The chicken Ig light chain 3′-enhancer is essential for gene expression and regulates gene conversion via the transcription factor E2A
Abstract
Expression of the rearranged chicken immunoglobulin light chain (IgL) gene is regulated by a V gene promoter, a matrix attachment region (MAR) in the J-C intron and an enhancer downstream of the Ig constant region. Using knockout analysis, we demonstrate that the 3′-enhancer is not only required for gene activation but is also essential for the maintenance of gene expression. Deletion of the MAR on the other hand increases IgL transcription, indicating that the MAR acts as negative regulator. We demonstrate that Id1 and Id3, dominant-negative regulators of basic-region helix-loop-helix (bHLH) transcription factors, are able to reduce chicken IgL 3′-enhancer activity in transient assays and strongly reduce the rate of gene conversion (GC) in DT40 clone 18 cells. Conversely, overexpression of avian E47, a bHLH transcription factor, leads to a dramatic increase in GC rates independent of IgL or activation-induced cytidine deaminase RNA levels. Thus, E47 is the first transcription factor to activate GC without an apparent increase in transcription.
Abbreviations:
-
- AID:
-
activation-induced cytidine deaminase
-
- bHLH:
-
basic-region helix-loop-helix
-
- GC:
-
gene conversion
-
- IgL:
-
immunoglobulin light chain
-
- MAR:
-
matrix attachment region
-
- SHM:
-
somatic hypermutation
Introduction
In order to be functionally expressed, Ig genes undergo a number of gene modification events. They are rearranged to generate diversity and they undergo class switching to elicit an immune response that is appropriate to the invading pathogen. In addition, they can undergo diversification of the already rearranged gene, either by gene conversion (GC) or by somatic hypermutation (SHM). Different species have evolved to use these mechanisms for different purposes. In mouse and humans, rearrangement generates the majority of the diversity of the naive immune repertoire. SHM is then used to generate further diversity from which antibodies with increased specificity can be selected in the secondary immune response 1. In the chicken on the other hand, a single functional V gene is recombined with a single J fragment to give a functional Ig with very limited diversity 2, 3. The immune repertoire is generated subsequently by a process of GC in which sequences from upstream V pseudogenes are copied into the functional Ig locus 4.
The regulation of all the gene modification events at the Ig loci is closely coupled to the regulation of gene expression, and the enhancer and promoter elements regulating transcription in different species are highly conserved. Like in the human and murine immunoglobulin light chain (IgL) loci, the chicken IgL locus contains a B cell-specific promoter and a 3′-enhancer 5–7. A matrix attachment region (MAR) but no enhancer has been identified in the J-C intron 8, making the avian IgL more reminiscent of the Igλ rather than the Igκ locus. Furthermore, the chicken V-J intron contains a silencer and a rearrangement-activating sequence whose combined action regulates rearrangement 5, 7, 9. While the recombination activation sequence is also present in the mouse, the silencer is only found in chicken, where it is thought to be required because the avian IgL locus is more compact, spanning only approximately 20 kb, placing the non-rearranged V and J elements in relatively close proximity. Most of the analysis of the regulatory regions in the chicken has been carried out by dissecting these sequences and testing them in transfection or transgenic mouse assays. As yet, no knockout analysis has been carried out in chicken to examine the function of these elements in their normal genetic context. In this paper, we describe the deletion of the IgL MAR and the IgL 3′-enhancer in the chicken B cell line DT40. We find that the MAR is a negative regulatory element, whereas the 3′-enhancer is essential for gene expression.
Although the role of transcription in regulation of SHM and GC has been studied in some detail 1, 10, it has been difficult to pinpoint the exact role of the multiple Ig enhancers. The chicken IgL locus is much more compact, with only a single enhancer. It therefore lends itself as a model system to study the regulation of GC or SHM. Both processes are initiated by the enzyme activation-induced cytidine deaminase (AID) 11–13, which functions to introduce the primary lesion into the DNA. This lesion is subsequently repaired by distinct repair pathways leading either to SHM or GC. Thus, in the absence of either homologous repair enzymes 14 or the homologous recombination substrate 15, DT40 cells no longer carry out GC but instead diversify their genes by SHM. This argues strongly that the initial events and the regulation of these two processes are the same. A potential candidate for regulating GC is the transcription factor E2A, which is highly conserved between human, mouse and chicken 16, 17. In the mouse, this transcription factor is essential for B cell development. In E2A knockout mice, B cell development arrests prior to Ig gene activation 18. Nevertheless, E2A is of great importance throughout B cell ontogeny and it has been shown to play an important role in both gene rearrangement 19 and class switching 20. Indirect evidence also supports a role for E2A in the regulation of SHM 21. Within the chicken IgL 3′-enhancer, multiple E2A consensus binding sites exist, although these have not yet been tested functionally. In this paper, we have used the dominant-negative regulators of E2A, Id1 and Id3 22, to reduce IgL 3′-enhancer activity. We find that reduced enhancer activity down-regulates GC. Furthermore, we demonstrate that the transcription factor E47 plays a crucial role in activating GC, since its overexpression leads to a dramatic increase in GC frequencies.
