Calcium channel blocker use and outcomes in patients with heart failure and mildly reduced and preserved ejection fraction
Abstract
Aims
Patients with heart failure (HF) and mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF) are often treated with calcium channel blockers (CCBs), although the safety of CCBs in these patients is uncertain. We aimed to investigate the association between CCB use and clinical outcomes in patients with HFmrEF/HFpEF; CCBs were examined overall, as well as by subtype (dihydropyridine and non-dihydropyridine).
Methods and results
We pooled individual patient data from four large HFpEF/HFmrEF trials. The association between CCB use and outcomes was assessed. Among the 16 954 patients included, the mean left ventricular ejection fraction (LVEF) was 56.8%, and 13 402 (79.0%) had HFpEF (LVEF ≥50%). Altogether, 5874 patients (34.6%) received a CCB (87.6% dihydropyridines). Overall, the risks of death and HF hospitalization were not higher in patients treated with a CCB, particularly dihydropyridines. The risk of pump failure death was significantly lower (hazard ratio [HR] 0.76, 95% confidence interval [CI] 0.60–0.96), while the risk of stroke was higher (HR 1.26, 95% CI 1.06–1.50) in patients treated with a CCB compared to those not. These risks remained different in patients treated and not treated with a CCB after adjustment for other prognostic variables. Although the majority of patients were treated with dihydropyridine CCBs, the pattern of outcomes was broadly similar for both dihydropyridine and non-dihydropyridine CCBs.
Conclusion
Although this is an observational analysis of non-randomized treatment, there was no suggestion that CCBs were associated with worse HF outcomes. Indeed, CCB use was associated with a lower incidence of pump failure death.
Introduction
Calcium channel blockers (CCBs) are used to treat a variety of cardiovascular problems including coronary artery disease, hypertension, and atrial fibrillation (AF).1-3 Patients with heart failure (HF) and preserved or mildly reduced ejection fraction (HFpEF/HFmrEF) have a high prevalence of the latter two comorbidities and, as a result, are often treated with CCBs.4-6 Indeed, in recent large trials in patients with HFmrEF/HFpEF around 35–40% of patients were taking a CCB at baseline.7-9 This clearly contrasts with HF and reduced ejection fraction (HFrEF) where the use of CCBs, especially non-dihydropyridine CCBs, is discouraged because of concerns that these drugs may worsen left ventricular systolic function.10, 11 Although, in theory, reduction in myocardial contractility should not be a concern in patients with a relatively normal left ventricular ejection fraction (LVEF), it may be in patients with HFmrEF. Furthermore, the vasodilatation induced by some dihydropyridine CCBs may cause reflex neurohumoral activation and secondary sodium and water retention which would be undesirable in any type of HF.12-14 Yet, curiously, the safety of CCBs has never been evaluated in patients with HF and a LVEF >40%15 with prior trials limited to those with a LVEF <30% and limited observational data from registries of patients with decompensated HF.16, 17 Ideally, this would be done in a prospective randomized placebo-controlled trial but unfortunately, such a trial has never been done (and is unlikely ever to be done). Therefore, we analysed outcomes in almost 6000 patients with HFmrEF/HFpEF treated with a CCB, compared to 11 000 not treated with a CCB, using individual participant data from four landmark randomized trials. In addition, we examined outcomes related to the use of different CCB subtypes that is, dihydropyridines and non-dihydropyridines.
Methods
Study population
We pooled the patients enrolled in four large randomized controlled trials: the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-Preserve), the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial (TOPCAT), the Prospective Comparison of ARNI [angiotensin receptor–neprilysin inhibitor] with ARB [angiotensin receptor blockers] Global Outcomes in HF with Preserved Ejection Fraction trial (PARAGON-HF), and the Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure trial (DELIVER).7, 8, 18, 19 Patients who were randomized into TOPCAT from Russia (n = 1066) and Georgia (n = 612) were excluded because of doubts about the diagnosis of HF and treatment adherence compared to participants in the Americas.9 Accordingly, we analysed 1767 patients enrolled in TOPCAT-Americas.
The key aspects of the design of these trials are summarized in online supplementary Table S1.7, 8, 18, 19 Briefly, I-Preserve compared irbesartan with placebo in 4128 patients with LVEF ≥45%. In TOPCAT, 3445 patients with LVEF ≥45% were allocated to spironolactone or placebo. PARAGON-HF enrolled 4796 patients with LVEF ≥45% who were randomized to valsartan or sacubitril/valsartan. DELIVER assessed the efficacy of the sodium–glucose cotransporter 2 inhibitor, dapagliflozin, compared to placebo, in 6263 patients with LVEF ≥40%. Each trial was approved by local ethics committees, and written informed consent was obtained from all participants.
Calcium channel blocker use at baseline
In each trial, data on the use of CCBs were collected on the case report form. Baseline characteristics and outcomes were analysed according to CCB use in a pooled dataset created using individual patient data from the four trials listed above. We also analysed characteristics and outcomes according to dihydropyridine and non-dihydropyridine CCB subtypes separately.
Outcomes examined
In the current study, the primary outcome examined was the time to the first occurrence of the composite of cardiovascular death or HF hospitalization. We also analysed each component of the composite outcome and all-cause mortality, sudden death, pump failure death (worsening HF death). In addition, fatal or non-fatal myocardial infarction (MI) and fatal or non-fatal stroke were evaluated. HF hospitalization and causes/modes of death were adjudicated by a central endpoint committee according to similar pre-specified criteria in each trial. With respect to death from pump failure (or from HF), similar definitions were used across the trials: defined as a death occurring within the context of clinically worsening symptoms and/or signs of HF without evidence of another cause of death (online supplementary Table S2). The definitions of sudden death, MI, and stroke were also summarized (online supplementary Table S2).
