Qiliqiangxin: A multifaceted holistic treatment for heart failure or a pharmacological probe for the identification of cardioprotective mechanisms?
Abstract
The active ingredients in many traditional Chinese medicines are isoprene oligomers with a diterpenoid or triterpenoid structure, which exert cardiovascular effects by signalling through nutrient surplus and nutrient deprivation pathways. Qiliqiangxin (QLQX) is a commercial formulation of 11 different plant ingredients, whose active compounds include astragaloside IV, tanshione IIA, ginsenosides (Rb1, Rg1 and Re) and periplocymarin. In the QUEST trial, QLQX reduced the combined risk of cardiovascular death or heart failure hospitalization (hazard ratio 0.78, 95% confidence interval 0.68–0.90), based on 859 events in 3119 patients over a median of 18.2 months; the benefits were seen in patients taking foundational drugs except for sodium–glucose cotransporter 2 (SGLT2) inhibitors. Numerous experimental studies of QLQX in diverse cardiac injuries have yielded highly consistent findings. In marked abrupt cardiac injury, QLQX mitigated cardiac injury by upregulating nutrient surplus signalling through the PI3K/Akt/mTOR/HIF-1α/NRF2 pathway; the benefits of QLQX were abrogated by suppression of PI3K, Akt, mTOR, HIF-1α or NRF2. In contrast, in prolonged measured cardiac stress (as in chronic heart failure), QLQX ameliorated oxidative stress, maladaptive hypertrophy, cardiomyocyte apoptosis, and proinflammatory and profibrotic pathways, while enhancing mitochondrial health and promoting glucose and fatty acid oxidation and ATP production. These effects are achieved by an action of QLQX to upregulate nutrient deprivation signalling through SIRT1/AMPK/PGC-1α and enhanced autophagic flux. In particular, QLQX appears to enhance the interaction of PGC-1α with PPARα, possibly by direct binding to RXRα; silencing of SIRT1, PGC-1α and RXRα abrogated the favourable effects of QLQX in the heart. Since PGC-1α/RXRα is also a downstream effector of Akt/mTOR signalling, the actions of QLQX on PGC-1α/RXRα may explain its favourable effects in both acute and chronic stress. Intriguingly, the individual ingredients in QLQX – astragaloside IV, ginsenosides, and tanshione IIA – share QLQX's effects on PGC-1α/RXRα/PPARα signalling. QXQL also contains periplocymarin, a cardiac glycoside that inhibits Na+-K+-ATPase. Taken collectively, these observations support a conceptual framework for understanding the mechanism of action for QLQX in heart failure. The high likelihood of overlap in the mechanism of action of QLQX and SGLT2 inhibitors requires additional experimental studies and clinical trials.
Introduction
Plant-based medicines have been used for the treatment of chronic heart failure for centuries. Digitalis, diuretics and sodium–glucose cotransporter 2 (SGLT2) inhibitors have their origins in plants, whereas angiotensin-converting enzyme and neprilysin inhibitors were derived from snake venom.1-3 Therefore, it should not seem peculiar that new ideas about the treatment of heart failure will continue to emerge from natural sources.
Chinese herbal medicines represent a diverse group of empirically-derived formulations that rely on natural sources to treat a broad group of illnesses. The goal is to restore a vital force of life (called Qi), which is composed of complementary but opposing forces (yin and yang). Disease is triggered by an imbalance in Qi, and health is restored when balance is regained through message, movement and concentration exercises, acupuncture and moxibustion, and herbal remedies. In traditional Chinese medicines, specific parts of different plants are combined at varying concentrations. Since heart failure is regarded as an imbalance in heart Qi due to a deficiency of yang, the goal is to boost yang and restore balance.4 Considered broadly, this concept does not differ philosophically from our current understanding that cardiomyocyte health relies on achieving a balance of opposing neurohormonal systems and opposing nutrient surplus and deprivation signalling.5-8 However, the core value of Chinese herbal medicine is holistic – a marked departure from the reductionist perspective of Western medicine.
Formulation and active ingredients of qiliqiangxin
Qiliqiangxin (QLQX) is a standardized commercial formulation that seeks to address the deficiency of yang to restore Qi balance in the heart. The drug was approved by the Chinese Food and Drug Administration in 2004 for the treatment of heart failure.9 QLQX consists of 11 different ingredients: (1) the root of Astragalus mongholicus Bunge (milkvetch root), (2) the root of Panax ginseng C.A. Mey. (ginseng), (3) the root of Aconitum carmichaeli Debx. or Aconitum lethale Griff. (wolfsbane/monkshood), (4) the root and rhizome of Salvia miltiorrhiza Bunge (danshen root), (5) the ripe seed of Descurainia Sophia (L.) Webb ex prantl (pepperweed seed), (6) the rhizome of Alisma plantagoaquatica subsp. orientale (Oriental waterplantian), (7) the rhizome of Polygonatum odoratum (Mill.) Druce (solomonseal), (8) the flower of Carthamus tinctorius (L.) (safflower), (9) the root bark of Periploca sepium Bunge (Chinese silkvine), (10) the pericarp of Citrus reticulata Blanco (dried tangerine peel), and (11) the branch of Cinnamomum cassia Perl (cassia twig). These ingredients are combined in capsules that are available for prescription.