Results and discussion
Deletion of the MAR in the chicken IgL locus
A knockout construct for the MAR (Fig. 1A) was transfected into DT40 cells; neomycin-resistant clones were selected and analysed for homologous recombination. DT40 cells contain a rearranged and a non-rearranged Ig allele, which, after Pst I digestion, yield a 3.0- and a 2.4-kb fragment using an IgL constant region probe in Southern blot analysis (Fig. 1B). Insertion of the neomycin gene at the rearranged allele leads to the formation of a 1.7-kb band instead of the 3.0-kb band. Homologous recombinants were analysed for IgM surface expression by FACS (Fig. 2A). Insertion of the neomycin gene in place of the MAR generated an IgM– phenotype. This is not surprising since the neomycin gene introduces a stop codon, which causes premature termination of transcription leading to a non-functional IgL message. To assess the effect of deleting the MAR without interference from the inserted neomycin gene, we stably transfected these cells with a tamoxifen-inducible form of Cre recombinase. Upon induction of the recombinase, approximately 15% of cells had become IgM+. These were likely to be the cells in which the Cre recombinase had catalysed the excision of the neomycin gene, leaving only the loxP sites in its place. To test this, IgM-positive and -negative cells were sorted and examined for their Ig genotype. Fig. 1C shows that indeed the IgM-positive cells had lost the neomycin gene, while the IgM-negative cells retained it. This phenotype remained stable after several weeks of culture. The level of surface expression in the Ig-positive population was very similar to that of the parental DT40 cells (Fig. 2A). However, we wished to examine RNA levels and utilised real-time PCR to quantitate IgL expression after deletion of the MAR. Fig. 2C shows that RNA levels are strongly reduced after the insertion of the neomycin gene (MAR neo). Similar expression levels were seen in cells that had been induced by tamoxifen, but failed to recombine out their neomycin gene (Tmx–). However, there was an approximately twofold increase in the levels of IgL RNA in MAR knockout clones in which the neomycin gene had been removed (MAR del) (Fig. 2C). Hence, the MAR in the chicken light chain locus acts as a negative regulator. This is in keeping with findings in the murine system where the MAR also acts as a negative regulator, lowering the probability of premature Igκ rearrangement 23.
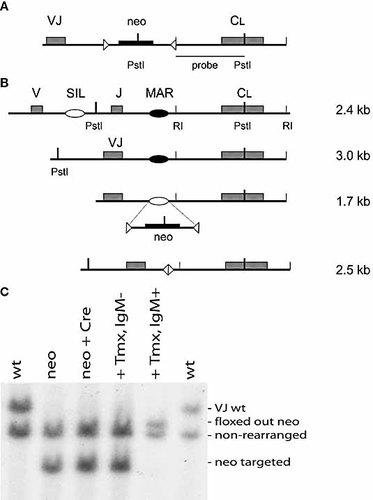
Creation of MAR knockout clones. (A) Schematic representation of the knockout construct. (B) Cartoon of the endogenous unrearranged, rearranged, targeted and Cre-recombined IgL locus, indicating fragment sizes obtained after a Pst I digestion. (C) Southern blot analysis of the clones generated: wild type (wt), neo targeted (neo), transfected with CRE recombinase (neo CRE) and IgM-negative and -positive populations sorted after CRE induction by tamoxifen (Tmx).
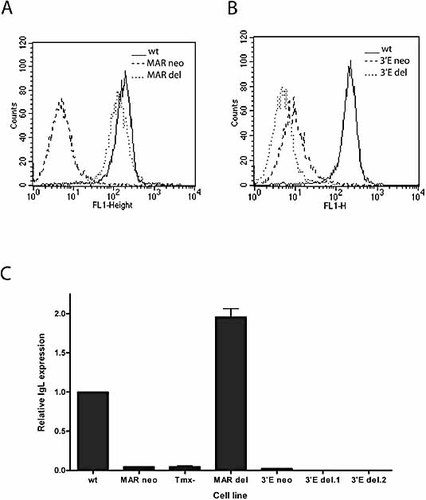
Ig expression in targeted clones. FACS analysis of IgM surface expression of (A) MAR knockout clones as indicated and (B) IgL 3′-enhancer knockout clones. (C) Real-time PCR analysis of IgL gene expression of targeted clones. Values were normalised to β-actin gene expression and are given relative to expression levels in the parental DT40 cells (wt).
