Canagliflozin reduces oral loop diuretic intensification in patients with type 2 diabetes: A participant-level pooled analysis of the CANVAS and CREDENCE trials
ABSTRACT
Aims
The sodium–glucose cotransporter 2 inhibitor canagliflozin reduces the risk of heart failure (HF) hospitalization or cardiovascular death and chronic kidney disease (CKD) progression among patients with type 2 diabetes at high cardiovascular risk or with CKD. Patients with type 2 diabetes commonly have coexisting HF or CKD that require treatment with loop diuretics; however, the prognostic implications of oral loop diuretic intensification are not well characterized.
Methods and results
In this participant-level pooled analysis of the CREDENCE and CANVAS trials (not including CANVAS-R), 1454/8731 (16.7%) patients were treated with loop diuretics at baseline. Over a median on-treatment follow-up of 2.2 years, 1264 patients (14.5%) required oral loop diuretic intensification, of whom 981 (77.6%) required initiation of oral loop diuretics and 283 (22.4%) required oral loop diuretic dose increase. Patients requiring oral loop diuretic intensification experienced rates of subsequent HF hospitalization, CKD progression and mortality that were 29.5-, 5.0-, and 3.5-fold higher, respectively, than those not requiring oral loop diuretic intensification. Treatment with canagliflozin reduced the need for oral loop diuretic intensification by 41% (hazard ratio [HR] 0.59; 95% confidence interval [CI] 0.53–0.66) including both new diuretic initiation (HR 0.65; 95% CI 0.57–0.74) and diuretic dose increase (HR 0.42; 95% CI 0.33–0.54). Inclusion of oral diuretic intensification in an expanded HF composite outcome inclusive of cardiovascular death and HF hospitalization approximately double the number of events, with similar observed treatment effect (HR 0.64; 95% CI 0.58–0.70).
Conclusion
Among high-risk patients with type 2 diabetes, new oral loop diuretic intensification was frequent and portended adverse prognostic significance. Treatment with canagliflozin significantly reduced the need for loop diuretic intensification.
Clinical Trial Registration: CANVAS (Canagliflozin Cardiovascular Assessment Study), ClinicalTrials.gov NCT01032629; CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation), ClinicalTrials.gov NCT02065791.
Introduction
Patients with type 2 diabetes face heightened risk for the development of comorbid cardiovascular and kidney disease including heart failure (HF).1 The sodium–glucose cotransporter 2 (SGLT2) inhibitor canagliflozin has been shown to reduce the risk of HF hospitalization or cardiovascular death among patients with type 2 diabetes at high cardiovascular and kidney risk across a broad range of patient subgroups in the CANVAS and CREDENCE trials, respectively.2, 3 Loop diuretics are a key component for the treatment of HF-related congestion.4 Prior studies have established the prognostic relevance of oral loop diuretic intensification in HF populations.5-7 Loop diuretic use, regardless of HF diagnosis, also identifies individuals at higher cardiovascular risk.8 However, the prognostic implications of oral loop diuretic intensification in type 2 diabetes, particularly those with chronic kidney disease (CKD), are less well studied. Furthermore, loop diuretics when used at high doses, which is more frequently the case in patients with CKD, are associated with deleterious effects and therefore any potential diuretic sparing effect of SGLT2 inhibitors in this population is important to further characterize.9
Worsening HF can manifest across a wide spectrum of clinical events including a HF hospitalization, emergency department visit, or in the outpatient setting via intravenous diuretic administration or intensification of oral diuretics.10 Since outpatient worsening events treated with oral diuretics occur much more frequently than those treated with intravenous therapy, inclusion of oral diuretic intensification as part of an expanded composite endpoint has the potential to increase the number of clinical events and may impact future clinical trial design.
The objective of this analysis was to assess the prognostic implications of oral loop diuretic intensification and the treatment effects of canagliflozin versus placebo on loop diuretic use in high-risk patients with type 2 diabetes. We further assessed the impact of the addition of oral diuretic intensification to an expanded composite cardiovascular outcome in a trial of patients with type 2 diabetes with or at high risk of cardiovascular disease and/or CKD.