Each plant part is the source of scores of compounds, and when 11 plants are combined, there are hundreds of potentially active molecules. When analysed by mass spectroscopy, QLQX may have as many as 173 active ingredients.10 Nevertheless, identification of the molecules that are critical to a formulation's therapeutic success is important for maintaining quality control when the drug is manufactured for clinical use.
Identification of active ingredients of qiliqiangxin
Elucidation of the active compounds in QLQX is challenging, since many may be poorly absorbed and have minimal bioavailability, and others may not achieve meaningful levels in target tissues. Furthermore, the stochiometric relationships between the concentration of specific ingredients in the capsules and actions on potentially relevant biological pathways remain to be fully elucidated.
One report noted high levels of the following agents in both plasma and cardiac tissue following oral administration of QLQX: astragaloside IV, ginsenoside Rb1, periplocymarin, benzoylmesaconine, benzoylhypaconine, and alisol A.11 A second report that relied on the identification of compounds with bioactivity on isolated cardiomyocytes yielded astragaloside, tanshinone IIA, ginsenoside Re, songorin, calycosin-7-O-β-D-glucopyranoside, hesperidin and alisol A.12 Similar listings of constituents (including other ginsenosides) have been published by others.13, 14
The common molecules in all reports are isoprene oligomers that have a diterpenoid (C-20) or triterpenoid (C-30) structure. Astragaloside IV is a cycloartane-type triterpene glycoside; ginsenosides are triterpene saponins (triterpenoid glycosides); alisol A is a protostane triterpenoid; periplocymarin is a triterpenoid-derived cardenolide-type steroid-like cardiac glycoside; and tanshinone IIA, benzoylmesaconine, benzoylhypaconine and songorine are diterpene alkaloids. It is generally believed that plants synthesize diterpenes and triterpenes as a mechanism of host defense, but these cytoprotective properties are evolutionarily conserved, i.e. they are mediated through convergent pathways that also ameliorate stress in cardiomyocytes. Several of the ingredients of QLQX have been used individually for the treatment of patients with heart failure.15-17
Randomized controlled clinical trials with qiliqiangxin in heart failure
There is considerable experience with QLQX in patients with heart failure, with reports of favourable effects on symptoms, functional capacity and outcomes. However, most trials have been small and or short duration and have not met accepted methodological standards that are routinely utilized to minimize bias and yield replicable results.
Sun et al.9 presented a summary of 129 randomized controlled trials that evaluated QLQX, involving 11 547 patients with heart failure. Most trials were considered by the authors to be of low quality, with concerns about the integrity of randomization, blinding of study medication, ascertainment of events, completeness of data collection, and absence of rigorous statistical methods. Treatment with QLQX was reportedly accompanied by an improvement in functional class and quality of life. Additionally, based on <10 trials that were judged to have a lower risk of bias, assignment to QLQX was accompanied by a 51% reduction in hospitalization for heart failure (risk ratio 0.49, 95% confidence interval [CI] 0.38–0.64) and a 47% reduction in all-cause mortality (risk ratio 0.53, 95% CI 0.27–1.07). However, these estimates were based on a sparse number of events. A meta-analyses by Xu et al.18 reached similar conclusions. Two smaller meta-analyses19, 20 that evaluated the efficacy of QLQX in patients with heart failure already receiving sacubitril/valsartan reported an improvement in ejection fraction and 6-min walk distance, but the authors raised important methodological concerns with the underlying trials.
The Qili Qiangxin Capsules for Chronic Heart Failure Study Group21 reported the effects of QLQX in 512 patients with heart failure with a reduced ejection fraction receiving diuretics, inhibitors of the renin–angiotensin system, beta-blockers and mineralocorticoid receptor antagonists. Patients were randomly assigned (double-blind) to placebo or QLQX (4 capsules three times daily) for 12 weeks. N-terminal prohormone B-type natriuretic peptide (NT-proBNP) was reported to be reduced by 25%, but the data indicated a 13% decrease in NT-proBNP. 6-min walk distance and quality-of-life scores improved in the QLQX group, but there were marginal between-group differences in ejection fraction and cardiac dimensions. Major adverse cardiovascular events (death, resuscitated cardiac death, stroke or worsening heart failure requiring hospitalization or intravenous therapy) occurred in 27 and 11 patients in the placebo and QLQX groups, respectively (p = 0.008), but the number of events was too small to generate a reliable estimate.
Primary results of the QUEST trial
The Qiliqiangxin in Heart Failure: Assessment of Reduction in Mortality (QUEST) investigators presented the findings of a randomized, double-blind, placebo-controlled, parallel-group, multicentre, event-driven trial at the 2023 annual meeting of the European Society of Cardiology. The trial protocol called for the 1:1 randomization of ≈3080 patients with heart failure and a reduced ejection fraction and NT-proBNP ≥450 pg/ml to double-blind treatment with QLQX (given as 4 capsules three times daily) or matching placebo, to be added to conventional therapy for heart failure.22 The prespecified primary endpoint was cardiovascular death and hospitalization for worsening heart failure. It was anticipated that 620 events would be needed to provide 80% power to discern a 20% reduction in risk, 2-sided α = 0.04628, adjusted for two interim analyses.