Deletion of the 3′-enhancer in the chicken IgL locus
Next, we examined the effect of deleting the 3′-enhancer. The knockout construct (Fig. 3A) was stably transfected into DT40 cells and homologous recombinants were identified by Southern blotting. Using the restriction enzymes Bam HI and Sac I, the non-rearranged and the rearranged IgL alleles give rise to a 7.4- and a 6.2-kb band, respectively (Fig. 3B). Insertion of the neomycin gene into the non-rearranged allele would yield a 4.9-kb band, although no such band was seen in any of the clones analysed. Deletion of the 3′-enhancer by neomycin insertion into the rearranged allele leads to the formation of a 6.2-kb band, which is the same size as the non-rearranged allele. The creation of a homologous recombinant (3′E neo) is therefore characterised by the loss of the 7.4-kb band. To detect recombination between the loxP sites after the induction of Cre recombinase expression, we used PCR to amplify the enhancer regions with the primers shown (Fig. 3B). The WT locus gives rise to a single 748-bp PCR product, while the loxP recombinant (3′E del) gives rise to an additional band of 321 bp (Fig. 3D). No detectable PCR product was generated from alleles containing the neomycin gene, as the resultant fragment is too big to be amplified efficiently. Using FACS to assess IgM expression (Fig. 2B), we determined that replacement of the enhancer by neomycin (3′E neo) was sufficient to give rise to an IgM– phenotype. After deletion of the neomycin gene by Cre recombinase, these cells remained IgM–. In fact, somewhat reduced surface expression is observed when compared to the neomycin-containing knockout (Fig. 2B). Real-time PCR analysis of IgL expression levels (Fig. 2C) revealed that the differences seen in the FACS analysis are also apparent at the RNA level. Upon insertion of the neomycin gene (3′E neo), transcription was much reduced, falling to 1/50th of the WT IgL. Once the neomycin gene had been removed, expression was reduced another 100-fold in two independent Cre transfectants (3′E del.1 and 3′E del.2). Presumably, the presence of the promoter driving the neomycin gene is able to maintain low levels of expression. In its absence, the locus becomes fully inactive. In future analyses, it will be interesting to determine whether or not deletion of the 3′-enhancer leads to full chromatin condensation. In conclusion, our experiments have shown that the 3′-enhancer is essential not only for the establishment of an active locus, but also for maintenance of IgL gene expression in a mature B cell.
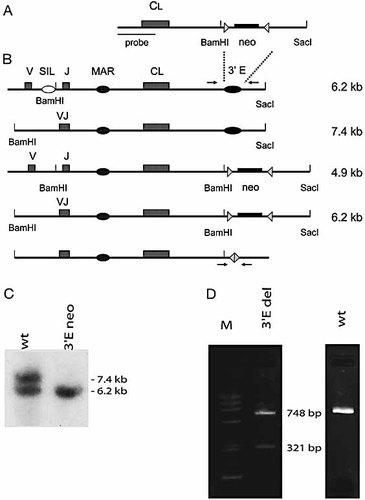
Creation of 3′-enhancer knockout clones. (A) Schematic representation of the knockout construct. (B) Cartoon of the IgL locus showing the sizes of the unrearranged, rearranged and neomycin-targeted alleles after Sac I × Bam HI digestion. Arrows denote the PCR primers used to identify Cre-recombined clones. (C) Southern blot analysis of WT and targeted clones. (D) PCR analysis to identify clones in which the neomycin gene has been deleted.
