane 19. Analyzing Ca2+ signals in the DN32.D3 cells in response to GSPL-treated and untreated BMDC(I), we found that increases in intracellular Ca2+ mobilization was associated with IS formation. Intracellular calcium mobilization was observed when T cells were treated with GSPL-BMDC(I). Only a slight mobilization was observed when stimulation was performed with BMDC(I).
Discussion
By bridging the innate and acquired immunity, NKT cells play an important role in modulating immunity to infectious agents 20. The lipid anchoring hydrophobic-binding groove of the CD1d molecule allows interactions of polar chains with the TCRs. Different kinds of glycolipids including GSLs presented by CD1d have been identified 21.
GSPL treatment and lipid raft localization of CD1d
Our earlier work had established that GSPL was expressed on sMϕ(I) 10. Yet the sMϕ(I) failed to stimulate DN32.D3 cells. Lipid rafts are required for efficient signal transduction by CD1d to the target NKT cells 9, 12, 22. Raft disruption during Leishmania infection compromises the ability of sMϕ to provide optimal TCR signaling to Ag-specific effector T cells 2, 5. Leishmania have evolved a variety of strategies to survive inside their host cells. It has been suggested that the alterations of the host cell raft architecture associated with Leishmania infection is probably achieved by the parasite uptake of host cholesterol 23. Paradoxically, though, the restoration of lipid rafts by the cholesterol-enhanced Ag-presenting function of sMϕ(I), cholesterol/dyslipidemia, is also known to support Leishmania infection inside the host 23-25. Since exogenous GSLs are also known to modulate raft composition 26, 27, in this study we used GSPL replenishment as an alternative to cholesterol treatment to restore raft structure and function.
We have confirmed that lipid raft disruption during Leishmania infection blocked efficient signaling through CD1d that could be corrected on GSPL pulsing. By virtue of their high affinity for liquid-ordered domains 28, GSLs are known to play an important role in microdomain formation. Self-association, driven by the hydrogen-bonding capacities of their sphingosine backbone and polar head-groups, enhances the ability of the GSLs to associate with rafts 28, 29. Incorporation of glycolipid between the two membrane leaflets may lead to morphological changes in the membranes 26, 27. GSL replenishment indeed resulted in the localization of GSPL, along with CD1d, and I-Ad into the lipid rafts of APC(I). Although MHC II localization in lipid rafts is well documented, only a limited number of studies have demonstrated the importance of membrane rafts on CD1d presentation by APC 30, 31. The requirement of a minimum concentration of GSPL for efficient translocation of CD1d within lipid rafts argues for the existence of a threshold concentration that when exceeded promotes translocation of CD1d from the non-raft to the raft domains. Though an IEF-binding assay indicated that GSPL binds to CD1d, in the absence of antibodies that can detect GSPL-CD1d complex we cannot unequivocally state that the raft-associated GSPL was loaded onto the CD1d molecules.
CD1d raft localization and antigen presentation
Evidence has indicated that glycolipid-enriched membrane rafts are important for efficient antigen presentation. Similar to MHC II antigen presentation 6, disruption of cholesterol-dependent microdomains inhibits CD1d-restricted antigen presentation to NKT cells 7, 12. If lipid rafts are required for efficient signaling by CD1d 12, the efficiency of the CD1d-NKT cell immune axis in the GSPL-treated cells would be expected to undergo a sharp change as compared to the sMϕ(I). Though GSPL was expressed on sMϕ(I), partitioning of CD1d into non-raft domains of sMϕ(I) explains the inability of the APC(I) to efficiently present the glycolipid-Ags to NKT cells. GSPL treatment of BMDC(I) resulted in the restoration of CD1d and MHC II molecules into lipid rafts. Restoration of CD1d molecules into raft domains restored the ability of the CD1d-mediated presentation of the GSL ligands to the DN32.D3 cells with comparable efficiency. Analogous to MHC II antigen presentation 4, the integrity of cholesterol-dependent lipid raft is important at limiting concentrations of antigen 13. Cholesterol extraction and LD infection inhibited CD1d-mediated presentation of low but not high concentrations of α-GalCer, confirming that raft integrity was essential for low concentrations of antigen. It has been suggested that association of CD1d molecules with membrane rafts is important for presentation of limiting quantities of glycolipid-Ags perhaps by stabilizing mCD1d/Ag structures on the plasma membrane and optimizing TCR engagement on NKT cells 7, 31. The Ag-presenting ability of the GSPL-treated APC(I) was comparable to that of the untreated uninfected macrophages. GSPL treatment also restored the ability of the sMϕ to present low concentrations of peptide-Ags. Similar to MHC class II-restricted peptide engaging either classical or re-cycling MHC class II pathways for presentation to T cells 32, glycolipid-Ag can be presented by either the endosomal or the non-endosomal pathway 15. Kinetics of GSL presentation indicated that α-GalCer preferentially loaded surface CD1d, while GSPL probably required endosomal trafficking.