The trial enrolled 3119 patients (1561 to QLQX and 1558 to placebo) with heart failure (mean ejection fraction of 32%); >60% were receiving a combination of an inhibitor of the renin–angiotensin system, a beta-blocker and a mineralocorticoid receptor antagonist, 50–60% were receiving sacubtril/valsartan, but <10% were treated with an SGLT2 inhibitor. During a median of 18.2 months of double-blind treatment (with a 5% dropout rate and <0.1% patients lost to follow-up for vital status), there were 467 primary events in the placebo group and 389 primary events in the QLQX group, yielding a hazard ratio of 0.78 (95% CI 0.68–0.90; p < 0.001).10 The hazard ratios were 0.76 (95% CI 0.64–0.90; p = 0.002) for hospitalizations for heart failure and 0.83 (95% CI 0.68–0.996; p = 0.045) for cardiovascular death. The effect on hospitalization for heart failure was dominant during the first 12–18 months of treatment. Death for any reason occurred in 262 and 221 patients in the placebo and QLQX groups, respectively (p = 0.058). The effects on systolic blood pressure, heart rate, NT-proBNP and renal function were not reported. These results have not yet been published.
Assuming that the results of the QUEST trial represent a replicable benefit of QLQX, how might the blend of 11 different herbs reduce major outcomes in patients with heart failure and a reduced ejection fraction? Despite its holistic origins, there has been intense interest in identifying the molecular and cellular mechanisms of action of Chinese herbal medicines. The QUEST investigators proposed that the benefits of QLQX were mediated by signalling through peroxisome proliferator-activated receptor-gamma (PPARγ),10 but this mechanism seems highly unlikely in light of clinical trials showing an increased risk of adverse heart failure outcomes with full PPARγ and dual PPARα/γ agonists.23-25
Cellular pathways that underlie the mechanism of action of qiliqiangxin
Numerous studies of the effect of QLQX have been performed in diverse experimental models of acute and chronic cardiac injury and stress. However, only a minority of reports carried out studies to determine if overexpression or silencing of a specific mechanism would abolish any cardioprotective effect. Such studies have been essential to deciphering the pathways by which SGLT2 inhibitors exert favourable effects in the heart.8
Diterpenes and triterpenes in traditional Chinese medicines influence signalling through nutrient deprivation and surplus pathways
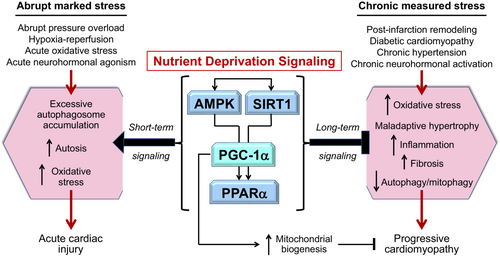
Mechanistic studies of QLQX have focused on nutrient surplus and deprivation signalling in the heart during abrupt marked injury and during sustained measured stress (e.g. heart failure). This focus is predicated on numerous reports demonstrating that diterpenes and triterpenes in traditional Chinese medicines have effects on (i) nutrient surplus signalling through the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR)/hypoxia inducible factor 1-alpha (HIF-1α)/nuclear factor erythroid 2-related factor 2 (NRF2) pathway; (ii) nutrient deprivation signalling through sirtuin-1 (SIRT1) and adenosine monophosphate protein kinase (AMPK); (iii) the cellular housekeeping mechanism known as autophagy (particularly the autophagic clearance of damaged mitochondria [mitophagy]); and (iv) signalling through PPAR coactivator-1alpha (PGC-1α) and its downstream transcription factors, PPARα and PPARγ.26-28
In general, an increase in environmental and cytosolic nutrients enhances signalling through PI3K-Akt–mTOR-HIF-1α-PGC-1α-NRF2, which prioritizes cardiomyocyte growth and replication, effects that are potentiated by neuregulin-1 (NRG-1) and its ligand, Erb-B2 receptor tyrosine kinase 2 (Erbb2).7, 29-31 In contrast, a depletion of environmental and cytosolic nutrients augments signalling through SIRT1-AMPK-PGC-1α, which prioritizes cardiomyocyte health and survival.6, 7, 29, 32 Both pathways can exert important cardioprotective effects, depending on the duration and severity of cardiac stress (Figures 1 and 2). During marked abrupt stress, cardioprotection relies on upregulation of the PI3K-Akt–mTORHIF-1α-PGC-1α-NRF2 pathway, which act to ameliorate autophagic hyperactivation and the deleterious accumulation of autophagosomes and to promote mitochondrial biogenesis and antioxidant defenses33-36; yet, such signalling is deleterious if it is sustained for long periods.7, 30, 37 Conversely, upregulation of SIRT1 and AMPK may be counterproductive during abrupt marked stress,38 but during sustained measured stress (as is typically the case in cardiomyopathy), prolonged SIRT1-AMPK-PGC-1α signalling exerts highly favourable effects on cardiomyocyte function and viability.6, 8, 29
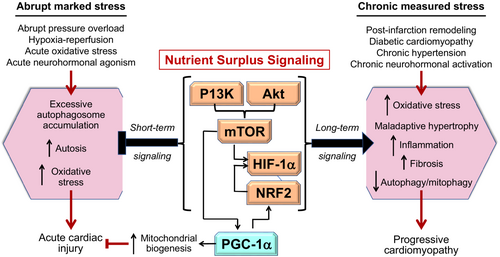
