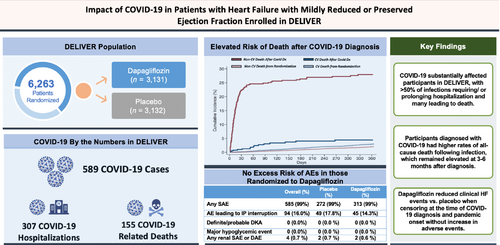
Impact of COVID-19 in patients with heart failure with mildly reduced or preserved ejection fraction enrolled in the DELIVER trial
Abstract
Aim
COVID-19 may affect clinical risk in patients with heart failure. DELIVER began before and was conducted during the COVID-19 pandemic. This study aimed to evaluate the association between COVID-19 and clinical outcomes among DELIVER participants.
Methods and results
Participants with chronic heart failure with mildly reduced or preserved ejection fraction (HFmrEF/HFpEF) were randomized to dapagliflozin or placebo across 350 sites in 20 countries. COVID-19 was investigator-reported and the contribution of COVID-19 to death was centrally adjudicated. We assessed (i) the incidence of COVID-19, (ii) event rates before/during the pandemic, and (iii) risks of death after COVID-19 diagnosis compared to risks of death in participants without COVID-19. Further, we performed a sensitivity analysis assessing treatment effects of dapagliflozin vs. placebo censored at pandemic onset. Of 6263 participants, 589 (9.4%) developed COVID-19, of whom 307 (52%) required/prolonged hospitalization. A total of 155 deaths (15% of all deaths) were adjudicated as definitely/possibly COVID-19-related. COVID-19 cases and deaths did not differ by randomized assignment. Death rate in the 12 months following diagnosis was 56.1 (95% confidence interval [CI] 48.0–65.6) versus 6.4 (95% CI 6.0–6.8)/100 participant-years among trial participants with versus without COVID-19 (adjusted hazard ratio [aHR] 8.60, 95% CI 7.18–10.30). Risk was highest 0–3 months following diagnosis (153.5, 95% CI 130.3–180.8) and remained elevated at 3–6 months (12.6, 95% CI 6.6–24.3/100 participant-years). After excluding investigator-reported fatal COVID-19 events, all-cause death rates in the 12 months following diagnosis among COVID-19 survivors (n = 458) remained higher (aHR 2.46, 95% CI 1.83–3.33) than rates for all trial participants from randomization, with censoring of participants who developed COVID-19 at the time of diagnosis. Dapagliflozin reduced cardiovascular death/worsening HF events when censoring participants at COVID-19 diagnosis (HR 0.81, 95% CI 0.72–0.91) and pandemic onset (HR 0.72, 95% CI 0.58–0.89). There were no diabetic ketoacidosis or major hypoglycaemic events within 30 days of COVID-19.
Conclusion
DELIVER is one of the most extensive experiences with COVID-19 of any cardiovascular trial, with >75% of follow-up time occurring during the pandemic. COVID-19 was common, with >50% of cases leading to hospitalization or death. Treatment benefits of dapagliflozin persisted when censoring at COVID-19 diagnosis and pandemic onset. Patients surviving COVID-19 had a high early residual risk.
Clinical Trial Registration: ClinicalTrials.gov Identifier NCT03619213.
Graphical Abstract
Impact of COVID-19 in patients with heart failure (HF) and mildly reduced and preserved ejection fraction. AE, adverse event; DAE, adverse events leading to discontinuation of study drug; DKA, diabetic ketoacidosis; IP, investigational product; SAE, serious adverse event. [Correction added on 15 January 2024, after first online publication: list of abbreviations have been added in this version.]