Statistical analysis
To compare patient characteristics according to CCB use, all baseline data are reported as frequencies and percentages (%) for categorical variables, and as means (± standard deviation) or medians with interquartile ranges (quartiles 1–3) for continuous variables. For continuous variables, differences between the two groups (no CCB vs. CCB) were compared by Student's t-test or Mann–Whitney U test, and those between the three groups (no CCB vs. dihydropyridine CCB vs. non-dihydropyridine CCB) were compared by one-way analysis of variance or Kruskal–Wallis test. Differences of the categorical variables were compared by the χ2 test.
Clinical outcomes are reported as the number of events. Cox proportional hazards models were used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for each outcome, using the no-CCB group as a reference. Competing-risks regression with the Fine–Gray method were used to analyse outcomes except for all-cause mortality. Cardiovascular death was tested for the competing risk for non-cardiovascular death. The first hospitalization for HF was tested for the competing risk of all-cause death. Sudden death was tested for the competing risk of non-sudden death, and pump failure death was tested for the competing risk of death not caused by worsening HF. Fatal and non-fatal MI and stroke were tested for the competing risk of all-cause death not due to MI or stroke. Survival curves with cumulative incidence function for each outcome considering the competing risk were drawn according to CCB use (and CCB subtypes). The survival differences in cumulative incidence function were evaluated with Gray test.
The associations between CCBs and outcomes were adjusted for other prognostic variables; age, sex, region, race, trials and treatment groups, body mass index, prior HF hospitalization, hypertension, MI, stroke, diabetes mellitus, AF, New York Heart Association (NYHA) functional class I or II versus III or IV, heart rate, systolic blood pressure, estimated glomerular filtration rate (eGFR), LVEF, and log-transformed N-terminal pro-B-type natriuretic peptide (NT-proBNP). Incidence rates for each outcome were plotted across the spectrum of LVEF as a continuous variable based on the Poisson regression model using restricted cubic splines. The number of knots was set as 3 (at 10, 50, and 90 percentiles) according to Akaike Information Criterion. Interactions between CCB use and LVEF were also evaluated.
In sensitivity analyses, baseline characteristics were evaluated according to each trial included. The adjusted HRs for cardiovascular death or HF hospitalization according to interest HF subgroups (HFmrEF or HFpEF, with or without AF, with or without hypertension, and with or without coronary artery disease) were also evaluated with the interaction assessments between CCB use and each HF subtype. In addition, given that the missing values of NT-proBNP (12.4%) were mainly observed in the cohort of TOPCAT-Americas, further Cox proportional hazards models excluding TOPCAT-Americas were conducted as a sensitivity analysis to examine the consistency of the main results. To test if the effect of CCB might vary by the included trials we included an interaction term between CCB use and trial and found no evidence of interaction for the primary composite endpoint (p for interaction = 0.42); therefore we did not separate the trials for our main analysis but have added these as supplementary analyses.
We conducted a further sensitivity analysis adjusting for baseline use of beta-blockers (renin–angiotensin–aldosterone system inhibitors such as ARBs and mineralocorticoid receptor antagonists were the randomized therapies in these trials and therefore could not be adjusted for consistently as they were already included in the randomized therapy arm). Furthermore, to adjust for the likelihood of receiving a CCB at baseline, we additionally performed propensity score matching. This involved generating a propensity score for CCB treatment through multivariable logistic regression using available baseline patient characteristics. Patients were then matched based on CCB treatments and similar baseline characteristics, using one-to-one nearest-neighbour matching without replacement. All variables listed in the baseline characteristics table (except glycated haemoglobin, as this was not recorded in two trials) were used for the propensity score matching analysis. The balance between the matched groups was evaluated by calculating the absolute standardized differences before and after matching. A difference of less than 10% was deemed acceptable for achieving balance. We also conducted a comparison using inverse probability weighting.
All analyses were performed using STATA version 17.0 (StataCorp., College Station, TX, USA) and R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Overall, 16 954 patients with HFpEF and HFmrEF were included, of whom 5874 (34.6%) were treated with a CCB at baseline. CCB subtype was not recorded in I-Preserve. Among the 12 788 participants in the remaining three trials excluding I-Preserve, 3678 patients (28.8%) were treated with a dihydropyridine and 521 (4.1%) with a non-dihydropyridine (i.e. among patients receiving a CCB, 87.6% were treated with a dihydropyridine). Overall, 15.4% and 20.2% of patients treated with a CCB at baseline discontinued this treatment by 1 and 2 years, respectively. The median duration of follow-up in the pooled four-trial cohort was 34.1 months.
Baseline characteristics
Baseline characteristics according to CCB use (and CCB subtypes) are shown in Table 1. As can be seen, baseline characteristics differed not only between patients treated or not treated with a CCB but also according to the type of CCB. There were some differences between the patients in the trial included. For example, the I-Preserve trial had a higher proportion of patients in NYHA class III or IV and a higher baseline eGFR whereas the DELIVER trial had a lower mean LVEF, both reflecting the specific inclusion criteria. The prevalence of common comorbidities associated with HFpEF/HFmrEF, such as diabetes mellitus, hypertension, AF, and coronary disease, varied somewhat from trial to trial. However, in each trial, the differences between patients treated with CCB and those who were not treated with a CCB were similar across the four trials (online supplementary Table S3).