The 3′-enhancer contributes to the regulation of GC
In the mouse model, deletion of the intron or the 3′ enhancer does not stop surface expression of Ig. This means that it has been possible to assess the role of the intron and 3′-enhancer in regulating the levels of somatic hypermutation 24, 25. We have demonstrated above that deletion of the IgL 3′-enhancer leads to a more than 1000-fold reduction in transcription. There is now extensive evidence that transcription is required for SHM or GC to occur (reviewed in 10). Thus, in order to examine the role of the IgL 3′-enhancer in the regulation of GC, we sought to reduce rather than abolish enhancer activity. Within the chicken 3′-enhancer, five CANNTG consensus sites for basic-region helix-loop-helix (bHLH) proteins have been identified. We wondered whether overexpression of the dominant-negative Id proteins would suffice to diminish enhancer activity. First, we carried out transient transfection experiments in mouse A20 cells. Fig. 4 demonstrates that co-expression of either mouse Id1 or Id3 will reduce enhancer activity by 50%, indicating that bHLH proteins contribute significantly to enhancer function. Overexpression of the Id proteins is therefore a valid approach to reduce 3′-enhancer function. For a convenient GC assay, we utilised clone 18 DT40 cells. This cell line transcribes its IgL gene at high levels, but is IgM surface negative since the light chain gene carries a frameshift nonsense mutation 26. Upon GC, this lesion is repaired using upstream pseudogenes and the cells revert to being IgM surface positive. Therefore, in clone 18 DT40 cells, GC can easily be quantified by measuring the appearance of IgM-positive cells. We generated stable Id1 and Id3 transformants of clone 18 cells. RT-PCR analysis of selected subclones demonstrated that Id1 and Id3 are transcribed (Fig. 5). In the case of Id1 where commercial antiserum is available, we confirmed by Western blotting that Id1 protein is present in the cells (data not shown). Subsequently, fluctuation analysis was carried out for WT clone 18 cells and two independent Id1- and Id3-expressing clones. Individual subclones were cultured for 4 wk and then analysed for IgM surface expression. Fig. 6A shows that in the presence of Id1 and Id3, the appearance of IgM-positive clones is reduced fivefold. Id3 transfectants appear to be more closely clustered. However, statistical analysis revealed that this spread is not significantly different from that seen for Id1 transformants. Id proteins have been reported to exert their effect via changes in the cell cycle 22. To ensure that reduced GC is not simply due to reduced proliferation rates, we measured cell growth in DT40 clone 18 and the Id1 and Id3 transformants. As Fig. 6B demonstrates, no changes in cell doubling times were observed. Taken together, our data indicate that the 3′-enhancer regulates GC, which is reduced by Id protein overexpression.
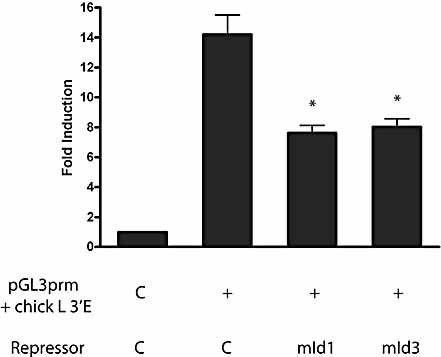
Inhibition of chicken IgL 3′-enhancer function by murine Id1 and Id3 proteins in A20 cells. Values are given relative to those obtained with the pGL3prm control vector. The data shows the means ± SEM of three to six independent transfections. * p ⩽0.01 vs. the pGL3prm + chick IgL 3′E vector.
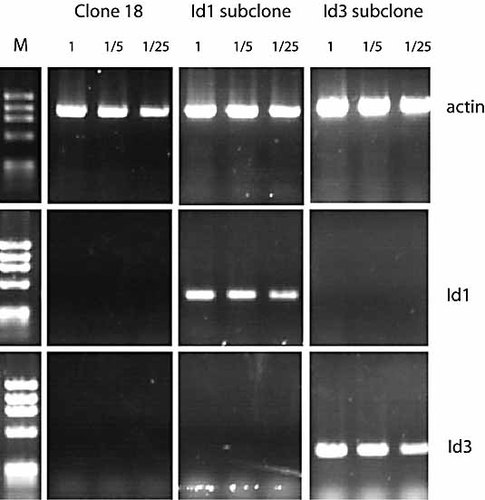
PCR analysis of murine Id1 and Id3 transcription in clone 18 subclones. cDNA dilutions were used as indicated. β-Actin was amplified as a control. M, PhiX Hae III DNA markers.
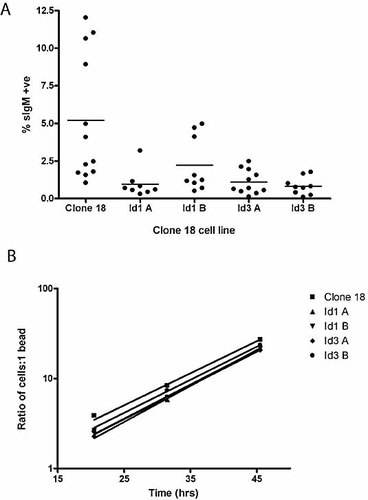
(A) Fluctution analysis for clone 18 DT40 cells stably transfected with Id1 or Id3. Values are given as the percentage of surface IgM-positive cells after culturing individual surface IgM-negative subclones for 1 month. Single surface IgM-negative cells were sorted from clone 18 and two independent Id1- and Id3-expressing clone 18 lines on a Mo-Flo cell sorter. (B) Analysis of cell doubling time for the clones analysed in (A).