Introduction
Patients with cardiovascular (CV) disease, including heart failure (HF), are at elevated risk of complications associated with COVID-191; however, few data exist describing the immediate and longer-term risks faced by individuals with HF who develop COVID-19. Large registries and trials may be well-suited to assess such risks; however, CV disease COVID-19 registries have generally limited enrolment to hospitalized COVID-19 patients, have had short follow-up periods, or have lacked central adjudication of clinical events.2, 3 In addition, CV trials during this period had largely completed enrolment and follow-up prior to pandemic onset,4, 5 enrolled North American participants only,6 and evaluated primary surrogate endpoints.6 In addition, the COVID-19 pandemic had a major effect on rates of death, CV outcomes and treatment effects in CV clinical trials.5, 7 DELIVER (Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure), the largest trial of HF with mildly reduced or preserved ejection fraction (HFmrEF/HFpEF), enrolled a population across four continents, was operational before and during the COVID-19 pandemic8 and observed a treatment effect favouring dapagliflozin. We sought to examine the incidence and impact of COVID-19 in the DELIVER trial.
Methods
Trial design and changes during the COVID-19 pandemic
DELIVER was an international, prospective, multicentre, double-blind, event-driven clinical trial assessing the safety and efficacy of dapagliflozin 10 mg once daily versus placebo in patients with HFmrEF or HFpEF. The design and primary results of the DELIVER trial have been previously published.8 In brief, patients with symptomatic HF, elevated natriuretic peptides (NP), and left ventricular ejection fraction (LVEF) >40% were randomized in a 1:1 fashion to dapagliflozin 10 mg once daily or a matching placebo. Patients were also required to have elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) and evidence of structural heart disease. The primary endpoint of DELIVER was a composite of time to first CV death or worsening HF event (including HF hospitalization or an urgent HF visit).
DELIVER randomized patients across 350 centers in 20 countries in North America, Latin America, Europe, and Asia. The first patient was randomized in September 2018 and the last patient completed the trial follow-up in March 2022. In response to the rise in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and the declaration of COVID-19 as a pandemic by the World Health Organization on 11 March 2020, the sponsor's clinical project team, in conjunction with the trial executive committee, took several steps to minimize risk to participants and trial staff. Trial enrolment was temporarily paused in all active sites on 27 March 2020 (except Chinese sites which initiated randomization in March 2020) and re-started at various time points depending on region and site; home delivery of study drug was initiated after the resumption of enrolment. Local sites were instructed to collect and report COVID-19 information as adverse events. Investigators reported COVID-19 diagnoses, COVID-19-related events leading to or prolonging hospitalization, and fatal COVID-19 events as adverse events. Infection status was determined by local protocols and could have included probable and lab-confirmed diagnoses. All deaths regardless of assigned cause were centrally adjudicated by an independent endpoints committee (Brigham and Women's Hospital, Boston, MA, and University of Glasgow, Scotland, UK) as definitely, possibly, or not related to COVID-19. Definite relatedness required a clinical diagnosis with a positive polymerase chain reaction or typical imaging of COVID-19 and possible cases required clinical features and treatment of COVID-19 without definitive test. Guidance to sites from trial leadership included recommendations to consider interruption of the investigational product (IP) in patients with type 2 diabetes and known diabetic ketoacidosis (DKA) risk and to continue IP in non-diabetic patients and those without risk factors or prior DKA.
Statistical analyses
The statistical analysis plan for the DELIVER study was modified prior to unblinding to incorporate a COVID-19 sensitivity analysis, censoring participants at the time of COVID-19 diagnosis, and has been previously reported.8 The primary objectives of this pre-specified analysis by amendment to the academic statistical analysis plan (SAP) were (1) to describe the incidence of COVID-19 in a large, international randomized clinical trial, (2) to assess the impact of the pandemic on clinical event accrual and treatment effects in DELIVER, and (3) to assess the health risks faced by participants with HFmrEF/HFpEF after COVID-19 diagnosis.
We described the incidence of COVID-19 associated, hospitalization, and death during the trial based on investigator-reported adverse events. Baseline characteristics at the time of randomization were compared between participants who did versus did not develop COVID-19 and those with severe versus non-severe disease as reported by investigators. Severe disease was defined as requiring or prolonging hospitalization or resulting in an investigator-reported fatal COVID-19 event. Baseline characteristics were summarized as means and standard deviations, medians and interquartile ranges, or frequencies and percentages, as appropriate. ANOVA, Kruskal–Wallis, and chi-square tests were used to test for between-group differences.