| Overall (n = 16 954) | p-value | Without I-Preserve (n = 12 788)a | p-value | ||||
|---|---|---|---|---|---|---|---|
| No CCB (n = 11 080) | CCB (n = 5874) | No CCB (n = 8589) | Dihydropyridine (n = 3678) | Non-dihydropyridine (n = 521) | |||
| Age (years) | 71.9 ± 8.9 | 72.0 ± 8.4 | 0.66 | 71.9 ± 9.3 | 72.1 ± 8.8 | 73.3 ± 9.1 | 0.003 |
| Age >70 years | 6432 (58.1) | 3423 (58.3) | 0.78 | 5050 (58.8) | 2212 (60.1) | 329 (63.1) | 0.08 |
| Male sex | 5601 (50.6) | 2754 (46.9) | <0.001 | 4566 (53.2) | 1883 (51.2) | 246 (47.2) | 0.008 |
| Region | <0.001 | <0.001 | |||||
| North America | 2011 (18.1) | 1261 (21.5) | 1800 (21.0) | 839 (22.8) | 242 (46.4) | ||
| Latin America | 1732 (15.6) | 825 (14.0) | 1351 (15.7) | 439 (11.9) | 47 (9.0) | ||
| Western Europe | 3937 (35.5) | 1917 (32.6) | 2994 (34.9) | 1317 (35.8) | 80 (15.4) | ||
| Eastern/Central Europe | 1992 (18.0) | 1203 (20.5) | 1082 (12.6) | 593 (16.1) | 40 (7.7) | ||
| Asia/Pacific and Other | 1408 (12.7) | 668 (11.4) | 1362 (15.9) | 490 (13.3) | 112 (21.5) | ||
| Race | <0.001 | <0.001 | |||||
| White | 8911 (80.4) | 4678 (79.6) | 6538 (76.1) | 2830 (76.9) | 352 (67.6) | ||
| Black | 339 (3.1) | 306 (5.2) | 313 (3.6) | 218 (5.9) | 31 (6.0) | ||
| Asian | 1318 (11.9) | 616 (10.5) | 1299 (15.1) | 463 (12.6) | 114 (21.9) | ||
| Other | 512 (4.6) | 274 (4.7) | 439 (5.1) | 167 (4.5) | 24 (4.6) | ||
| BMI (kg/m2) | 29.9 ± 5.9 | 31.1 ± 6.2 | <0.001 | 30.1 ± 6.1 | 31.4 ± 6.2 | 31.1 ± 7.1 | <0.001 |
| BMI category | <0.001 | <0.001 | |||||
| <18.5 | 72 (0.7) | 25 (0.4) | 57 (0.7) | 18 (0.5) | 1 (0.2) | ||
| 18.5–24.9 | 2114 (19.1) | 838 (14.3) | 1665 (19.4) | 512 (13.9) | 109 (21.0) | ||
| 25.0–29.9 | 3956 (35.8) | 1936 (33.0) | 2895 (33.7) | 1101 (30.0) | 153 (29.4) | ||
| ≥30 | 4918 (44.5) | 3059 (52.2) | 3962 (46.2) | 2041 (55.6) | 257 (49.4) | ||
| NYHA class III or IV | 4013 (36.2) | 2363 (40.2) | <0.001 | 2067 (24.1) | 914 (24.9) | 134 (25.7) | 0.50 |
| Heart rate (bpm) | 71.2 ± 11.7 | 70.3 ± 11.3 | <0.001 | 71.2 ± 12.0 | 69.4 ± 11.5 | 73.4 ± 12.4 | <0.001 |
| Systolic blood pressure (mmHg) | 129.1 ± 15.7 | 133.9 ± 15.2 | <0.001 | 127.3 ± 15.4 | 133.3 ± 15.0 | 127.0 ± 15.7 | <0.001 |
| Systolic blood pressure >140 mmHg | 2320 (20.9) | 1766 (30.1) | <0.001 | 1559 (18.2) | 1077 (29.3) | 102 (19.6) | <0.001 |
| Diastolic blood pressure (mmHg) | 74.8 ± 10.4 | 75.2 ± 10.7 | 0.01 | 73.7 ± 10.4 | 74.0 ± 11.0 | 72.5 ± 11.0 | 0.01 |
| Pressure pulse (mmHg) | 54.3 ± 13.7 | 58.7 ± 14.2 | <0.001 | 53.6 ± 13.8 | 59.3 ± 14.6 | 54.4 ± 15.3 | <0.001 |
| eGFR (ml/min/1.73 m2) | 65.0 ± 20.6 | 63.9 ± 20.9 | 0.001 | 62.9 ± 19.6 | 60.2 ± 19.4 | 62.0 ± 18.6 | <0.001 |
| eGFR <60 ml/min/1.73 m2 | 4781 (43.4) | 2729 (46.6) | <0.001 | 4020 (46.8) | 1951 (53.0) | 272 (52.2) | <0.001 |
| LVEF (%) | 56.0 ± 8.7 | 58.4 ± 8.6 | <0.001 | 55.2 ± 8.5 | 57.4 ± 8.2 | 58.4 ± 8.3 | <0.001 |
| ≥50% | 8348 (75.3) | 5054 (86.0) | <0.001 | 6224 (72.5) | 3066 (83.4) | 460 (88.3) | <0.001 |
| ≥60% | 4075 (36.8) | 2788 (47.5) | <0.001 | 2945 (34.3) | 1615 (43.9) | 238 (45.7) | <0.001 |
| NT-proBNP (pg/ml) | 912 (465–1678) | 715 (365–1354) | <0.001 | 1022 (584–1779) | 843 (478–1492) | 1055 (655–1740) | <0.001 |
| NT-proBNP–AF | 1265 (775–2040) | 1148 (703–1804) | <0.001 | 1296 (826–2066) | 1176 (741–1858) | 1286 (826–1982) | <0.001 |
| NT-proBNP–No AF | 576 (340–1135) | 468 (266–846) | <0.001 | 691 (435–1276) | 596 (393–1012) | 603 (418–906) | <0.001 |
| HbA1cb (%) | 6.5 ± 1.3 | 6.7 ± 1.4 | <0.001 | 6.5 ± 1.3 | 6.8 ± 1.5 | 6.4 ± 1.2 | <0.001 |
| Medical history | |||||||
| Diabetes mellitus | 4111 (37.1) | 2679 (45.6) | <0.001 | 3435 (40.0) | 2014 (54.8) | 191 (36.7) | <0.001 |
| Hypertension | 9697 (87.5) | 5678 (96.7) | <0.001 | 7595 (88.4) | 3632 (98.7) | 460 (88.3) | <0.001 |