These striking intensity- and duration-dependent differences in the effects of cellular signals are particularly characteristic of PGC-1α and its downstream effector NRF239-41 and may be related to the background level of autophagy. NRF2 signalling is adaptive in marked abrupt stress when autophagy is exaggerated, but is maladaptive during prolonged measured stress when autophagy is suppressed.41 Similarly, low levels of mTOR expression seen immediately after injury promote PGC-1α-mediated mitochondrial biogenesis,39, 42 whereas marked sustained mTOR upregulation inhibits PGC-1α-mediated mitophagy.30, 31, 40, 42 Measured PGC-1α upregulation protects against sustained cardiac injury,43 whereas PGC-1α overexpression results in excessive mitochondrial proliferation and cardiomyopathy.44
PGC-1α is a convergence point for nutrient surplus and deprivation signalling, mediating differential effects on PPARα and PPARγ during short- and long-term stress
PGC-1α represents a point of convergence of these mutually antagonist pathways, being activated by mTOR during the high energy demands of acute cardiac stress and by SIRT1/AMPK during the chronic stress of cardiomyopathy (Figure 3).40, 45
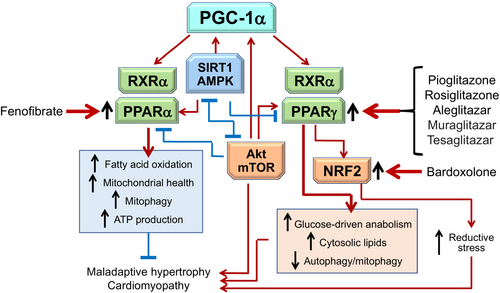
When activated by Akt–mTOR during acute intense stress, PGC-1α signals primarily through PPARγ to enhance the entry of glucose (which is directed towards anabolism mediated by the pentose phosphate pathway) and the accumulation of cytosolic lipids, thereby mitigating excessive autophagy.46, 47 PPARγ agonism ameliorates hypoxia–reperfusion by reinforcing Akt–mTOR signalling, by enhancing mitochondrial biogenesis, and by upregulating NRF2 and its downstream antioxidant actions.48, 49 However, during chronic measured stress, mTOR and PPARγ activation promotes the development of maladaptive hypertrophy and cardiomyopathy.30, 50
In contrast when activated by SIRT1/AMPK during prolonged measured stress, PGC-1α signals primarily through PPARα to augment clearance of cytosolic lipids, fatty acid oxidation and ATP generation and to promote mitochondrial health and mitophagy.51, 52 PPARα silencing promotes and PPARα agonism ameliorates maladaptive hypertrophy and experimental cardiomyopathy.53-56 However, enhanced SIRT1 and PPARα signalling is detrimental during acute pressure overload stress.38
Therefore, PPARα and PPARγ reflect oppositional responses to PGC-1α signalling during abrupt and prolonged stress. Chronic cardiac stress leads to upregulation of PPARγ, but downregulation of PPARα.55-58 PPARα and PPARγ are differentially influenced by Akt/mTOR and SIRT1/AMPK. mTOR activation causes upregulation of PPARγ, but suppression of PPARα,59 and dual PPARα/PPARγ agonism suppresses SIRT1.60 AMPK can inhibit cardiac hypertrophy by signalling through PPARα,61 and SIRT1 binds to PPARα to promote its activity38, 62 and binds to PPARγ to suppress its activity.63
These counterbalancing interactions are likely mediated through a PGC-1α branch point,64 because both PPARα and PPARγ compete with each other for binding to PGC-1α (Figure 3).59, 64 Both PPARα and PPARγ form heterodimers with nuclear transcription factor retinoid X receptor-alpha (RXRα), which is coactivated by PGC-1α. When activated by SIRT1 and AMPK during chronic stress, PGC-1α promotes the entry and oxidation of fatty acids and augments mitochondrial health.65 PGC-1α-deficient mice show impaired mitochondrial function, maladaptive hypertrophy, fibrosis and accelerated heart failure,43 and the PPARα agonist fenofibrate ameliorates cardiac injury (in a SIRT1- and PGC-1α-dependent manner).55, 56 In contrast, during acute stress, PGC-1α coactivates a heterodimer of RXRα and PPARγ under the influence of the transcription factor yin-yang 1 (YY1), a target of mTOR and a promoter of mitochondrial biogenesis and oxidative functions.30, 39 Short-term upregulation of mTOR, PPARγ, RXRα and YY1 (and their downstream effector, NRF2) suppresses excessive autophagosome formation and exerts cardioprotective effects in acute ischaemia or pressure overload.34, 66-68 However, prolonged upregulation of PGC-1α, RXRα and PPARγ leads to mitochondrial oxidative dysfunction, lipid accumulation, maladaptive hypertrophy and cardiomyopathy,50, 69, 70 and experimental diabetic cardiomyopathy is alleviated by PPARγ silencing.57 mTOR and PPARγ also act to upregulate NRF2,41 and sustained NRF2 activation produces excessive reductive stress with adverse effects on cardiomyopathy.71, 72
The balance of PPARα/PPARγ signalling has important implications for patients with cardiomyopathy (Figure 3). The cardiac expression of PGC-1α, PPARα and downstream effectors is downregulated, but the expression of PPARγ is upregulated, in patients with hypertensive, coronary artery disease or heart failure.73-77 The PPARα agonist fenofibrate restores PPARα signalling and reduced heart failure events in a large-scale clinical trial.78 Conversely, both selective PPARγ and dual PPARα/PPARγ agonists have been shown to increase the risk of heart failure events in clinical trials,23-25 and long-term activation of NRF2 (the downstream effector of PPARγ76) with bardoxolone increased the risk of death or hospitalization for heart failure in a large-scale trial.79 Taken collectively, these observations suggest that the balance of PGC-1α/PPARα and PGC-1α/PPARγ signalling has important effects on the course of clinical cardiomyopathy.