E47 strongly up-regulates GC
The major target of Id proteins in B cells is E47, which is inducible in mature B cells 20, 27. We have previously demonstrated that Id1 and Id3 are able to inhibit chicken E47 17, which is the most likely mediator of the effect observed here. As Id acts as a negative regulator of GC, we wondered whether transfection of E47 would suffice to activate GC. A chicken E47 expression vector 17 was stably transfected into clone 18 cells and fluctuation analysis was once again used to assay GC rates. As shown in Fig. 7A, E47 had a dramatic effect on clone 18 cells. In individual E47-expressing subclones, up to 90% of cells became IgM+ within the 4 wk of our analysis. The average rate of GC increased from 5% in clone 18 cells to 35% in clone 18 + E47 cells. Two additional, independent transfectants expressing E47 yielded average conversion rates of 31% and 34%. Once again, the growth rates of E47-expressing cells were similar to those of untransfected cells. Thus, E47 is a strong positive regulator of GC.
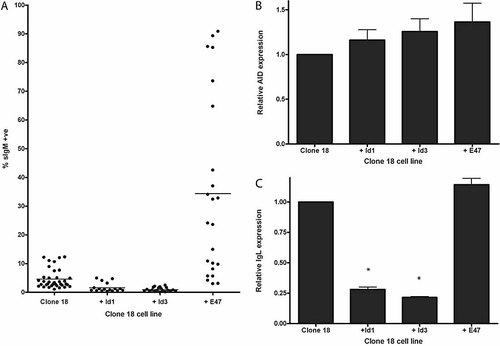
(A) Fluctuation analysis for clone 18 DT40 cells stably transfected with avian E47 and mouse Id1 or mouse Id3. Percentages of IgM-positive clones after 4 wk of culture are given. (B, C) Real-time PCR analysis of AID gene expression (B) and IgL gene expression (C) in representative transfectants of mouse Id1 and Id3 and avian E47. Values were normalised to β-actin gene expression and are given relative to expression levels in clone 18 cells. The data shows the means ± SEM from PCR carried out in triplicate on three independent mRNA for each line. * p ⩽0.01 vs. clone 18 line.
We wondered whether indirect pathways might be responsible for the effects of Id and E47 on GC. Id3 has previously been reported to prevent the induction of AID gene expression in mouse primary B cells, while E47 activated expression 28. We therefore used real-time PCR to analyse whether chicken AID gene expression is affected by the overexpression of Id or E47 in DT40 cells. We find that, relative to WT clone 18 cells, AID expression levels in the Id1, Id3 and E47 transformants are unchanged (Fig. 7B). Although there appears to be a small up-regulation in all three transformants, statistical analysis of the data using an unpaired t-test indicates that the increase is not significant, yielding p values of 0.36, 0.19 and 0.11 for Id1, Id3 and E47, respectively. Furthermore, we see the same increase for both the Id and E47 transformants while of course GC is down-regulated in the Id- and up-regulated in the E47-expressing clones. We conclude that AID gene expression in the chicken is not significantly affected by the levels of Id1, Id3 or E47 expressed in our system. Several factors could explain the apparent discrepancy between our results and those obtained in murine cells 28. Firstly, the chicken AID gene may be regulated by distinct sequences. Secondly, mouse Id proteins may be less effective in repressing chicken rather than mouse E47. Alternatively, the protein level of Id in our experimental system may not suffice to see a reduction in AID expression levels, although this is unlikely since the expression levels suffice to strongly affect GC. Furthermore, recent reports indicate that transcription factors other than E47 act as critical regulators of AID gene expression. AID gene activity correlates with occupancy of the Pax5 but not the E2A binding sites in this gene 29. In addition NF-κB and STAT-6 have been shown to regulate AID gene expression in activated human B cells 30.