Methods
Study design
The study design and primary results of the CANVAS Program and CREDENCE trials have been previously reported.11, 12 The CANVAS Program comprised two parallel companion trials, CANVAS and CANVAS-R, enrolling participants with type 2 diabetes (glycated haemoglobin [HbA1c] ≥7.0% and ≤10.5%) who were aged ≥30 years with established atherosclerotic vascular disease or aged ≥50 years with two or more cardiovascular risk factors.11 Because diuretic dose information was only available in CANVAS but not CANVAS-R, this analysis is limited to the CANVAS trial. CREDENCE included participants aged ≥30 years with type 2 diabetes (HbA1c 6.5% to 12.0%) and CKD (defined as an estimated glomerular filtration rate [eGFR] of 30 to <90 ml/min/1.73 m2 and urinary albumin to creatinine ratio >300 to 5000 mg/g) who were receiving a maximum tolerated dose of renin–angiotensin system blockade for at least 4 weeks prior to randomization.12
Participants enrolled in CANVAS were randomized to receive canagliflozin 100 mg daily, canagliflozin 300 mg daily or placebo, and participants in CREDENCE were randomly assigned to canagliflozin 100 mg or placebo once daily. Institutional ethics committees provided approval for trial protocols at each site with all participants providing written informed consent.
Clinical endpoints
In this analysis, the primary outcome was oral diuretic intensification defined as any new loop diuretic initiation (among those not on loop diuretics at baseline) or loop diuretic dose increase (among those on loop diuretics at baseline). In order to provide a broader picture of treatment effects on the total burden of worsening HF inclusive of outpatient worsening, an extended composite outcome was developed including cardiovascular death, hospitalization for HF or loop diuretic intensification. We also assessed an alternative extended composite outcome inclusive of HF death, hospitalization for HF or loop diuretic intensification. The kidney outcome was defined as a composite of doubling of serum creatinine from baseline sustained for at least 30 days, end-stage kidney disease or death due to kidney failure.
Statistical analysis
Baseline characteristics were compared among patients requiring and not requiring diuretic intensification. Data are reported as mean ± standard deviation, median (interquartile range) for non-normal distributions, and frequency (percentage) for categorical variables. Student's t-test and Pearson χ2 test were used where appropriate. All patients with diuretic dose information were included in this analysis. A total daily dose equivalent to 80 mg of oral furosemide was calculated according to the following equivalency: bumetanide 1 mg, torsemide 20 mg, azosemide 60 mg, and ethacrynic acid 100 mg were considered equivalent to 80 mg of oral furosemide. We evaluated the cumulative incidence of all-cause mortality following loop diuretic intensification relative to those not requiring loop diuretic intensification. The time scale used for patients experiencing loop diuretic intensification was time after loop diuretic intensification and the time scale for patients not requiring loop diuretic intensification was time from randomization. We also assessed the treatment effect of canagliflozin on loop diuretic intensification including loop diuretic initiation and loop diuretic dose increase as well as on an exploratory post hoc expanded composite of cardiovascular death, hospitalization for HF or loop diuretic intensification in time-to-first event analyses using Cox regression and displayed in Kaplan–Meier curves. In a sensitivity analysis, we additionally assessed the treatment effect of canagliflozin on sustained increase in loop diuretic dose of ≥28 days. eGFR was calculated according to the Modification of Diet in Renal Disease equation in the CANVAS trial and the 2009 Chronic Kidney Disease Epidemiology Collaboration equation in the CREDENCE trial.
All analyses were conducted using STATA 17 (Stata Corp., College Station, TX, USA). A p-value of <0.05 was considered statistically significant.
Results
Baseline characteristics
Of the total 8731 patients randomized across the CANVAS and CREDENCE trials, 1454 (16.7%) were treated with loop diuretics at baseline. Among those requiring loop diuretic at baseline, 26.5% had a recorded diagnosis of HF. Compared to patients not requiring oral diuretic intensification (n = 7467, 85.5%) those requiring oral diuretic intensification (n = 1264, 14.5%) were older, and more frequently had a history of prior HF and cardiovascular disease, a longer duration of diabetes as well as lower eGFR and higher level of albuminuria (Table 1).