| AF | 5401 (48.8) | 2522 (43.0) | <0.001 | 4601 (53.6) | 1717 (46.7) | 372 (72.5) | <0.001 |
| Angina pectoris | 3191 (28.8) | 1960 (33.4) | <0.001 | 2093 (24.4) | 1123 (30.5) | 155 (27.7) | <0.001 |
| Myocardial infarction | 2788 (25.2) | 1262 (21.5) | <0.001 | 2140 (24.9) | 855 (23.2) | 79 (15.2) | <0.001 |
| Coronary artery bypass graftc | 1065 (12.4) | 592 (14.0) | 0.01 | 1065 (12.4) | 529 (14.4) | 53 (10.2) | 0.002 |
| Percutaneous coronary interventionc | 1936 (22.5) | 1005 (23.7) | 0.14 | 1936 (22.5) | 914 (24.9) | 83 (15.9) | <0.001 |
| Peripheral arterial diseasec | 494 (5.8) | 351 (8.3) | <0.001 | 494 (5.8) | 316 (8.6) | 34 (6.6) | <0.001 |
| Asthmac | 572 (6.7) | 360 (8.5) | <0.001 | 572 (6.7) | 269 (7.3) | 89 (17.1) | <0.001 |
| COPDc | 1319 (11.9) | 725 (12.3) | 0.41 | 1086 (12.7) | 463 (12.6) | 95 (18.2) | <0.001 |
| Asthma or COPD | 1501 (17.5) | 828 (19.5) | 0.005 | 1501 (17.5) | 653 (17.8) | 164 (31.5) | <0.001 |
| Strokec | 792 (9.2) | 471 (11.1) | <0.001 | 792 (9.2) | 425 (11.6) | 41 (7.9) | <0.001 |
| Stroke or TIAd | 1158 (11.6) | 695 (13.4) | 0.001 | 1016 (13.4) | 538 (16.9) | 52 (12.9) | <0.001 |
| Medication | |||||||
| ACE-I | 4189 (37.8) | 1993 (33.9) | <0.001 | 3477 (40.5) | 1492 (40.6) | 161 (30.9) | <0.001 |
| ACE-I or ARBd | 7318 (85.2) | 3705 (87.4) | <0.001 | 7318 (85.2) | 3297 (89.6) | 378 (72.6) | <0.001 |
| Beta-blocker | 8707 (78.6) | 4105 (69.9) | <0.001 | 7116 (82.9) | 3003 (81.6) | 245 (47.0) | <0.001 |
| MRAd | 3337 (33.4) | 1202 (23.2) | <0.001 | 2892 (38.5) | 885 (28.3) | 116 (29.5) | <0.001 |
| Loop diureticsd | 7183 (71.9) | 3539 (68.2) | <0.001 | 5852 (78.0) | 2381 (76.1) | 313 (79.6) | 0.07 |
| Any diuretics without MRA | 9914 (89.7) | 5330 (90.8) | 0.02 | 7851 (91.7) | 3466 (94.4) | 474 (91.2) | <0.001 |
| Digoxin | 1121 (10.1) | 388 (6.6) | <0.001 | 729 (8.5) | 151 (4.1) | 65 (12.5) | <0.001 |
| Statin | 6095 (55.0) | 3357 (57.2) | 0.008 | 5385 (62.7) | 2540 (69.1) | 296 (56.8) | <0.001 |
| Anticoagulants | 4358 (39.3) | 1995 (34.0) | <0.001 | 3835 (44.7) | 1392 (37.8) | 317 (60.8) | <0.001 |
| Anticoagulants in AF patients | 3903 (72.3) | 1803 (71.5) | 0.48 | 3469 (75.4) | 1263 (73.6) | 301 (80.9) | 0.01 |
| Any antiplatelets including aspirin | 5231 (47.2) | 3083 (52.5) | <0.001 | 3783 (44.1) | 1884 (51.2) | 214 (41.1) | <0.001 |
- Data are presented as mean ± standard deviation or median (interquartile range) for continuous measures, and n (%) for categorical measures.
- ACE-I, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; TIA, transient ischaemic attack.
- a Thirty-eight patients with both dihydropyridine and non-dihydropyridine drugs are excluded.
- b Not available in TOPCAT-Americas and I-Preserve.
- c Not available in I-Preserve.
- d Not available in TOPCAT-Americas.
Demographics and physiologic measures
Calcium channel blocker users were more often female than male and non-dihydropyridine CCBs were used more commonly in North America and the Asia Pacific region than in other regions. CCB users overall had a higher blood pressure than non-users, but this difference was driven by dihydropyridine use.
Medical history and comorbidity
Calcium channel blocker users overall more often had a history of hypertension than non-users, but this difference was driven by dihydropyridine use. This was also true for a history of diabetes in both these respects and diabetes was less common in patients receiving a non-dihydropyridine CCB than in patients not receiving a CCB. However, the reverse was true for AF which was much more common among users of non-dihydropyridine CCBs than dihydropyridine CCBs. Similarly, a history of asthma or chronic obstructive pulmonary disease was more frequent among those treated with a non-dihydropyridine CCB.