Qiliqiangxin in experimental models of marked abrupt cardiac stress
Marked abrupt cardiac injury is typically produced by hypoxia–reoxygenation injury, by exposure to angiotensin II, isoproterenol or cardiotoxic agents or by severe transverse aortic constriction. Following sudden depletion of ATP, exaggerated upregulation of the low-ATP sensor AMPK leads to extreme autophagic stimulation and cell death by autosis.80 Autosis occurs not because of enhanced autophagic flux and self-digestion, but as a result of the excessive accumulation of autophagosomes.36 Autosis is dependent on the interaction of the autophagy gene Beclin 1 with Na+-K+-ATPase, and it is inhibited by cardiac glycosides.80, 81 Upregulation of AMPK promotes Na+-K+-ATPase activity and autosis82; conversely, excessive accumulation of autophagosomes their cardiac deleterious effects can be muted by activation of the PI3K-Akt–mTOR pathway.30
Mechanistic studies of qiliqiangxin in marked abrupt cardiac injury
Qiliqiangxin has been shown to attenuate experimental doxorubicin cardiotoxicity, while enhancing PI3K-Akt–mTOR signalling and suppressing autophagic hyperactivation and autophagosome accumulation.83 In cardiac or cardiomyocyte injury produced by hypoxia–reperfusion, acute ischaemia, isoproterenol or hydrogen peroxide, QLQX ameliorated adverse structural and functional changes, apoptosis and autophagosome-mediated cell death while increasing Akt and mTOR phosphorylation and muting AMPK phosphorylation; the drug's cardioprotective effects were abolished by PI3K, Akt or mTOR inhibition or by AMPK hyperactivation.84-88 QLQX prevented hyperglycaemia-induced apoptosis in neonatal rat ventricular cardiomyocytes, an effect that was accompanied by increased expression of PGC-1α, PPAR-γ and NRF2 and was attenuated by PPAR-γ antagonism.89 In the border zone of experimental myocardial infarction,90 QLQX ameliorated cardiomyocyte apoptosis and induced the expression of NRF2; this protective effect was abolished when NRF2 was knocked out.
Studies by the group led by Ge et al.91-99 highlighted a role of enhanced Akt/mTOR/HIF-1α/NRG-1/Erbb2 signalling in mediating QXQL cardioprotection during acute marked injury (Figure 4). In hypoxic cardiac microvascular endothelial cells, QLQX alleviated oxidative stress, mitochondrial dysfunction and apoptosis; these benefits were accompanied by upregulation of Akt/mTOR/HIF-1α/NRG-1 signalling and downregulation of AMPK and were abolished by HIF-1α, NRG-1 and Akt silencing and by AMPK activation.94-96 In abrupt transverse aortic constriction, QLQX attenuated apoptosis, suppressed autophagosome formation and enhanced Erbb2 signalling, effects that were abrogated by Erbb2 inhibition.97, 99 Similar responses were seen in cardiac microvascular endothelial cells exposed to angiotensin II; excessive autophagosome formation and apoptosis was suppressed by QLQX, and these actions were abolished by mTOR or Erbb2 inhibition.98 In acute myocardial infarction, the cardioprotective effects of QLQX were associated with enhanced glucose uptake, upregulation of Akt, HIF-1α and NRG-1 in the border zone, and increased expression of biomarkers of vascularization; these effects were abrogated by HIF-1α inhibition.91-93
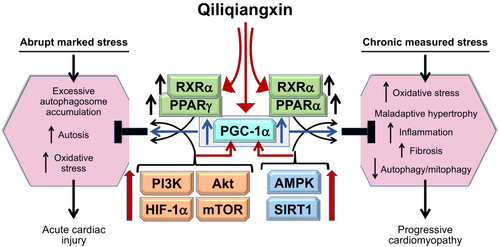
Ingredients of qiliqiangxin responsible for effects in marked abrupt cardiac stress
Several constituents of QLQX contribute to its effect on PI3K-Akt–mTOR-HIF-1α signalling. In injury produced by hypoxia–reperfusion or doxorubicin, astragaloside IV prevented apoptosis while enhancing glucose uptake and activating the PI3K-Akt–mTOR-HIF-1α pathway.100-102 Similarly, tanshione IIA and ginsenosides (Rb1 and Rg1) augmented glucose uptake and upregulated P13K–Akt–mTOR and HIF-1α signalling, while inhibiting autophagosome accumulation and cell death in pressure overload and hypoxia–reperfusion injury.103-106 Acting through mTOR, tanshione IIA promoted the transcription and stability of NRF2, and its antiapoptotic effects were dampened by NRF2 silencing.34
Additionally, in human pluripotent stem cell-derived cardiomyocytes, QLQX increased Ca2+ spark frequency, amplitude and duration, indicative of enhanced Ca2+ release.107 Similar effects have been reported with digitalis and other plant-derived compounds with positive inotropic actions.108 Several QLQX ingredients (e.g. the cardiac glycoside periplocymarin and ginsenosides) inhibit Na+-K+-ATPase.109, 110 Inhibition of Na+-K+-ATPase may explain the action of QLQX to enhance PI3K-Akt–mTOR signalling,80, 111, 112 suppress excessive autophagosome formation,80 and prevent injury following acute pressure overload.112 Given these observations, it is perplexing that the effect of QLQX on cardiac Na+-K+-ATPase has not yet been described in the medical literature.