To assess the impact of the COVID-19 pandemic on changes in event rates, we compared event rates (per 100 participant-years) between a pre-pandemic and pandemic period. The pre-pandemic period was defined as before the World Health Organization's declaration of COVID-19 as a pandemic on 11 March 2020. Clinical outcomes assessed included (1) the primary composite outcome, (2) components of the primary composite, and (3) all-cause death. We further compared the proportion of all deaths attributable to CV causes (vs. non-CV causes) and the proportion of primary events due to worsening HF events (vs. CV death) between the two periods. Comparisons were adjusted for time since randomization. The associations between COVID-19 and risks of time-to-event outcomes were estimated by creating time-updated covariates to represent pre- versus post-COVID-19 diagnosis status at the patient level, as well as pre-pandemic and pandemic period (i.e. set to 0 for all patient follow-up prior to 11 March 2020 and set to 1 for all follow-up subsequent to 11 March 2020), such that comparisons were made between participants based on the time they have spent in the study from randomization or from 11 March 2020. We also conducted a dedicated sensitivity analysis evaluating the treatment effects of dapagliflozin versus placebo on clinical outcomes with censoring at the time of pandemic onset.
To assess the health and safety risks faced by participants after COVID-19 diagnosis, we evaluated (1) drug interruptions and withdrawals and (2) adverse events in the 30 days following COVID-19 diagnosis overall and by treatment assignment. In addition, we assessed the risk of clinical outcomes in the 12 months after incident COVID-19 using Cox proportional hazards models. To evaluate excess short-term risk after COVID-19 diagnosis, we compared these risks to those faced by all trial participants from the time of enrolment, censoring participants who developed COVID-19 at the time of diagnosis. Cox models were adjusted for age, sex, race, geographic region, body mass index, HF duration, NT-proBNP, glycated haemoglobin, estimated glomerular filtration rate, baseline use of loop diuretics and angiotensin receptor–neprilysin inhibitor, and history of type 2 diabetes, chronic obstructive pulmonary disease, sleep apnoea, atherosclerotic vascular disease, prior HF hospitalization, smoking, atrial fibrillation/atrial flutter.
All analyses were performed in Stata version 16 (StataCorp, College Station, TX, USA). A nominal p-value of ≤0.05 was considered statistically significant.
Results
COVID-19 among participants in DELIVER
At the time of first reported COVID-19 infection in the trial (16 March 2020), 5153 (82.3%) of the total trial population had been randomized, 363 (5.8%) had already experienced a primary endpoint event (out of a total of 1122 primary events in the trial), and 168 (2.7%) had died; >75% of trial follow-up occurred on or after 11 March 2020. Among the full study population of 6263 participants in DELIVER, 589 (9.4%) were diagnosed with COVID-19 during the trial, 314/3131 (10.0%) of which were randomized to dapagliflozin and 275/3132 (8.7%) randomized to placebo (Graphical Abstract). Most participants with COVID-19 had symptomatic disease (92%) and the majority (n = 307, 52.1%) required hospitalization or had prolongation of an existing hospitalization (Figure 1). Reported COVID-19 cases occurred across all four enrolling regions: Europe and Saudi Arabia (n = 377), Asia (n = 18), Latin America (n = 128) and North America (n = 66).
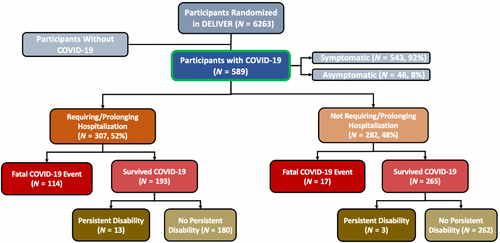
Patients who developed COVID-19 during the trial were similar in age and sex but were more likely to be white, enrolled in Europe/Saudi Arabia, and had greater symptomatic impairment by baseline Kansas City Cardiomyopathy Questionnaire total symptom score (KCCQ-TSS) than those who did not develop COVID-19. Mean LVEF was similar between those who did (54.1 ± 8.4%) and did not (54.2 ± 8.8%) develop COVID-19. Rates of pulmonary comorbidities, including chronic obstructive pulmonary disease and smoking, were also similar between groups; however, participants with COVID-19 were more likely to have cardiometabolic comorbidities, including diabetes, atherosclerotic cardiovascular disease, and obesity. Older patients and those with higher baseline NT-proBNP levels were more likely to have severe COVID-19 (requiring/prolonging hospitalization or resulting in a fatal COVID-19 event, n = 324) versus non-severe COVID-19 (Table 1). Baseline characteristics in the COVID-19 population were similar by treatment assignment (online supplementary Table S1). COVID-19 diagnosis rates over time are summarized in online supplementary Figure S1.