Heart failure characteristics
Calcium channel blocker users overall had a higher average LVEF than non-users. Among those treated with a CCB, more patients had an LVEF ≥50% compared with people not treated with a CCB. Median NT-proBNP level was also lower among patients treated with a CCB compared to those who were not, regardless of the presence of atrial fibrillation.
Other medications
Beta-blockers were used less frequently among patients treated with a CCB, especially a non-dihydropyridine CCB.
Outcomes according to baseline treatment with or without a calcium channel blocker
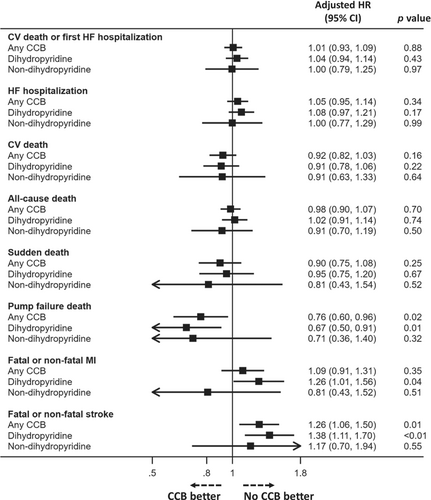
Outcomes according to CCB use versus no use and by type of CCB are shown in Figure 1 and online supplementary Figure S2, with adjusted risk shown in Figure 2 and online supplementary Figure S1.
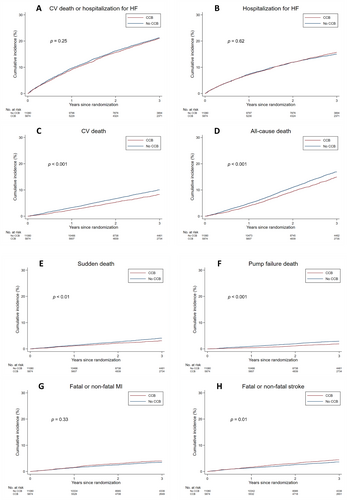
Any calcium channel blocker versus no calcium channel blocker
Overall, the unadjusted risk of the primary composite outcome did not differ between patients treated or not treated with a CCB. Similarly, the risk of HF hospitalization did not differ between patients treated or not treated with a CCB (Table 2 and Figure 1). However, patients treated with a CCB had a trend to a lower unadjusted risk of cardiovascular death (with significantly lower risks of sudden death and pump failure death) and a significantly lower risk of all-cause mortality (Table 2 and Figure 1). By contrast, the unadjusted risk of stroke was higher in patients treated with CCBs.
| Overall (n = 16 954) | Without I-Preserve (n = 12 788)a | ||||
|---|---|---|---|---|---|
| No CCB (n = 11 080) | CCB (n = 5874) | No CCB (n = 8589) | Dihydropyridine (n = 3678) | Non-dihydropyridine (n = 521) | |
| CV death or hospitalization for HF | |||||
| No. of events | 2419 (21.8%) | 1287 (21.9%) | 1743 (20.3%) | 786 (21.4%) | 115 (22.1%) |
| Event rate per 100 py (95% CI) | 8.3 (7.9–8.6) | 7.9 (7.4–8.3) | 8.7 (8.3–9.1) | 8.9 (8.3–9.6) | 9.2 (7.7–11.1) |
| HR (95% CI) | Reference | 0.96 (0.90–1.03) | Reference | 1.03 (0.95–1.12) | 1.07 (0.88–1.29) |
| Adjusted HRb (95% CI) | Reference | 1.01 (0.93–1.09) | Reference | 1.04 (0.94–1.14) | 1.00 (0.79–1.25) |
| Hospitalization for HF | |||||
| No. of events | 1698 (15.3%) | 948 (16.1%) | 1277 (14.9%) | 610 (16.6%) | 91 (17.5%) |
| Event rate per 100 py (95% CI) | 5.8 (5.5–6.1) | 5.8 (5.4–6.2) | 6.4 (6.0–6.7) | 6.9 (6.4–7.5) | 7.3 (6.0–9.0) |
| HR (95% CI) | Reference | 1.02 (0.95–1.11) | Reference | 1.10 (0.99–1.21) | 1.17 (0.95–1.45) |
| Adjusted HRb (95% CI) | Reference | 1.05 (0.95–1.14) | Reference | 1.08 (0.97–1.21) | 1.00 (0.77–1.29) |
| CV death | |||||
| No. of events | 1197 (10.8%) | 547 (9.3%) | 800 (9.3%) | 285 (7.7%) | 44 (8.4%) |
| Event rate per 100 py (95% CI) | 3.8 (3.6–4.0) | 3.1 (2.8–3.3) | 3.7 (3.4–3.9) | 2.9 (2.6–3.3) | 3.2 (2.3–4.2) |
| HR (95% CI) | Reference | 0.81 (0.73–0.90) | Reference | 0.80 (0.70–0.92) | 0.84 (0.62–1.14) |
| Adjusted HRb (95% CI) | Reference | 0.92 (0.82–1.03) | Reference | 0.91 (0.78–1.06) | 0.91 (0.63–1.33) |
| All-cause mortality | |||||
| No. of events | 2011 (18.1%) | 971 (16.5%) | 1445 (16.8%) | 574 (15.6%) | 79 (15.2%) |
| Event rate per 100 py (95% CI) | 6.3 (6.0–6.6) | 5.4 (5.1–5.8) | 6.6 (6.3–7.0) | 5.9 (5.5–6.4) | 5.7 (4.5–7.1) |
| HR (95% CI) | Reference | 0.85 (0.79–0.92) | Reference | 0.89 (0.81–0.98) | 0.83 (0.66–1.05) |
| Adjusted HRb (95% CI) | Reference | 0.98 (0.90–1.07) | Reference | 1.02 (0.91–1.14) | 0.91 (0.70–1.19) |
| Sudden death | |||||
| No. of events | 465 (4.2%) | 205 (3.5%) | 307 (3.6%) | 118 (3.2%) | 12 (2.3%) |
| Event rate per 100 py (95% CI) | 1.5 (1.3–1.6) | 1.1 (1.0–1.3) | 1.4 (1.3–1.6) | 1.2 (1.0–1.5) | 0.9 (0.5–1.5) |