| No loop diuretic intensification (n = 7467) | Loop diuretic intensification (n = 1264) | p-value | |
|---|---|---|---|
| Age, years | 63 ± 9 | 64 ± 9 | 0.03 |
| Male sex, n (%) | 4919 (65.9) | 849 (67.2) | 0.37 |
| Race, n (%) | 0.05 | ||
| White | 5263 (70.5) | 847 (67.0) | |
| Asian | 1431 (19.2) | 24 (19.1) | |
| Black | 270 (3.6) | 59 (4.7) | |
| Other | 503 (6.7) | 117 (9.3) | |
| HbA1c, % | 8.2 ± 1.1 | 8.3 ± 1.2 | 0.004 |
| BMI, kg/m2 | 31.5 ± 6.1 | 32.8 ± 6.6 | <0.001 |
| Baseline SBP, mmHg | 137.6 ± 15 | 141.2 ± 17.0 | <0.001 |
| Duration of diabetes, years | 14.4 ± 8.2 | 15.6 ± 8.1 | <0.001 |
| Atherosclerotic cardiovascular disease, n (%) | 4016 (53.8) | 753 (59.6) | <0.001 |
| Heart failure, n (%) | 956 (12.8) | 211 (16.7) | <0.001 |
| eGFR, ml/min/1.73 m2 | 68 ± 21 | 60 ± 21 | <0.001 |
| ≥60, n (%) | 4794 (64.2) | 591 (46.8) | <0.001 |
| 45 ≤ 60, n (%) | 1492 (20.0) | 319 (25.2) | |
| <45, n (%) | 1178 (15.8) | 354 (28.0) | |
| UACR, mg/g, median [IQR] | 181 [11–862] | 615 [36–1740] | <0.001 |
| <300, n (%) | 4102 (55.1) | 483 (33.3) | <0.001 |
| ≥300, n (%) | 3347 (44.9) | 778 (61.7) |
- BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; IQR, interquartile range; SBP, systolic blood pressure; UACR, urine albumin to creatinine ratio.
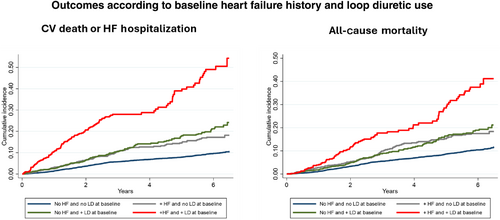
Cardiovascular outcomes according to baseline heart failure history and loop diuretic use
The cumulative incidence of clinical outcomes including the composite of cardiovascular mortality or HF-related hospitalization and all-cause mortality was lowest among patients without a recorded history of HF receiving no diuretics at baseline (Figure 1). This was followed by patients with HF not receiving loop diuretics at baseline and patients without HF receiving loop diuretics, who had a similarly higher risk for all outcomes. Patients with a recorded history of HF receiving diuretics at baseline had the highest incidence of cardiovascular death or HF hospitalization and all-cause mortality.
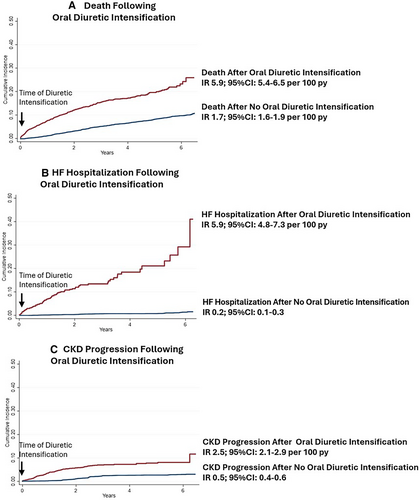
Association between loop diuretic intensification and subsequent outcomes
Over a median follow-up time of 2.2 years, 1264 patients (14.5%) experienced oral loop diuretic intensification of which 981 patients (77.6%) required initiation of oral loop diuretics and 283 (22.4%) required oral loop diuretic dose increase. Patients requiring oral loop diuretic intensification experienced rates of subsequent all-cause death that were 3.5-fold higher (incidence rate [IR] 5.9, 95% confidence interval [CI] 5.4–6.5 per 100 patient-years) than those not requiring oral loop diuretic intensification (IR 1.7, 95% CI 1.6–1.9 per 100 patient-years) (Figure 2). Patients requiring oral loop diuretic intensification also experienced 29.5-fold higher rates of subsequent HF-related hospitalization (loop diuretic intensification: IR 5.9; 95% CI 4.8–7.3 per 100 patient-years vs. no loop diuretic intensification: IR 0.2; 95% CI 0.1–0.3) and 5.0-fold higher rates of CKD progression (loop diuretic intensification: IR 2.5; 95% CI 2.1–2.9 per 100 patient-years vs. no loop diuretic intensification: IR 0.5; 95% CI 0.4–0.6) (Figure 2). Results are presented for each trial separately in online supplementary Figure S1 with a consistent pattern of increase seen across the CANVAS and CREDENCE trials for subsequent all-cause mortality and HF-related hospitalization. The higher rates of CKD progression were driven largely by the higher kidney risk population enrolled in the CREDENCE trial (online supplementary Figure S1).