GSPL treatment, membrane fluidity and antigen presentation
Anergic immune responses associated with leishmaniasis has been correlated with a defective Ag-presentation, which in turn correlates with altered physical properties of sMϕ(I) cell membranes 3 and restoration of these physical properties by an exogenous supply of cholesterol to host cells leads to recovery in Ag-presenting ability of sMϕ(I) 5, 23. The addition of exogenous gangliosides to cells can also lead to an induction of raft clustering 33. Raft clustering may lead to an alteration of membrane properties, leading to the initiation of immune modulation by recruiting specific membrane proteins and lipids in membrane microdomains.
The functions of membrane-bound proteins are regulated by the physical properties of the cell membrane. It is widely accepted that the membrane fluidity plays an important role in regulating the function of membrane-bound proteins and their localization or endocytosis, although how this happens is not altogether clear. Increased membrane fluidity associated with Leishmania infection leads to reduced clustering of MHC class II-peptide complexes, diminished Ag-presenting ability of sMϕ(I) and impairment of PKC translocation and phagosome fusion 34. Changes in lipid composition have been correlated with changes in membrane microviscosity 35. Even at low concentrations, gangliosides reduce fluidity and hydrocarbon chain mobility in phosphatidylcholine bilayers, due to lateral cooperative interactions between ganglioside molecules 36, 37. The ceramide moiety of GSLs can be considered a rigid structure, and addition of GSLs to cells was shown to reduce the original membrane fluidity 37-40. The increased membrane fluidity of LD-infected macrophages could indeed be corrected by GSPL treatment.
Lipid rafts and the immune synapse
Successful glycolipid Ag-presentation requires an interaction of the TCR with the glycolipid-bound CD1d 17, 18. A notable feature of T-lymphocyte recognition on other cell surfaces is the formation of a stable mature IS. Central to the synapse is the interaction between the presented antigen and its cognate receptor 41. TCR ligation induces rapid lipid raft clustering and accumulation of signaling proteins at the area of contact between APC and T cells 8, 42. As a consequence, CD1d and TCR become concentrated in the IS 17. To address the question whether, analogous to MHC class II presentation, successful presentation of GSPL by lipid-raft-associated CD1d molecules results in the formation of IS, confocal microscopy was performed. Most of the GSPL-pulsed cells (60–80%) showed strong co-localization of mCD1d and GM1 at the IS. Elevation of cytoplasmic calcium is one of the earliest events in T-cell activation 19. The rapid calcium elevation that is seen after T-cell activation by GSPL-BMDC(I) was blocked when BMDC(I) were used for Ag presentation.