| HR (95% CI) | Reference | 0.79 (0.67–0.93) | Reference | 0.87 (0.71–1.08) | 0.95 (0.75–1.20) |
| Adjusted HRb (95% CI) | Reference | 0.90 (0.75–1.08) | Reference | 0.95 (0.75–1.20) | 0.81 (0.43–1.54) |
| Pump failure death | |||||
| No. of events | 344 (3.1%) | 123 (2.1%) | 264 (3.1%) | 67 (1.8%) | 11 (2.1%) |
| Event rate per 100 py (95% CI) | 1.1 (1.0–1.2) | 0.7 (0.6–0.8) | 1.2 (1.1–1.4) | 0.7 (0.5–0.9) | 0.8 (0.4–1.4) |
| HR (95% CI) | Reference | 0.64 (0.52–0.79) | Reference | 0.57 (0.44–0.74) | 0.65 (0.35–1.19) |
| Adjusted HRb (95% CI) | Reference | 0.76 (0.60–0.96) | Reference | 0.67 (0.50–0.91) | 0.71 (0.36–1.40) |
| Fatal or non-fatal MI | |||||
| No. of events | 406 (3.7%) | 240 (4.1%) | 304 (3.5%) | 174 (4.7%) | 17 (3.3%) |
| Event rate per 100 py (95% CI) | 1.3 (1.2–1.4) | 1.4 (1.2–1.6) | 1.4 (1.3–1.6) | 1.8 (1.6–2.1) | 1.2 (0.8–2.0) |
| HR (95% CI) | Reference | 1.09 (0.93–1.27) | Reference | 1.32 (1.09–1.59) | 0.91 (0.56–1.48) |
| Adjusted HRb (95% CI) | Reference | 1.09 (0.91–1.31) | Reference | 1.26 (1.01–1.56) | 0.81 (0.43–1.52) |
| Fatal or non-fatal stroke | |||||
| No. of events | 418 (3.8%) | 279 (4.7%) | 300 (3.5%) | 174 (4.7%) | 24 (4.6%) |
| Event rate per 100 py (95% CI) | 1.3 (1.2–1.5) | 1.6 (1.4–1.8) | 1.4 (1.2–1.6) | 1.8 (1.6–2.1) | 1.8 (1.2–2.6) |
| HR (95% CI) | Reference | 1.22 (1.05–1.42) | Reference | 1.33 (1.10–1.61) | 1.29 (0.85–1.95) |
| Adjusted HRb (95% CI) | Reference | 1.26 (1.06–1.50) | Reference | 1.38 (1.11–1.70) | 1.17 (0.70–1.94) |
- CCB, calcium channel blocker; CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; py, person-years.
- a Thirty-eight patients with both dihydropyridine and non-dihydropyridine drugs are excluded.
- b Adjusted for age, sex, region, race, trials and treatment groups, body mass index, prior HF hospitalization, hypertension, MI, strokec, diabetes mellitus, atrial fibrillation, NYHA class I or II vs. III or IV, heart rate, systolic blood pressure, estimated glomerular filtration rate, left ventricular ejection fraction, and log-transformed NT-proBNP.
- c Stroke or transient ischeamic attack in I-Preserve.
After adjustment for other prognostic variables, only the risks of pump failure death (HR 0.76, 95% CI 0.60–0.96) and stroke (HR 1.26, 95% CI 1.06–1.50) remained significantly different (Table 2 and Figure 2). Analysis of the dataset excluding the TOPCAT-Americas patients and an additional multivariable analysis model including baseline beta-blocker treatment gave consistent findings (online supplementary Tables S4, S5, and Figure S1).
Dihydropyridine versus non-dihydropyridine calcium channel blockers
The pattern of events was broadly similar for dihydropyridine and non-dihydropyridine CCBs (Table 2, Figure 2, and online supplementary Figure S2). These results of the sensitivity analyses excluding TOPCAT were consistent with the primary analysis (online supplementary Table S4 and Figure S1).
Outcomes according to trials included
Analysis of each included trial suggested consistent findings with overall analysis (online supplementary Tables S6–S9). The interaction for the primary outcome between CCB treatment and the trials included was not significant (p for interaction = 0.42).
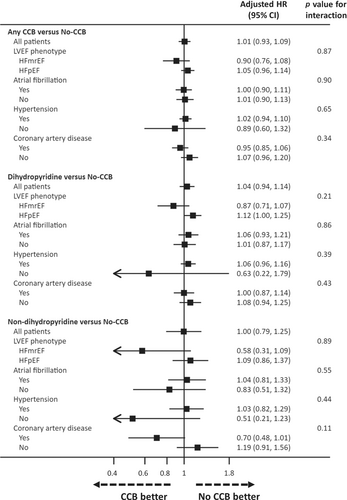
Outcomes according to baseline treatment with or without a calcium channel blocker in patient subgroups
The risks of the primary outcome according to CCB use versus no use (and by type of CCB) in relevant patient subgroups are shown in Figure 3. The pattern in each subgroup was consistent with the overall pattern of risk related to CCB treatment for this outcome.