| Characteristics | Developed COVID-19 (n = 589) | Did not develop COVID-19 (n = 5674) | p-value | Severe COVID-19 (n = 324) | Non-severe COVID-19 (n = 265) | p-value |
|---|---|---|---|---|---|---|
| Age (years) | 71.2 ± 9.6 | 71.7 ± 9.5 | 0.17 | 72.1 ± 9.2 | 70.0 ± 10.1 | 0.009 |
| Age group (years) | 0.31 | 0.021 | ||||
| ≤65 | 154 (26.1%) | 1350 (23.8%) | 70 (21.6%) | 84 (31.7%) | ||
| >65–75 | 229 (38.9%) | 2183 (38.5%) | 135 (41.7%) | 94 (35.5%) | ||
| >75 | 206 (35.0%) | 2141 (37.7%) | 119 (36.7%) | 87 (32.8%) | ||
| Men | 319 (54.2%) | 3197 (56.3%) | 0.31 | 186 (57.4%) | 133 (50.2%) | 0.08 |
| Race | < 0.001 | 0.11 | ||||
| White | 508 (86.2%) | 3931 (69.3%) | 281 (86.7%) | 227 (85.7%) | ||
| Asian | 19 (3.2%) | 1255 (22.1%) | 10 (3.1%) | 9 (3.4%) | ||
| Black or African American | 21 (3.6%) | 138 (2.4%) | 10 (3.1%) | 11 (4.2%) | ||
| American Indian or Alaska Native | 20 (3.4%) | 169 (3.0%) | 7 (2.2%) | 13 (4.9%) | ||
| Other | 21 (3.6%) | 181 (3.2%) | 16 (4.9%) | 5 (1.9%) | ||
| Geographic region | < 0.001 | 0.26 | ||||
| Europe and Saudi Arabia | 377 (64.0%) | 2628 (46.3%) | 219 (67.6%) | 158 (59.6%) | ||
| Asia | 18 (3.1%) | 1208 (21.3%) | 9 (2.8%) | 9 (3.4%) | ||
| Latin America | 128 (21.7%) | 1053 (18.6%) | 63 (19.4%) | 65 (24.5%) | ||
| North America | 66 (11.2%) | 785 (13.8%) | 33 (10.2%) | 33 (12.5%) | ||
| Comorbidities | ||||||
| History of: | ||||||
| AFF | 321 (54.5%) | 3231 (56.9%) | 0.25 | 180 (55.6%) | 141 (53.2%) | 0.57 |
| Stroke | 56 (9.5%) | 541 (9.5%) | 0.98 | 33 (10.2%) | 23 (8.7%) | 0.54 |
| Dyslipidemia | 412 (69.9%) | 3578 (63.1%) | < 0.001 | 218 (67.3%) | 194 (73.2%) | 0.12 |
| Type 2 diabetes mellitus | 304 (51.6%) | 2502 (44.1%) | < 0.001 | 172 (53.1%) | 132 (49.8%) | 0.43 |
| Chronic obstructive pulmonary disease | 73 (12.4%) | 619 (10.9%) | 0.27 | 46 (14.2%) | 27 (10.2%) | 0.15 |
| Peripheral artery disease | 42 (7.1%) | 337 (5.9%) | 0.24 | 27 (8.3%) | 15 (5.7%) | 0.21 |
| Sleep apnoea | 59 (10.0%) | 426 (7.5%) | 0.029 | 28 (8.6%) | 31 (11.7%) | 0.21 |
| Myocardial infarction | 180 (30.6%) | 1459 (25.7%) | 0.011 | 102 (31.5%) | 78 (29.4%) | 0.59 |
| Prior HF hospitalization flag | 219 (37.2%) | 2320 (40.9%) | 0.08 | 132 (40.7%) | 87 (32.8%) | 0.048 |
| Any atherosclerotic cardiovascular disease | 356 (60.4%) | 3196 (56.3%) | 0.06 | 201 (62.0%) | 155 (58.5%) | 0.38 |
| Smoking status | 0.61 | 0.67 | ||||
| Current | 41 (7.0%) | 443 (7.8%) | 20 (6.2%) | 21 (7.9%) | ||
| Former | 207 (35.1%) | 2054 (36.2%) | 113 (34.9%) | 94 (35.5%) | ||