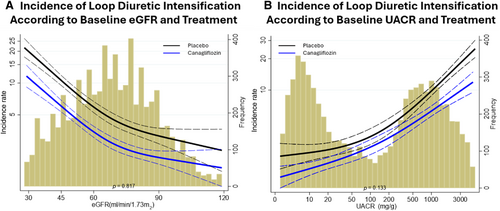
Treatment effect of canagliflozin on loop diuretic intensification and extended composite outcome
Canagliflozin relative to placebo significantly reduced the need for oral loop diuretic intensification by 41% (HR 0.59; 95% CI 0.53–0.66) including both new diuretic initiation (HR 0.65; 95% CI 0.57–0.74, p < 0.001) and diuretic dose increase (HR 0.42; 95% CI 0.33–0.54, p < 0.001) (Table 2). Results remained consistent in sensitivity analysis where diuretic dose increase was defined as an increase sustained for ≥28 days (online supplementary Table S1). The treatment effect on oral loop diuretic intensification was consistent across the spectrum of kidney function when both eGFR and UACR were analysed as continuous variables (Figure 3). The addition of oral loop diuretic intensification to the composite outcome of cardiovascular death or HF-related hospitalization increased the number of events from 859 to 1796, an overall 110% increase. Treatment with canagliflozin resulted in a 36% risk reduction in the extended composite outcome of cardiovascular death, HF-related hospitalization or oral loop diuretic intensification (HR 0.64; 95% CI 0.58–0.70, p < 0.001) (Figure 4). Results remained consistent when assessed by each trial separately (online supplementary Table S2). Results were also consistent when assessing the treatment effect of canagliflozin on the composite of HF death, HF hospitalization or oral loop diuretic intensification (HR 0.60; 95% CI 0.54–0.67) (Table 2).
| Outcome | Canagliflozin | Placebo | Hazard ratio (95% CI) | p-value |
|---|---|---|---|---|
| Oral diuretic intensificationa | 585 | 679 | 0.59 (0.53–0.66) | <0.001 |
| New oral diuretic initiation | 477 | 504 | 0.65 (0.57–0.74) | <0.001 |
| Oral diuretic dose increase | 108 | 175 | 0.42 (0.33–0.54) | <0.001 |
| HF hospitalization | 174 | 194 | 0.67 (0.54–0.82) | <0.001 |
| HF hospitalization or HF death | 189 | 209 | 0.64 (0.53–0.79) | <0.001 |
| Oral diuretic intensification or HF hospitalization or HF death | 660 | 752 | 0.60 (0.54–0.67) | <0.001 |
- CI, confidence interval; HF, heart failure.
- a New diuretic initiation or dose increase.
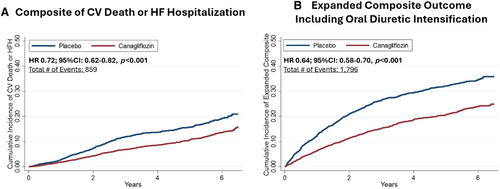
Discussion
In this post hoc patient-level pooled analysis of the CANVAS and CREDENCE trials, among patients with type 2 diabetes with or at high risk of cardiovascular disease and/or CKD, we found that the requirement for oral loop diuretic intensification was a frequent occurrence (1 in 7 patients) and was associated with increased risk of subsequent mortality, HF hospitalization and CKD progression. Furthermore, canagliflozin significantly reduced the need for oral loop diuretic intensification alone and as part of an expanded worsening HF composite outcome. Taken together, these data highlight the prognostic relevance of oral loop diuretic intensification in high-risk patients with type 2 diabetes and the clinical benefits of canagliflozin in preventing diuretic intensification and potentially worsening HF (Graphical Abstract).