Qiliqiangxin in experimental models of prolonged measured cardiac injury
In contrast to the adaptive responses during acute injury, sustained upregulation of the PI3K-Akt–mTOR pathway for long periods (as in chronic injury) leads to ventricular remodelling, maladaptive hypertrophy, apoptosis, inflammation and fibrosis.6, 7 The evolution and progression of cardiomyopathy is further driven by suppression of nutrient deprivation signalling (AMPK-SIRT1-PGC-1α),7 thus abrogating its action to mute cellular stress and proinflammatory pathways.6-8, 29, 32 Furthermore, AMPK, SIRT1 and PGC-1α are the guardians of mitochondrial health – repairing structure and function, disposing of dysfunctional organelles (through mitophagy), and promoting the biogenesis of healthy mitochondria – thereby promoting fatty acid oxidation and ATP production.
In the failing heart, cardiomyocyte metabolism shifts from long-chain fatty acids to glucose. Glucose entry into the cell is markedly upregulated, as glucose transporter 1 (GLUT1) becomes the dominant isoform, and the insulin-regulated glucose transporter 4 (GLUT4) is suppressed.29 Heightened intracellular levels of glucose are directed towards anabolism (through the pentose phosphate pathway and the hexosamine biosynthetic pathway), but activation of these pathways in high-pressure normoxic conditions promotes oxidative stress, maladaptive hypertrophy, inflammation and organellar dysfunction.7 Impaired long-chain fatty acid oxidation due to mitochondrial dysfunction also enhances the intracellular accumulation of toxic lipid intermediates.29 Both glucotoxicity and lipotoxicity can be alleviated if mitochondrial fatty acid oxidation can be augmented pharmacologically,113, 114 e.g. by upregulation of PGC-1α and formation of the PPARα-RXRα heterodimer.115 The latter mechanism appears to underlie the cardioprotective effects of many traditional Chinese medicines.27, 116
Descriptive reports of the effects of qiliqiangxin in chronic sustained cardiac injury
The effects of QLQX have been studied in diverse models of chronic cardiac injury, characterized by ventricular remodelling and systolic dysfunction and accompanied by oxidative stress, apoptosis, inflammation and fibrosis. In experimental myocardial infarction, aortic banding, doxorubicin injury, prolonged atrial pacing and streptozotocin-induced diabetic cardiomyopathy and in spontaneously hypertensive rats, QLQX mitigated the degree of ventricular remodelling and prevented the decline in systolic function.84, 117-127 These changes were associated with a reduction in apoptosis and muting of proinflammatory and profibrotic pathways84, 117-127 and enhanced autophagic flux.127 QLQX prevented angiotensin II-mediated differentiation of cardiac fibroblasts,128 abolished phenylephrine-induced hypertrophy in neonatal rat ventricular cardiomyocytes,124 and reversed the aortic endothelial dysfunction in diabetic rats.129 In experimental heart failure and preserved ejection fraction, QLQX mitigated myocardial hypertrophy with decreased expression of proinflammatory and profibrotic biomarkers.130 The effects were comparable to those seen with sacubitril/valsartan, but QLQX did not produce meaningful changes in blood pressure or left ventricular filling pressure.