Concluding remarks
CD1d-restricted NKT cells possess a wide range of effector and regulatory activities that are related to their ability to secrete both Th1- and Th2-type cytokines. It has been suggested that Th2-type cytokine-biasing ligands are rapidly and directly loaded onto cell-surface CD1d proteins. In contrast glycolipid ligands that require endosomal loading preferentially induce Th1-type cytokine profiling 30. Our lipid loading experiments indicated that GSPL probably required endosomal trafficking. This corroborated with our earlier observation that GSPL could stimulate untreated uninfected human PBMC to produce Th1 cytokines 10. The importance of CD1d for full host resistance to LD in liver and spleen during mouse infection has been documented by Amprey et al. 43. Though LPG was identified as a potential glycolipid antigen that binds to CD1d and stimulates IFN-γ production in naive hepatic lymphocytes, the phenotype of the responsive NKT cells remained a fundamental unanswered question 43. Vα14Jα18 NKT hybridoma cells and splenic iNKT cells failed to respond to CD1d-bound LPG. In contrast, our data, for the first time, demonstrate that a parasite-derived GSL binds to CD1d, restores the defective APC function in LD-infected cells, and stimulates robust IL-2 production in Vα14Jα18 NKT hybridoma cells. The impact of this glyclipid antigen on host immunity is currently under investigation.
Materials and methods
Abs and other reagents
The anti-CD1d mAb was purified from the 1B1 hybridoma supernatant using protein G-agarose columns (Life Technologies, Grand Island, NY, USA). An irrelevant rat IgG2b mAb was used as a control. PE-anti-mouse MHC class II antibody (clone AMS-32.1), and isotype control antibodies were obtained from BD Pharmingen. FITC-conjugated CTX-B and HRP-conjugated CTX-B was obtained from Sigma Chemical (St. Louis, MO, USA). The peptide LEDARRLAIYEKK (λR12–26, N-terminal 12–26 amino acid residues of repressor protein) was obtained from Invitrogen Life Technologies.
Generation of BMDCs
BMDCs were generated from BM progenitors (106 cells/mL) in the presence of rmGM-CSF (150 U/mL) and rmIL-4 (75 U/mL) according to the method of Ahuja et al. 44. The DC culture medium was changed every 2 days to remove non-adherent cells. Loosely adherent clustured cells expressing CD11c assessed by flow cytometry (data not shown) were collected on day 10. During the last 24 h of BMDC culture, the cells were grown in the presence of rmTNF-α (20 ng/mL), providing a DC maturation stimulus.
Parasites and infection
LD strain AG83 (MHOM/IN/83/AG83) was used for experimental infections 45. Parasites were maintained in golden hamsters as previously described 45. Promastigotes obtained after transforming amastigotes from infected spleen were maintained in M199 (Invitrogen Life Technologies) as previously described 45. For in vitro infection, BMDC or sMϕ cells were allowed to adhere to 35 mm coverslips for 24 h 46. Non-adherent cells were removed and APC were counted by direct immunofluorescence for the CD11b/CD11c Mϕ/BMDC markers respectively, using labeled anti-CD11b/CD11c antibodies and infected with 2nd passage stationary phase promastigotes (1:20 cell/parasite ratio). After 12 h at 37°C, free promastigotes were removed and cells were cultured for 48 h post infection. To quantify infection level, coverslips were washed, dried, stained with Geimsa and mounted on microscopic slides. The proportion of cells infected and the number of amastigotes per 100 host cells was calculated from triplicate cultures.
Forty-eight hour parasitized APC are used throughout the study. Experiments using animals were done with the permission of the animal ethics committee of the Institute (accreditation no. of the Institute 147/1999/CPSEA).
Purification of GSPL
GSPL was prepared from AG83 promastigote membrane as described earlier 10, 47. The anionic glycolipids were separated from the non-acid GSLs by ion-exchange chromatography on a DEAE-Sephadex column. GSPL-enriched fraction from silicic acid columns was further purified on a RCA-1-Sepharose 4B affinity column. GSPL was radio-labeled with the galactose oxidase-sodium boro[3H]hydride method.