Previous results in cell lines have indicated that SHM in cell lines correlates directly with the rates of transcription of the Ig genes 1, 10. We therefore wished to examine whether the changes in GC observed here correlate with IgL transcription rates. Real-time PCR on the IgL gene demonstrates that both Id1 and Id3 transformants displayed a fivefold decrease in IgL transcription (Fig. 7C). This result is consistent with our transient transfection data showing that Id reduces enhancer activity. Interestingly, reduced expression did not lead to significant changes in surface expression. Presumably, other factors, which operate independently of IgL message levels, control the levels of Ig surface expression. For example, Ig heavy chain, the Igα or Igβ proteins, which are required for Ig surface expression, could be limiting. Our findings that Id overexpression reduces IgL RNA levels in chicken cells are entirely consistent with data obtained in other systems. For example, IgL gene expression is reduced in Id1-transgenic mice 31. The down-regulation observed in the stable transformants is higher (fivefold) than that seen in transient transfections (twofold). One reason for this may lie in the ability of E2A to modify chromatin. E2A is able to interact directly with the SAGA complex, which catalyses histone acetylation 32. This would have a more pronounced effect in stable transfections where the introduced DNA is assembled into higher-order chromatin. In contrast, transiently transfected DNA is unlikely to have acquired such a structure and the effects would not be seen. Secondly, we cannot rule out that there are additional elements within the IgL gene that can respond to bHLH proteins, although no such binding sites have been described.
We next wondered whether the increase in GC after E47 transfection is once again mirrored by IgL transcription rates. As Fig. 7C demonstrates, overexpression of E47 did not cause a significant increase in IgL RNA levels. This is surprising since E47 is thought to be a strong transcriptional activator in mature B cells and there are five potential E2A binding sites within the IgL 3′-enhancer. However, these results are entirely consistent with transient transfection experiments carried out in our laboratory. Using either A20 or HEK293 cells, we were unable to up-regulate IgL 3′-enhancer activity by cotransfection of murine or chicken E47 or E12 (data not shown). These data indicate that E47 protein levels in the cell are not limiting for Ig transcription in mature B cells. In conclusion, our experiments demonstrate that E47 is an important positive regulator of GC. E47 is the first transcription factor identified as an activator of GC whose function does not depend on increasing the rate of transcription.
Upon transfection of E47, GC rates increased sevenfold. We wondered whether such a large increase was due to an increase in the number of cells attempting GC or whether there is an increase in the number of GC events per V gene. To this end, we sequenced the V genes of three clones of unsorted clone 18 + E47 cells, which had been cultured for 4 wk. These clones had become 40.2%, 85% and 90.8% IgM surface positive. GC events are shown in Fig. 8. Our data clearly demonstrate that the increase in surface IgM-positive cells correlates with an increase in the number of GC events per V gene sequence. While the data set is relatively small, there is no doubt that the number of GC events per sequence is dramatically higher than those obtained with unsorted, untransfected clone 18 cells, where less than five conversion events would be expected per 100 sequences.
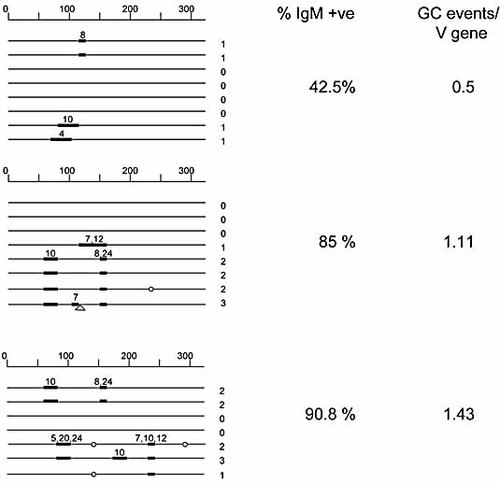
GC tracts identified in three independent clones of DT40 clone 18 + E47 cells. Sequences were obtained from unsorted cells grown for 4 wk. Conversions are shown relative to the consensus obtained amongst each sequence set. This is likely to under- rather than overestimate the number of conversions identified. Conversions (black bar) contain at least two bp changes with a minimum of 9 bp homology to the assigned pseudogene. The most likely GC donor is shown above each bar. Single mutations (circle) and untemplated insertions (open triangle) are also shown.