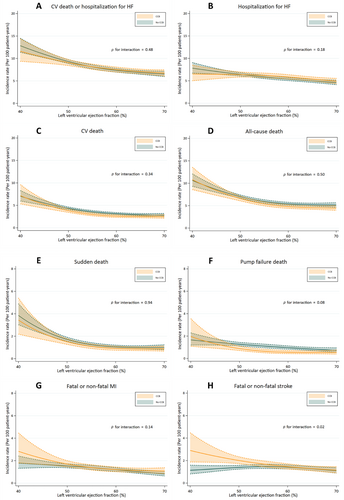
Outcomes according to baseline treatment with or without a calcium channel blocker across the range of left ventricular ejection fraction
Incidence rates for the outcomes of interest across the range of LVEF are shown in Figure 4 according to treatment with or without a CCB. Overall, the risk of all outcomes was higher in patients with a LVEF at the lower end of the range of LVEF that is, in patients with HFmrEF. The risk of stroke was higher in patients treated with a CCB (p for interaction = 0.02) (Figure 4). AF was not more prevalent in patients with HFmrEF, and its prevalence did not differ between patients treated with or without a CCB (online supplementary Table S10).
Propensity-score matching and inverse probability weighting
After matching based on CCB treatment and available baseline characteristics, the matched groups consisted of a total of 7042 patients. The baseline variables showed acceptable standardized differences between patients with and without CCB use (online supplementary Table S11). Online supplementary Table S12 shows clinical outcomes in the matched groups. The findings were consistent with the main results, with the exception of fatal or non-fatal stroke where no significant difference was observed between the groups. Online supplementary Table S13 shows the results of inverse probability weighting analysis. The results were also consistent with the primary results, but the lower risk of pump failure death in CCB users was not statistically significant.
Discussion
Although widely prescribed in people with HF and a LVEF >40%, the safety of CCBs has never been formally evaluated in these patients. Given that patients with HFmrEF are clinically more similar to patients with HFrEF than HFpEF, and respond to treatments effective in left ventricular systolic dysfunction, the concerns related to the use of CCBs in patients with HFrEF might also extend to those with HFmrEF.11, 20 Furthermore, although a negative inotropic effect should be of less concern in patients with HFpEF, in some patients with combined post- and pre-capillary pulmonary hypertension, CCB-induced pulmonary vasodilatation could flood the pre-capillary vasculature causing pulmonary oedema.21 Consequently, we examined outcomes according to CCB use in an individual patient data analysis of nearly 17 000 individuals with HF and a LVEF >40% enrolled in four landmark randomized controlled trials. We found that baseline use of a CCB was not associated with a higher risk of cardiovascular death or HF hospitalization, even in patients with HFmrEF. Moreover, this also was the case for patients treated with a non-dihydropyridine CCB, despite these CCBs causing the greatest concern in patients with impaired left ventricular systolic function.22, 23 Indeed, the outcome most likely to be sensitive to a negative inotropic action of CCBs, pump failure death, was nominally significantly less common in patients treated with a CCB compared to those not. This difference remained after adjustment for other prognostic variables. Although these included LVEF, we cannot exclude residual confounding related to left ventricular systolic function given the higher baseline LVEF in patients treated with a CCB compared to those not.
Surprisingly, we found that CCB treatment was associated with a higher risk of stroke (and trend to a higher risk of MI). Although this excess risk persisted after adjustment for a variety of recognized stroke risk factors including a history of hypertension, stroke and MI, there may be residual confounding given the indication for CCBs in patients with hypertension and coronary heart disease. Interestingly, the restricted cubic spline analysis suggested the elevated risk of stroke with CCB treatment was confined to the lower end of the range of LVEF that is, in patients with HFmrEF. Scrutiny of the baseline characteristics of participants with HFmrEF, compared to HFpEF, did not reveal any obvious explanation in terms of relevant risk factors such as a prior history of stroke/transient ischaemic attack, hypertension, coronary disease, or AF (or use of anticoagulants in patients with AF). Only diabetes and peripheral artery disease were relatively more common in individuals treated with a CCB, versus those not, among participants with HFmrEF versus HFpEF. These differences could be relevant to the finding of a higher risk of stroke observed among HFmrEF patients treated with a CCB.
Although our findings overall suggest CCBs are not associated with a higher risk of HF hospitalization or death, the need for treatment with these drugs should always be reviewed in patients with HFmrEF/HFpEF.11, 20, 24, 25 There are better alternatives for treating hypertension, specifically mineralocorticoid receptor antagonists26, 27 and sacubitril/valsartan.28 For the treatment of angina, the alternatives are less good. Oral nitrates caused a reduction in exercise tolerance in the Nitrate's Effect on Activity Tolerance in Heart Failure with Preserved Ejection Fraction (NEAT-HFpEF) trial29 and ivabradine and beta-blockers may also impair functional capacity in some patients with HFmrEF/HFpEF with chronotropic incompetence.30-32 By extrapolation from trials in HFrEF, amlodipine and felodipine appear to be the dihydropyridine CCBs with the best-studied safety profile in HF.16, 33, 34 Percutaneous coronary intervention is also an option in some patients with angina. Non-dihydropyridine CCBs are recommended as first-line therapy for the control of the ventricular rate in patients with AF, as are beta-blockers, with digoxin as a second-line treatment.24, 35 Again, by extrapolation from MI trials, verapamil22, 23 appears a safer option than diltiazem.15
Limitations
We studied patients enrolled in randomized clinical trials with specific inclusion and exclusion criteria and our results may not be generalizable to all patients with HFmrEF/HFpEF.36 Because this was an observational analysis, we cannot infer a causal relationship between CCBs and the clinical outcomes examined; this can only be done in a prospective randomized controlled trial. The number of patients who received a non-dihydropyridine CCB was relatively modest. As a common limitation of analysis of continuous measures, the association between LVEF and patient outcomes was less certain at the extremes of the LVEF range.