Qiliqiangxin-mediated upregulation of PGC-1α signalling, enhanced mitochondrial autophagy and biogenesis, and normalization of nutrient metabolism
Numerous studies have evaluated the role of PGC-1α, PPARα and PPARγ in mediating the effects of QLQX in chronic cardiomyopathy (Figure 4). In chronic isoproterenol-induced injury, QLQX prevented apoptosis, remodelling and fibrosis, while upregulating PGC-1α and PPAR-γ with no effect on PI3K-Akt–mTOR signalling.131 In post-infarction remodelling, QLQX restored cardiac structure and function and prevented apoptosis and fibrosis; these benefits were accompanied by upregulation of PPARα132 and PPARγ132, 133 and of genes critical to glucose and fatty acid metabolism.133, 134 In spontaneous hypertensive rats and streptozotocin-induced diabetic cardiomyopathy, QLQX attenuated ventricular dysfunction, hypertrophy, apoptosis, fibrosis and mitochondrial abnormalities; these benefits were accompanied by enhanced AMPK phosphorylation93 and upregulation of PGC-1α, PPARα and PPARγ along with enzymes responsible for nutrient oxidation.89, 93, 127, 135 In three reports,131-133 the effects of QLQX were abrogated by T0070907, often considered to be a PPARγ inhibitor. However, T0070907 is an inverse agonist of PPARγ,136 and thus, it interrupts basal PPARγ activity, thus enhancing cardiac vulnerability to injury independent of blocking QLQX-related effects.69, 137 Full PPARγ agonism is not expected to exert cardioprotective effects.23-25
A key mechanistic role for PGC-1α is supported by several experimental studies. In monocrotaline-induced pulmonary hypertension, QLQX ameliorated right ventricular hypertrophy and fibrosis, while enhancing PGC-1α expression and mitophagy,138 and QLQX also upregulated mitophagy in post-infarction ventricular dysfunction.139 Exposure of H9C2 cardiomyocytes to QLQX increased the expression of PGC-1α and promoted mitochondrial biogenesis, effects that were abolished when signalling through PGC-1α was suppressed.140 QLQX mitigated phenylephrine-induced hypertrophy in neonatal rat ventricular cardiomyocytes, while increasing the expression of PGC-1α; the antihypertrophic effect of QLQX was abrogated by silencing of PGC-1α.141
Qiliqiangxin also promoted changes in cardiomyocyte nutrient metabolism that corresponded closely to those that follow PGC-1α upregulation. In cultured H9c2 cardiomyocytes, in post-infarction and pressure-overload remodelled ventricles and in spontaneous hypertensive rats, QLQX reduced the expression of GLUT1, while enhancing the expression of GLUT4.93, 134, 140, 142 Normalization of glucose transporter expression was accompanied by upregulation of PGC-1α and AMPK and restoration of long-chain fatty acid uptake and oxidation in the heart.91, 93, 133, 134, 140, 142 These actions are features of enhanced PGC-1α and PPARα signalling.115, 143
Effect of qiliqiangxin constituents on SIRT1-AMPK-PGC-1α-PPARα signalling
Several constituents of QLQX can cause upregulation of the SIRT1/AMPK/PGC-1α pathway and autophagic flux and modulate PPARα/PPARγ signalling in chronic cardiac injury and heart failure. Traditional Chinese medicines have been a rich resource of SIRT1, PGC-1α and RXRα activators (Figure 4).26, 27
Astragaloside IV has been shown to protect against isoproterenol-induced cardiac hypertrophy144; lipopolysaccharide-induced cardiomyocyte dysfunction and apoptosis145; streptozotocin-induced diabetic cardiomyopathy146; and angiotensin II-induced mitochondrial dysfunction147 – effects that were accompanied by increased PGC-1α and PPARα expression and ATP production. In murine chronic heart failure produced by abdominal aortic constriction, astrogaloside IV preserved ventricular function and enhanced fatty acid oxidation, while increasing PPARα expression.148
Tanshione IIA has been used for the treatment of patients with heart failure,17 and in experimental models, tanshione IIA attenuated doxorubicin cardiotoxicity,149 alleviated the development of pressure overload-induced heart failure,150 and preserved cardiac function and minimized oxidative stress and apoptosis in post-infarction heart failure.151 The effects of tanshione IIA to prevent cardiomyopathy were accompanied by increased AMPK phosphorylation, decreased mTOR phosphorylation and enhanced autophagic flux – effects that were abolished by mTOR activation.152-154 Tanshione IIA also ameliorated diabetic cardiomyopathy and palmitic acid-induced endoplasmic reticulum stress in neonatal rat cardiomyocytes; these effects were accompanied by upregulation of SIRT1 and were abolished by SIRT1 inhibition.155 In ischaemic myocardium, neocryptotanshinone promoted mitochondrial biogenesis, fatty acid oxidation and ATP production by binding directly to RXRα to upregulate both RXRα and PPARα.156
Several ginsenosides have favourable effects in experimental chronic heart failure, primarily by signalling through SIRT1, AMPK and PGC-1α. Ginsenoside Rb3 mitigated ventricular remodelling and fibrosis and exerted antiapoptotic effects in models of pressure-overload heart failure and in osmotically stressed H9C2 cardiomyocytes, and these effects were accompanied by upregulation of PGC-1α, PPARα and RXRα and restoration of enzymes critical to fatty acid oxidation.157, 158 Ginsenoside Rb3 binds directly to RXRα,27 and its cellular benefits are abrogated by coadministration of a PPARα inhibitor.27, 157 Ginsenoside Rg3 enhanced PPARα signalling and reduced oxidative stress,159 and it augmented mitophagy and alleviated experimental heart failure.160 Similar effects were seen with ginsenoside Rg1, which reduced hypertrophy, oxidative stress and proinflammatory signalling in streptozotocin induced diabetic hearts, while upregulating AMPK and PGC-1α.161 In post-infarction heart failure, ginsenoside Rg1 alleviated left ventricular dilatation and fibrosis and promoted mitophagy, and the enhancement of mitophagy by ginsenoside Rg1 in hydrogen-peroxide treated cardiomyocytes was prevented by SIRT1 inhibition.162 In isoproterenol-induced heart failure, ginsenosides Rb1 and Re ameliorated cardiac injury and fibrosis, while upregulating PPARα and improving mitochondrial function and fatty acid oxidation.163, 164