Our results show that Id reduces both GC and transcription, while E47 increases GC but does not alter Ig message levels. One reason for this differential regulation may be the ability of Id proteins to interact with other families of transcription factors such as Ets and Pax proteins 29, 33, 34. Extensive functional analysis has not been carried out on the IgL 3′-enhancer and it may well be possible that members of the Pax and Ets families contribute to IgL 3′-enhancer activity. Interference with these could cause reduced Ig transcription. Furthermore, the differential regulation of GC and gene transcription could be achieved if E47 is not limiting for Ig transcription but is limiting for GC. In this scenario, Id would reduce expression through a combination of inhibiting E47 and other transcription factors. Reduced transcription levels would then correlate with reduced GC. Upon overexpression of E47, transcription levels would not increase since E47 is not limiting, yet GC would increase in line with higher E47 levels. Similarly, Storb and colleagues observed that the inclusion of a CANNTG consensus bHLH binding site in a synthetic V transgene increased the level of somatic hypermutation in this substrate without a notable increase in transcription rates 21, supporting the notion that distinct mechanisms regulate transcription and gene modification. However, it is also possible that E47 does increase the rate with which RNA polymerase is recruited. This could lead to increased recruitment of AID, introducing lesions that render the newly transcribed message unstable. The steady state level of the Ig RNA would therefore not change. However, this is unlikely since treatment of clone 18 cells with trichostatin A increases GC with a concomitant increase in transcription 35, suggesting that increased rates of transcription would be detectable in DT40 clone 18 cells. We therefore favour a model whereby the recombination in GC can be activated independently of transcription. In line with this model, deletion of the recombinase-activating sequence in the mouse Igκ locus reduces κ rearrangement without affecting the levels of transcription 9.
As yet, we do not understand the mechanism by which E47 acts to increase GC. We have excluded the possibility that the observed effect is due to an increase in AID expression and have demonstrated that IgL message levels do not change. E47 may also act directly to recruit the AID complex to the locus. Such an interaction may require intermediary proteins. Alternatively, E47 may exert its effect through changes in the chromatin structure. Effects of E47 on chromatin structure are well documented 32, 36, 37, although we do not as yet understand how changes in chromatin structure affect AID activity. There is evidence that another epigenetic modification, namely DNA demethylation, is associated with increased mutability 38, suggesting that other changes in chromatin structure may also affect GC and SHM.
Concluding remarks
In this paper, we demonstrate that the IgL 3′-enhancer is essential for maintenance of gene expression in a mature B cell and plays an important role in the regulation of GC. This is in sharp contrast to the situation in mice and humans where the regulation of Igκ or Igλ gene expression depends on multiple elements. We therefore believe that the chicken IgL locus is a very good model to further elucidate the regulation of gene diversification, especially as DT40 cells carry an already rearranged Ig gene. We also demonstrate that E47 is a strong, positive regulator of GC. E47 is the first transcription factor to be identified as an activator of GC whose function does not appear to depend on increasing the rate of transcription. Moreover, it is able to exert its effect on the endogenous locus rather than on a synthetic reporter gene. Overexpression of E47 may have important biotechnological applications when using GC or SHM to select high-affinity antibodies. Our results also underscore the importance of E2A in all aspects of Ig gene expression, as E2A has been shown to play a critical role in gene expression and rearrangement, class switching and now in diversification of the rearranged gene. We believe our findings are likely to be relevant to the regulation of both GC and SHM since the initiating events of the two processes are the same.
Materials and methods
Plasmids
To generate the MAR knockout vector, the rearranged IgL V gene was amplified by PCR using primers P1 and P2 (see Table 1 for all primer sequences). The flanking restriction enzyme sites Xho I and Sal I were used to subclone the resultant 1.2-kb fragment into a pBSII(KS)+ vector, in which the Hind III site had been replaced by a Bgl II site. Into this vector, we inserted a 2.5-kb genomic Eco RI fragment spanning the CL region of the IgL gene. A Bam HI fragment carrying the neomycin gene with flanking loxP sites (obtained from J. M. Buerstedde) was subsequently inserted into the Bgl II site (see Fig. 1A). To generate the 3′-enhancer knockout construct, the primer pairs P3/P4 and P5/P6 were used to amplify the sequences 3′ and 5′ of the IgL 3′-enhancer. These were inserted into the pBSII(SK+) vector using the restriction sites engineered into the primers. The floxed neomycin gene was inserted into the Bam HI site of the resultant vector. A 2.5-kb Eco RI fragment spanning the CL gene was subsequently inserted into the 5′ Eco RI site of our construct.