Conclusion
In an individual patient data analysis of nearly 17 000 individuals with HF and a LVEF >40%, the risks of death and HF hospitalization were not higher in patients treated with a CCB, particularly dihydropyridines. Although cause-and-effect cannot be inferred in an observational analysis, we believe these findings are reassuring regarding the safety of using CCBs in patients with HFpEF and even HFmrEF. However, to be certain of this, would require conduct of a prospective randomized controlled trial.
Conflict of interest: S.M. has received research grants and personal fees from Abbott, Bayer Pharma, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Novartis, Ono Pharma, Orbus Neich, Otsuka Pharma, and the Uehara Memorial Foundation. T.K. received speaker fees from Abbott, Ono Pharma, Otsuka Pharma, Novartis, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, and Abiomed. M.Y. has received a grant from AstraZeneca to attend a medical congress. R.T.C. has received consultancy honoraria from Bayer for speaking honoraria from AstraZeneca. K.F.D. reports receiving honoraria from AstraZeneca and a research grant to his institution from Boehringer Ingelheim. R.A.d.B. has received research grants and fees (outside the submitted work) from AstraZeneca, Abbott, Boehringer Ingelheim, Cardio Pharmaceuticals Gmbh, Ionis Pharmaceuticals, Inc, Novo Nordisk, and Roche; has received speaker fees from Abbott, AstraZeneca, Bayer, Novartis, and Roche (outside the submitted work). A.S.D. has received grants and personal fees from AstraZeneca during the conduct of the study; personal fees from Abbott, Biofourmis, Boston Scientific, Boehringer Ingelheim, Corvidia, DalCor Pharma, Relypsa, Regeneron, and Merck; grants and personal fees from Alnylam and Novartis; and personal fees from Amgen, outside the submitted work. C.S.P.L. is supported by a clinician scientist award from the National Medical Research Council of Singapore; has received research support from AstraZeneca, Bayer, Boston Scientific, and Roche Diagnostics; has served as a consultant or on the advisory board or steering committee or executive committee for Actelion, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc, Us2.ai, Janssen Research & Development LLC, Medscape, Merck, Novartis, Novo Nordisk, Radcliffe Group Ltd, Roche Diagnostics, Sanofi, and WebMD Global LLC; and serves as the cofounder and nonexecutive director of Us2.ai. M.P. has received consulting fees from AbbVie, Actavis, Amgen, Amarin, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Casana, CSL Behring, Cytokinetics, Johnson & Johnson, Eli Lilly and Company, Moderna, Novartis, ParatusRx, Pfizer, Relypsa, Salamandra, Synthetic Biologics, and Theravance; and is a Trial Executive Committee member of Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance (trial sponsor). B.P. reports consultancy fees from: Bayer, AstraZeneca, Boehringer Ingelheim, Merck, Lexicon, KBP BioSciences (stock options), Vifor (stockoptions), Sarfez (stock options), scPharmaceuticals (stock options), SQInnovation (stock options), G3pharkaceuticslŝ, ProtonIntel (stock options), Cereno Scientific (stock options), Brainstorm Medical (stock options). US patent 9931422–site specific delivery of eplerenone to the myocardium. US patent pending 63/045783 Histone acetylation modulating agents for the protection and treatment of organ damage. J.L.R. reports grants and consulting fees from Novartis and consulting fees from AstraZeneca. M.V. has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, and Sanofi, and Tricog Health, speaker engagements with AstraZeneca, Novartis and Roche Diagnostics, and participates in clinical trial committees for studies sponsored by AstraZeneca, Occlutech, Impulse Dynamicx, Galmed and Novartis. F.Z. reports personal fees from Boehringer Ingelheim, Janssen, Novartis, Boston Scientific, Amgen, CVRx, AstraZeneca, Vifor Fresenius, Cardior, Cereno pharmaceutical, Applied Therapeutics, Merck, Bayer, and Cellprothera. M.R.Z. has received grants, consulting fees, and participation in a data safety monitoring board or advisory board as part of the committees for the PARAGON-HF and PARADIGM-HF trials. S.D.S. has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, Ionis, Lilly, Mesoblast, MyoKardia, National Institutes of Health/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, US2.AI; and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GlaxoSmithKline, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellPro- Thera, Moderna, American Regent, and Sarepta. P.S.J. reports speaker fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, Sun Pharmaceuticals, Intas Pharmaceuticals; advisory board fees from AstraZeneca, Boehringer Ingelheim, Novartis; research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices Inc; Dr Jhund's employer the University of Glasgow has been remunerated for clinical trial work from AstraZeneca, Bayer AG, Novartis and NovoNordisk. Director, Global Clinical Trial Partners. J.J.V.M. reports payments through Glasgow University from work on clinical trials; consulting and other activities from Amgen, AstraZeneca, Bayer, Cardurion, Cytokinetics, GSK, KBP Biosciences, and Novartis; personal consultancy fees from Alnylam Pharma., Bayer, BMS, George Clinical PTY Ltd., Ionis Pharma., Novartis, Regeneron Pharma., River 2 Renal Corporation. J.J.V.M. receives personal lecture fees from Abbott, Alkem Metabolics, AstraZeneca, Blue Ocean Scientific Solutions Ltd., Boehringer Ingelheim, Canadian Medical and Surgical Knowledge, Emcure Pharma. Ltd., Eris Lifesciences, European Academy of CME, Hikma Pharmaceuticals, Imagica health, Intas Pharma, J.B. Chemicals & Pharma. Ltd., Lupin Pharma, Medscape/Heart.Org, ProAdWise Communications, Radcliffe Cardiology, Sun Pharma, The Corpus, Translation Research Group, and Translational Medicine Academy. J.J.V.M is a director of Global Clinical Trial Partners Ltd.