Insertion of GSPL and cholesterol depletion of cells
Stock solution of GSPL (100 μg/100 μL) was prepared by evaporating to dryness 100 μg of GSPL dissolved in 2:1 chloroform:methanol, under sterile conditions and reconstituting in culture (100 μL) media by vortexing immediately prior to use. To test for effects of the solvent, equal volumes of chloroform:methanol were evaporated to dryness and dissolved in media and used as a no-antigen control. Cells were loaded with 0.05–100 μg GSPL in medium for 1 h at 37°C. To remove excess GSPL, cells were washed extensively with PBS containing 2 mg/mL defatted BSA. To deplete APC of cholesterol, cells were incubated for 1 h at 37°C with 10 mM methyl-β-cyclodextrin.
Antigen presentation
For the assays, DN32.D3 cells were cultured for 24 h at 1×105 cells/well in the presence of GSPL-treated or untreated APCs (1×105 BMDC or sMϕ) that had been prepulsed for 12 h with the indicated concentrations of glycolipids or with the no-antigen control. IL-2 release was evaluated in a sandwich ELISA using rat anti–mouse IL-2 mAbs (BD-Pharmingen), and the levels of cytokine release were evaluated using a recombinant IL-2 standard (BD-Pharmingen).
Antigenic peptide-presenting ability of sMϕ was studied by their ability to present λR12–26 to T-cell hybridoma (9H 3.5) to produce interleukin IL-2 3.
Measurement of FA
The membrane fluorescence and lipid fluidity of cells were measured following the method described by Shinitzky and Barenholz 48. Briefly, 106 cells were mixed with an equal volume of DPH in PBS (1 μM) and incubated for 2 h at 37°C. Unlabeled organisms served as a scattering reference (less than 3% of the intensity of the labeled sample). Steady-state FA was measured at 37°C with a Perkin-Elmer LS50B spectrofluorometer (Perkin-Elmer, Beaconsfield, England) with excitation at 360 nm and emission at 430 nm. The FA value was calculated using the equation: FA=[(I∣∣–I⟂)/(I∣∣+2I⟂)], where I∣∣ and I⟂ are the fluorescent intensities oriented, respectively, parallel and perpendicular to the direction of polarization of the excited light 48.
IEF electrophoresis
Six micromolar CD1d was used in measuring lipid interactions. CD1d loaded with the trisialo ganglioside GT1b (net negative charge) was used as the positive control 14. Each binding assay was repeated a minimum of three times, independently.
Immunofluorescent staining
Cells (1×106 cells in 100 μL PBS) were chilled to 4°C in 0.1% w/v BSA in PBS, which was mock-treated or treated with GSPL at an appropriate concentration for 30 min at 37°C, before being washed in ice-cold PBS. For surface staining, cells were incubated with 2.4G2 FcRγ blocking Ab (1 μg/106 cells), followed by staining with biotinylated-anti-mCD1d antibody 1B1 (1 μg/106 cells) (Anti-CD1d antibody was biotinylated using Pierce EZ biotinylation kit), PE-anti-mouse-I-Ad (1 μg/106 cells) or FITC-CTX-B (0.2 μg/106 cells) respectively for 1 h. PE-conjugated streptavidin was used to detect biotinylated 1B1. Cells were washed three times in ice-cold PBS (200 g, 5 min, 4°C) before fixing in 1.0% paraformaldehyde in PBS. Background staining was determined using FITC-conjugated isotype control Ab.
For intracellular staining of mCD1d, surface-stained cells were further treated with excess unlabeled cold 1B1 (10 μg/mL), washed thoroughly and re-suspended in BD Cytofix/Cytoperm Plus solution for 20 min at 4°C, and then stained (30 min) with anti-mCD1d-FITC (anti-CD1d-FITC was prepared using the Pierce FITC labeling kit). Samples were analyzed using a FACS Calibur flow cytometer (BD Bioscience) and analyzed with CELLQuest software (BD Bioscience).