|
Primer |
Sequence |
|---|---|
|
P1 |
ctc gag atg gcc tgg gct cct ctc |
|
P2 |
gtc gac ccc aag gac act gtg tc |
|
P3 |
aag ctt gca ggc att cat gac ac |
|
P4 |
gga tcc cgg ggc tat tct gag |
|
P5 |
gga tcc aca ctg ctc cat ccg tgt c |
|
P6 |
gag ctc atg ccc ggg ccc acg ctg |
|
P7 |
gag ctc gaa ggc aca gcg ctg |
|
P8 |
aga tct gcg tgg tgg gag cgg gc |
|
P9 |
cat ggt aca gct cca tca caa gag |
|
P10 |
ctg ctc tgc att ttg ggg cat ctc |
|
P11 |
gag gag ctg aac gaa gcc acc |
|
P12 |
tgc agg tgt agg tct cgt ggc ttg |
|
P13 |
gtt tct gtg cac cag agg gct gaa cag tca |
|
P14 |
ctc ctt tct tgg ctg ggt gag agg tcc ata |
|
P15 |
ccc caa gct tac tcc cac agc cag cca tgg |
|
P16 |
ggc tct aga tag tcc gtc agg tca cgg cca |
|
P17 |
tca gcg aca caa gat gcg |
|
P18 |
atg aag gtc gcc agt ggc ag |
|
P19 |
atg aag gcg ctg agc ccg gtg |
|
P20 |
tca gtg gca aaa gct cct ctt g |
|
P21 |
gtg ctg tgt tcc cat cta tcg t |
|
P22 |
tgg aca atg gag ggt ccg gat t |
In order to introduce the Cre recombinase into the knockout cell lines, we subcloned the Hind III fragment of the pANmerCREmer vector 39 into the Hind III site of the pcDNA3.1/hyg vector (Invitrogen). Chicken E47 and mouse Id1 and Id3 expression constructs have been described 17. The chicken IgL 3′-enhancer was amplified using primer pair P7/P8, subcloned into the pCR2.1TOPO vector (Invitrogen) and then transferred to the pGL3promoter luciferase reporter vector (Promega) using Sac I and Bgl I.
Transfection and cell culture
Subclones of the chicken bursal B cell line DT40 26, 40 were maintained in RPMI medium with 7% foetal calf serum and 3% chicken serum, penicillin and streptomycin, and 50 µM β-mercaptoethanol. The subclone used carries a heterozygous mutation of the XRCC2 gene. Heterozygocity in this locus does not have any known phenotype with regard to gene expression. For stable transfection, 1 × 107 cells were electroporated (500 V, 25 µF) with 20 µg linearised plasmid and selected using 2 mg/mL Geneticin or 1.0–1.4 mg/mL hygromycin. Induction of Cre expression was carried out at 100 nM 4-hydroxy-tamoxifen for 3 days. Homologous recombinants were identified either by Southern blotting (Maniatis) or by PCR using primer pair P9/P10. A20 cells (mouse B lymphocytes) were maintained and transfected as described 17 using 10 µg reporter plasmid, 10 µg effector plasmid and 5 µg of the internal control vector CMV-β-gal. Luciferase and β-galactosidase assays were carried out as reported 17.
FACS analysis
Cells were stained with FITC-conjugated goat anti-chicken IgM antibody (Bethyl Laboratories), at a dilution of 1 : 100. The percentage of FITC-positive whole cells (as determined by forward scatter and side scatter) from a total of 10 000 events was determined using a FACSCalibur (BD) analyser. FITC-conjugated goat anti-IgM antibody-stained cells were also sorted on a Mo-Flo cell sorter (Dakocytomation). To determine the rate of cell growth, equal volumes of cells from the various subclones were analysed on a BD-FACSCalibur flow cytometer with an equal volume of BD-Calibrite beads. The number of live cells counted while acquiring 5000 beads was recorded at the time points shown in Fig. 6B, with the ratio of cells to one bead displayed.
PCR analysis
To assess gene expression levels, we isolated RNA on RNeasy columns (Qiagen) and carried out reverse transcription reactions using 2 µg RNA. The RNA was annealed to oligo(dT) and reverse-transcribed using Super RT enzyme (HT Biotechnology, UK). Real-time PCR was carried out using the QuantiTect SYBR Green PCR kit (Qiagen, UK) in a volume of 50 µL. Samples were denatured at 94°C for 15 min. Then, 40 cycles of 15 s denaturation at 95°C, 30 s annealing at 65°C and 30 s extension at 72°C were carried out. The primer pair P11/P12 was used to detect IgL expression and the primer pair P13/P14 to detect AID expression. Values were normalised to β-actin expression detected by primer pair P15/16. Samples were run and analysed on the 7000 Sequence Detection System (Applied Bioscience). Id expression in stably transfected cells was analysed as described 17. The primer pair P17/18 was used to detect Id1 expression, primer pair P19/20 Id3 expression, and primer pair P21/22 β-actin expression.
Acknowledgements
We wish to thank Javier di Noia and Michael Neuberger for cell lines and helpful discussions and Gareth Williams for help with sequencing. This work was funded by the Medical Research Council and the Royal Society.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