The rates of oral diuretic intensification observed in this analysis (~1 in 7 patients) where only 14–15% of patients had an investigator-reported history of HF at baseline, were similar to those observed in contemporary trials of chronic HF including DAPA-HF and DELIVER (~1 in 8 patients).5, 6 Many patients who are prescribed loop diuretics in clinical practice do not carry a diagnosis of HF.13 The extent to which such patients may in fact have unrecognized subclinical HF is an important clinical question. In this analysis, approximately 73.5% of patients prescribed loop diuretics at baseline did not have a recorded history of HF. However, patients receiving loop diuretics at baseline faced higher rates of cardiovascular outcomes irrespective of a history of HF. This is consistent with data from patients with type 2 diabetes enrolled in the EMPA-REG OUTCOME8 trial and highlights potential underdiagnosis of subclinical HF in such high-risk patients with type 2 diabetes.
Outpatient worsening HF characterized by the requirement for oral loop diuretic intensification has been demonstrated to be of prognostic significance in HF populations across the spectrum of ejection fraction.5, 6 Our results extend these findings to patients with type 2 diabetes at high cardiovascular and kidney risk. Furthermore, treatment with canagliflozin significantly reduced such events (across the spectrum of kidney function) underscoring an important incremental clinical benefit of canagliflozin to prevent oral loop diuretic intensification and potential worsening HF in such patients.
The diuretic-sparing effect of SGLT2 inhibitors, now demonstrated across the spectrum of cardio-kidney-metabolic conditions, has potentially important therapeutic implications in CKD. Endothelin receptor antagonists have been shown to reduce CKD progression in type 2 diabetes,14 with ongoing evaluation in a range of glomerular diseases. However, their use has been limited by fluid retention, leading to an increased risk of HF. The observed reduction in diuretic intensification with canagliflozin suggests that SGLT2 inhibitors are likely to enable safer use of endothelin receptor antagonists in CKD. This strategy is being evaluated in the ZENITH-HP trial (NCT06087835), which is evaluating the effect of zibotentan and dapagliflozin versus dapagliflozin alone on a primary outcome of change in eGFR over 24 months in 1500 individuals with albuminuric CKD.
The US Food and Drug Administration standardized definitions for cardiovascular and stroke endpoint events in clinical trials include events requiring intravenous therapies administered outside of hospital settings as a potential cardiovascular endpoint.15 Contemporary HF therapy trials now include such events as part of the primary composite endpoint.16, 17 Since outpatient worsening HF events characterized by oral diuretic intensification occur more frequently than those requiring intravenous diuretic administration, inclusion of such events in the composite outcome may be particularly useful in HF trials. In fact, newer HF Academic Research Consortium expert consensus definitions more broadly characterize outpatient worsening HF events as ‘unscheduled medical contact associated with changes in HF therapy’ and PARADISE-MI adjudicated HF events based on oral diuretic intensification.18, 19
In line with similar data from HF trials, the addition of oral diuretic intensification in an expanded cardiovascular composite endpoint in this pooled analysis more than tripled the total number of events. Such events were prognostically important and modified by treatment with canagliflozin. Indeed, in the FLOW trial, which evaluated the effects of semaglutide in patients with type 2 diabetes and CKD, augmentation of oral diuretic therapy was included as part of the definition of a worsening HF event.20 Taken together, the data suggest a role for adapting oral diuretic intensification for use in a broader cardiovascular endpoint in trials of patients with type 2 diabetes, including those with CKD.
The following limitations should be acknowledged. This was not a pre-specified analysis therefore the findings must be considered hypothesis generating. Oral diuretic intensification events did not undergo formal adjudication. The specific reason for diuretic dose changes were not available and therefore may not simply reflect volume status alone.
Overall, these data highlight oral diuretic intensification as a frequent (occurring in 1 in 7 patients) and prognostically relevant event in the clinical trajectory of patients with type 2 diabetes at high cardiovascular and kidney risk. Furthermore, treatment with canagliflozin significantly reduced oral diuretic intensification assessed alone and as part of an expanded cardiovascular endpoint supporting potential adaption in future clinical trials.
Funding
CANVAS and CREDENCE were funded by Janssen Research and Development. This analysis was supported by an unrestricted publication grant from Menarini that was not involved in the design, analysis, writing of the manuscript, or decision to submit the manuscript for publication. Open access publishing facilitated by University of New South Wales, as part of the Wiley - University of New South Wales agreement via the Council of Australian University Librarians.