| Never | 341 (57.9%) | 3177 (56.0%) | 191 (59.0%) | 150 (56.6%) | ||
| Time from diagnosis of HF | 0.024 | 0.88 | ||||
| 0–3 months | 41 (7.0%) | 527 (9.3%) | 23 (7.1%) | 18 (6.8%) | ||
| >3–6 months | 47 (8.0%) | 545 (9.6%) | 28 (8.6%) | 19 (7.2%) | ||
| >6–12 months | 66 (11.2%) | 776 (13.7%) | 40 (12.3%) | 26 (9.8%) | ||
| >1–2 years | 109 (18.5%) | 886 (15.6%) | 58 (17.9%) | 51 (19.2%) | ||
| >2–5 years | 146 (24.8%) | 1423 (25.1%) | 80 (24.7%) | 66 (24.9%) | ||
| >5 years | 180 (30.6%) | 1512 (26.7%) | 95 (29.3%) | 85 (32.1%) | ||
| Body mass index (kg/m2) | 31.9 ± 6.1 | 29.6 ± 6.1 | < 0.001 | 31.8 ± 6.3 | 32.1 ± 5.8 | 0.63 |
| Body mass index group (kg/m2) | < 0.001 | 0.18 | ||||
| <18.5 (underweight) | 2 (0.3%) | 52 (0.9%) | 0 (0.0%) | 2 (0.8%) | ||
| 18.5–24.9 (normal weight) | 62 (10.5%) | 1281 (22.6%) | 41 (12.7%) | 21 (7.9%) | ||
| 25.0–29.9 (overweight) | 182 (30.9%) | 1891 (33.4%) | 100 (30.9%) | 82 (30.9%) | ||
| 30.0–34.9 (Class I obesity) | 179 (30.4%) | 1395 (24.6%) | 94 (29.0%) | 85 (32.1%) | ||
| 35.0–39.9 (Class II obesity) | 101 (17.1%) | 697 (12.3%) | 51 (15.7%) | 50 (18.9%) | ||
| ≥40 (Class III obesity) | 63 (10.7%) | 352 (6.2%) | 38 (11.7%) | 25 (9.4%) | ||
| NYHA class at baseline | 0.92 | 0.06 | ||||
| I | 0 (0.0%) | 1 (0.0%) | – | – | ||
| II | 441 (74.9%) | 4272 (75.3%) | 232 (71.6%) | 209 (78.9%) | ||
| III | 147 (25.0%) | 1384 (24.4%) | 92 (28.4%) | 55 (20.8%) | ||
| IV | 1 (0.2%) | 17 (0.3%) | 0 (0.0%) | 1 (0.4%) | ||
| KCCQ-TSS | 66.2 ± 21.5 | 70.4 ± 22.2 | < 0.001 | 64.1 ± 20.6 | 68.7 ± 22.4 | 0.015 |
| LVEF (%) | 54.1 ± 8.4 | 54.2 ± 8.8 | 0.93 | 54.2 ± 8.5 | 54.1 ± 8.3 | 0.85 |
| LVEF group (%) | 0.52 | 0.87 | ||||
| ≤49 | 200 (34.0%) | 1916 (33.8%) | 110 (34.0%) | 90 (34.0%) | ||
| 50–59 | 201 (34.1%) | 2055 (36.2%) | 108 (33.3%) | 93 (35.1%) | ||
| ≥60 | 188 (31.9%) | 1703 (30.0%) | 106 (32.7%) | 82 (30.9%) | ||
| NT-proBNP (ng/L) | 975 [569–1621] | 1017 [626–1768] | 0.019 | 1050 [638–1686] | 824 [502–1452] | < 0.001 |
| NT-proBNP in AFF | 1351 [951–2309] | 1402 [964–2208] | 0.61 | 1399 [1003–2286] | 1274 [882–2318] | 0.43 |
| NT-proBNP when no AFF | 666 [451–1187] | 718 [470–1299] | 0.14 | 768 [491–1292] | 610 [406–1074] | 0.002 |
| ECG AFF | 227 (38.5%) | 2417 (42.6%) | 0.06 | 135 (41.7%) | 92 (34.7%) | 0.08 |
| Systolic blood pressure (mmHg) | 130.0 ± 15.5 | 128.1 ± 15.3 | 0.003 | 129.4 ± 15.5 | 130.7 ± 15.5 | 0.29 |
| Diastolic blood pressure (mmHg) | 73.9 ± 10.2 | 73.9 ± 10.4 | 0.85 | 74.1 ± 10.4 | 73.5 ± 10.0 | 0.50 |
| HbA1c (%) | 6.9 ± 1.7 | 6.6 ± 1.4 | < 0.001 | 7.0 ± 1.8 | 6.7 ± 1.5 | 0.10 |