Little is known about the effect of astragaloside IV, tanshione IIA and ginsenosides on PPARγ in the heart. However, in non-stimulated adipocytes, ginsenosides Rb1 and Re reduced inflammatory signalling and promoted the release of adiponectin, while increasing the expression of PGC-1α and PPARγ; these effects were abrogated by PPARγ antagonism.165-167 At the same time, ginsenoside Rg3 signaled through AMPK to function as a PPARγ antagonist in adipocytes prestimulated by rosiglitazone.168 Taken together, these observations suggest that ginsenosides may function as partial PPARγ agonists, i.e. stimulating PPARγ when expression is low and inhibiting PPARγ when PPARγ is activated. Many traditional Chinese medicines act as partial PPARγ agonists,169-171 and astragaloside IV and tanshione IIA function as PPARγ agonists or antagonists depending on the experimental setting, a profile typical of partial agonists.172-174 Importantly, when PPARγ is upregulated (as in chronic heart failure), partial PPARγ agonists act primarily as PPARγ antagonists, thus interfering with the cardiotoxicity of full PPARγ agonism.175 The possibility that QLQX acts as a partial PPARγ agonist remains to be explored.
Periplocymarin is a digitalis-like cardiac glycoside that inhibits Na+-K+-ATPase, but there is limited information about its effects in heart failure models. Nevertheless, it is noteworthy that a broad range of cardiac glycosides have been shown to stimulate autophagy via an AMPK-dependent mechanism.176 The effects of periplocymarin on PPARα and PPARγ signalling have not been studied, but cardiac glycosides increase PPARδ signalling and the expression of enzymes involved in fatty acid metabolism, both in isolated cardiomyocytes and in diabetes-related cardiomyopathy.177, 178
Conclusions: Qiliqiangxin as a pharmacological probe for identification of mechanisms relevant to the progression of heart failure
The key ingredients in many traditional Chinese medicines are isoprene oligomers with a diterpenoid (C-20) or triterpenoid (C-30) structure, which are known to exert cardiovascular effects by signalling through nutrient surplus and nutrient deprivation pathways. QLQX, a dipterpene- and tripterpene-replete combination of traditional Chinese herbs, was reported to reduce the combined risk of cardiovascular death or hospitalization for heart failure by 22% (p < 0.001) based on 859 events observed in 3119 patients over a median of 18.2 months; the benefits were seen in patients taking foundational drugs except for SGLT2 inhibitors.
Due to the similarity of their chemical structures, diterpenes and triterpenes influence specific closely-overlapping evolutionarily-conserved cellular pathways that are used for cytoprotection in plants, and potentially in cardiomyocytes. Therefore, QLQX may be less of a multifaceted holistic medicine than a focused pharmacological probe that is capable of elucidating pathways that are relevant to the development and progression of heart failure. In prolonged cardiac injury, QLQX ameliorates oxidative stress, maladaptive hypertrophy, apoptosis and proinflammatory and profibrotic pathways, while enhancing mitochondrial health and mitophagy, thereby promoting healthy glucose and fatty acid metabolism and ATP production. These effects are achieved by an action of QLQX to upregulate nutrient deprivation signalling through SIRT1/AMPK/PGC-1α/RXRα, and silencing of SIRT1, PGC-1α or RXRα abrogates the favourable effects of QLQX during sustained cardiac stress. Therefore, the findings with QXQL act to reinforce our understanding of the important role of the SIRT1/AMPK/PGC-1α/RXRα pathway in the development of experimental and clinical cardiomyopathy.
It should be noted that the nutrient deprivation pathways (i.e. SIRT1/AMPK/PGC1α signalling) involved in QLQX have also been implicated in the mechanism of action of SGTL2 inhibitors (Figure 4),8 which were also derived from a plant source. Both QLQX and SGLT2 inhibitors act to improve glucose and fatty acid oxidation, reduce oxidative stress, mitigate proinflammatory and profibrotic signalling, and enhance mitochondrial health and mitophagy.29 The magnitude and pattern of the benefits reported in the QUEST trial are similar to those reported for SGLT2 inhibitors in patients with heart failure and a reduced ejection fraction.10, 179 A potential interaction between QLQX and SGLT2 inhibitors has not been evaluated experimentally and could not be examined in the QUEST trial because the prevalence of use of SGLT2 inhibitors was very low. Given the high likelihood of a mechanistic overlap, the nature of an interaction between QLQX and SGLT2 inhibitors requires further experimental work and clinical trials.
Conflict of interest: During the past 3 years, M.P. reports personal fees for consulting from 89bio, Abbvie, Actavis, Ardelyx, Alnylam, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Medtronic, Moderna, Novartis, Reata, Relypsa, Salamandra.