Conflict of interest: S.C. is supported by the Canadian Child's Scholarship from the Libin Institute of Alberta/Cumming School of Medicine. M.V. has received research grant support, served on advisory boards, or had speaker engagements with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, BMS, Boehringer Ingelheim, Chiesi, Cytokinetics, Fresenius Medical Care, Idorsia Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Milestone Pharmaceuticals, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, and participates in clinical trial committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics. V.P. has received fees for Advisory Boards, Steering Committee roles, or Scientific Presentations from Abbvie, Astellas, AstraZeneca, Bayer, Baxter, BMS, Boehringer Ingelheim, Dimerix, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Retrophin, Sanofi, Servier, Vifor and Tricida. H.H. has served as a consultant for Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Janssen, Merck and Mitsubishi-Tanabe, and has received grant support from Abbvie, AstraZeneca, Boehringer Ingelheim and Janssen. R.A.F has received awards from the HDRUKTuring Wellcome Programme in Health Data science. V.P. serves as a board director for St Vincent's Health Australia, Victor Chang Cardiac Research Institute, and Mindgardens; and has received honoraria for Steering Committee roles, scientific presentations, or advisory board attendance, or a combination, from Abbvie, Amgen, Astra Zeneca, Bayer, Baxter, Boehringer Ingelheim, Chinook, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Pharmalink, Pfizer, Reata, Travere, Relypsa, Roche, Sanofi, Servier, and Tricida. H.J.L.H. has received grants or contracts from AstraZeneca, Boehringer Ingelheim, Janssen, and Novo Nordisk; consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, CSL Behring, Dimerix, Eli Lilly, Gilead, Janssen, Novo Nordisk, Novartis, and Travere Therapeutics; and payment or honoraria for speaking from AstraZeneca and Novo Nordisk. C.A. has received honoraria or sat on Advisory Boards/ Steering Committees for AstraZeneca, Novo Nordisk and Amgen. C.P. holds an NHMRC Investigator Grant and has received honoraria from Boehringer Ingelheim-Lilly, Amgen, AstraZeneca, Janssen, Bayer, CSL-Sequiris, Otsuka, and Novo Nordisk for advisory committes, steering committees and scientific presentations. K.W.M has received research support from Afferent, Amgen, Apple, AstraZeneca, Cardiva Medical, Daiichi, Ferring, Google (Verily), Johnson & Johnson, Luitpold, Medtronic, Merck, National Institutes of Health, Novartis, Sanofi, St Jude, and Tenax; and has served as a consultant (speaker fees for continuing medical education events only) for Abbott, Ablynx, AstraZeneca, Baim Institute, Boehringer Ingelheim, Bristol-Myers Squibb, Elsevier, GlaxoSmithKline, Johnson & Johnson, MedErgy, Medscape, Mitsubishi Tanabe, Myokardia, the National institutes for Health, Novartis, Novo Nordisk, Portola, Radiometer, Regeneron, Springer Publishing, and the University of California, San Francisco. B.N. has received grants for CANVAS and CREDENCE; is on an advisory board and has received honoraria and travel reimbursement all from Janssen and all paid to his institution; has received research support from the Australian National Health and Medical Research Council Principal Research Fellowship and Janssen; and has served on advisory boards or has had involvement in continuing medical education programmes for Janssen, with any consultancy, honoraria, or travel support paid to his institution; and has institutional relationships with AbbVie, Actelion, and Janssen. M.J.J. is supported by an National Health and Medical Research Council investigator grant; is responsible for research projects that have received funding from Amgen, Baxter, CSL, Dimerix, Eli Lilly, Gambro, and MSD; and has received fees for advisory, steering committee, or scientific presentations, or a combination, from Akebia, Amgen, Astra Zeneca, Baxter, Bayer, Boehringer Ingelheim, Cesas Linx, Chinook, CSL, Janssen, Medscape, MSD, Occuryx, Roche, and Vifor, with any consultancy, honoraria, or travel support paid to her institution. S.D.S. has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, US2.AI and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, Puretech Health. B.L.N. reports fees for travel support, research support, advisory boards, scientific presentations, and steering committee roles from AstraZeneca, Alexion, Bayer, Boehringer and Ingelheim, CSL-Sequiris, Menarini, Novo Nordisk, and Travere Therapeutics. B.LN. has received fees for advisory boards, steering committee roles, scientific presentations, and travel support from AstraZeneca, Bayer, Boehringer Ingelheim, Cambridge Healthcare Research, Cornerstone Medical Education, the Limbic, Medscape, and Janssen, Travere Therapeutics and Novo Nordisk with all honoraria paid to The George Institute for Global Health. All other authors have nothing to disclose.