| Pulse rate (bpm) | 70.5 ± 11.4 | 71.6 ± 11.8 | 0.028 | 71.1 ± 10.9 | 69.7 ± 12.0 | 0.13 |
| Creatinine (μmol/L) | 105.5 ± 33.2 | 102.2 ± 30.8 | 0.013 | 108.6 ± 33.6 | 101.7 ± 32.3 | 0.011 |
| eGFR (ml/min/1.73 m2) | 59.3 ± 18.5 | 61.2 ± 19.2 | 0.018 | 57.3 ± 18.3 | 61.6 ± 18.5 | 0.005 |
| Medication use | ||||||
| Loop diuretics | 463 (78.6%) | 4348 (76.7%) | 0.29 | 268 (82.7%) | 195 (73.6%) | 0.007 |
| ACEi | 230 (39.0%) | 2065 (36.4%) | 0.21 | 136 (42.0%) | 94 (35.5%) | 0.11 |
| ARB | 220 (37.4%) | 2052 (36.2%) | 0.57 | 107 (33.0%) | 113 (42.6%) | 0.016 |
| ARNI | 17 (2.9%) | 284 (5.0%) | 0.022 | 9 (2.8%) | 8 (3.0%) | 0.86 |
| Beta-blocker | 484 (82.2%) | 4693 (82.7%) | 0.73 | 274 (84.6%) | 210 (79.2%) | 0.09 |
| MRA | 239 (40.6%) | 2428 (42.8%) | 0.30 | 143 (44.1%) | 96 (36.2%) | 0.05 |
| Pacemaker | 75 (12.7%) | 587 (10.3%) | 0.07 | 40 (12.3%) | 35 (13.2%) | 0.75 |
| ICD | 15 (2.5%) | 98 (1.7%) | 0.15 | 7 (2.2%) | 8 (3.0%) | 0.51 |
- Values are presented as mean ± standard deviation or median [25th–75th percentile] unless otherwise indicated.
- Severe COVID-19 defined as COVID-19 infection requiring hospitalization and/or resulting in a fatal COVID-19 event as determined by investigators. COVID-19 infection was determined by investigator report and may or may not have been confirmed with laboratory testing.
- ACEi, angiotensin-converting enzyme inhibitor; AFF, atrial fibrillation/flutter; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HF, heart failure; ICD, implantable cardioverter-defibrillator; KCCQ-TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
Rates of trial outcomes prior to and during the COVID-19 pandemic
The primary composite endpoint occurred at a rate of 11.0 (95% confidence interval [CI] 9.9–12.2) per 100 participant-years during the pre-pandemic period (prior to 11 March 2020); rates were numerically lower during the pandemic (7.9, 95% CI 7.4–8.5 per 100 participant-years), but not statistically different when adjusting for the time from randomization (p = 0.34). However, the proportion of primary composite events attributable to worsening HF events (HF hospitalization or urgent HF visit) was lower during the pandemic period compared with the pre-pandemic period (68.7% vs. 82.8%, p < 0.01). All-cause death rates were higher during the pandemic (8.2, 95% CI 7.6–8.7 per 100 participant-years) versus pre-pandemic periods (5.0, 95% CI 4.3–5.8 per 100 participant-years; p = 0.05); the proportion of deaths related to non-CV causes also rose during the pandemic period (Table 2).