Conjugate formation and cell staining
For studying the conjugate formation, 2×105 BMDCs, either treated or untreated with 10 μg GSPL/105 cells, were pulsed with 10 μg/mL GSL for 6 h at 37°C. GSL-pulsed cells were incubated with 2×105 NKT cells for 45 min at 37°C/5% CO2 in conical tubes containing 1 mL of pre-warmed culture medium. Cells were briefly centrifuged before fixation to ensure that the cells had sedimented before decanting excess medium. Conjugates were then fixed and stained. FITC-CTB-X was used to stain GM1. CD1d was labeled with biotinylated anti-CD1d and then streptavidin-PE. Fixed conjugates were imaged by confocal microscopy. GM1 or CD1d was defined as clustered at the IS if the intensity at the intracellular contact was twice that of the unconjugated membrane. Contacts were defined as areas where co-localization of both markers occurs between two cells. Clustering of CD1d and GM1 at the IS was judged by yellow staining at the intracellular contact. It was confirmed that the secondary Abs used did not show any non-specific binding to the cells in the absence of primary Ab (data not shown).
Intracellular calcium mobilization in DN32.D3 cells
Intracellular calcium mobilization in DN32.D3 cells was monitored in response to GSPL-treated and untreated BMDC(I) using fura 2-AM 49. In brief, 1×106 BMDCs were incubated with 6 μM fura 2-AM for 1 h at 37°C in the dark with gentle shaking. Excess dye was removed by washing and Fura 2-loaded cells were resuspended to a final density of 1×106 cells/mL in HBSS containing 0.5 μM EGTA. Fluorescence was measured at λex 1=340 nm, λex 2=380 nm and λem=510 nm at a bandwidth of 10 nm, and the data were represented as the relative ratio of fluorescence excited at 340 and 380 nm.
Confocal microscopy
Cells were mounted on poly L-lysine-coated chamber slides and analyzed using a Zeiss LSM 510 laser-scanning confocal microscope. Images were analyzed using Adobe Photoshop software (Mountain View, CA, USA).
Membrane preparation and fractionation
Membrane fractionation was carried out according to the method of Prieschl et al. 50. A total of 4×106 LD-infected BMDCs (mock-treated or [3H]GSPL-treated) cells were lysed and loaded onto a step wise sucrose gradient of 80, 60, 40 (containing the cell lysate), 30, 20 and 10% sucrose (2 mL of each).
SDS-PAGE and western-immunoblot analysis
Representative amounts of each fraction from the sucrose gradient were analyzed by SDS-PAGE on a 10% acrylamide gel (Laemmli). Separated proteins were transferred on to a nitrocellulose membrane for immunoblot analysis. Membranes were blocked in 5% w/v milk in TBS-Tween (0.05% Tween 20 in 10 mM Tris/100 mM NaCl, pH 7.5), incubated with the primary Ab (anti-CD1d or anti-MHC class II), followed by incubation with HRP-conjugated goat anti-rat IgG secondary Ab. Specific interactions were revealed by using the Pierce ECL (enhanced chemiluminescence) detection system (Thermo Scientific).
Analysis of lipid-raft-associated GM1
Lipid-raft-associated GM1 was determined using an enzyme assay as described by Blank et al. 51. Cells (4×106) labeled with 500 m-units of CTB-X-HRP were subjected to sucrose density gradient as described above and the fractions assayed using the chromogenic peroxidase substrate, ABTS (2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonic acid)).
Statistical analysis
A paired two-tailed Student's t-test was used for statistical analysis of the data. Differences between means were assessed for statistical significance and p values of <0.05 were considered statistically significant.
Acknowledgements
The authors thank Prof. Randy R. Brutkiewicz, Indiana University School of Medicine, USA for his gift of DN32.D3 hybridoma, Dr. M. Kronenberg, La Jolla Institute for Allergy and Immunology, San Diego, CA for the gift of 1B1 hybridoma and Mr. Pranab Dhar for efficient technical support. This work was partly supported by The Department of Science and Technology, Government of India (Grant number, SR/SO/HS-46/2004 and SR/SO/HS-52/2007). S. K. and J. P. are the recipients of the Council of Scientific and Industrial Research fellowship.
Conflict of interest: The authors declare no financial or commercial conflict of interest.