| Before 11 March 2020 | On or after 11 March 2020 | RR pandemic period to pre-pandemic perioda (95% CI) | Time-stratified p-value | |
|---|---|---|---|---|
| Event rates, per 100 participant-years (95% CI) | ||||
| Primary composite | 11.0 (9.9–12.2) | 7.9 (7.4–8.5) | 0.93 (0.79–1.09) | 0.34 |
| All-cause death | 5.0 (4.3–5.8) | 8.2 (7.6–8.7) | 1.24 (1.00–1.54) | 0.05 |
| CV death | 2.9 (2.4–3.5) | 3.8 (3.4–4.2) | 1.10 (0.82–1.48) | 0.52 |
| Worsening HF event | 9.1 (8.1–10.2) | 5.5 (5.0–5.9) | 0.86 (0.72–1.03) | 0.11 |
| Hospitalization for HF | 7.9 (7.0–8.9) | 5.0 (4.6–5.5) | 0.90 (0.75–1.09) | 0.30 |
| Urgent HF visit | 1.5 (1.1–2.0) | 0.8 (0.7–1.0) | 0.59 (0.36–0.94) | 0.026 |
| Proportion of all-cause death attributable to CV causes | 98/164 (59.8%) | 394/853 (46.2%) | – | <0.001 |
| Proportion of the primary composite due to a worsening HF event | 299/361 (82.8%) | 523/761 (68.7%) | – | <0.001 |
| Proportion of all worsening HF events which were due to HF hospitalization | 257/299 (86.0%) | 461/524 (88.0%) | – | 0.40 |
- CI, confidence interval; CV, cardiovascular; HF, heart failure; RR, risk ratio.
- a The associations between COVID-19 and risks of time-to-event outcomes were estimated by creating time-updated covariates to represent pre- versus post-COVID-19 diagnosis status at the patient level.
Health risks after COVID-19 diagnosis
Adverse events
Among DELIVER participants on study drug at the time they developed COVID-19 (n = 533), 91 (17.1%) had study drug interruption or withdrawal after reported infection; most (n = 84, 92%) were temporary interruptions with subsequent resumption of study drug. Study drug interruption/withdrawal did not differ by treatment assignment to dapagliflozin versus placebo (15.9% vs. 18.4%, p = 0.44), but was more common overall in patients with severe COVID-19 (28.6%) versus non-severe COVID-19 (3.7%) (online supplementary Table S2). Adverse events in the 30 days following COVID-19 diagnosis are listed in Table 3. There were four investigator-reported renal adverse events which were serious in nature or resulted in IP discontinuation and two episodes of volume depletion among participants within 30 days of COVID-19 diagnosis. There were no reported hypoglycaemia or DKA events among trial participants within 30 days of COVID-19 diagnosis. Adverse event rates did not differ by treatment assignment (Table 3). Adverse events by COVID-19 severity and by region of enrolment are listed in online supplementary Tables S3 and S4, respectively.
| Adverse Events | Overall | Randomized to placebo | Randomized to dapagliflozin |
|---|---|---|---|
| Any SAE | 585 (99.3%) | 272 (98.9%) | 313 (99.7%) |
| Any AE leading to discontinuation of IP | 11 (1.9%) | 5 (1.8%) | 6 (1.9%) |
| Any AE leading to interruption of IP | 94 (16.0%) | 49 (17.8%) | 45 (14.3%) |
| Any amputation | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Any definite or probable diabetic ketoacidosis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Any major hypoglycaemic event | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Any SAE or DAE suggestive of volume depletion | 2 (0.3%) | 2 (0.7%) | 0 (0.0%) |
| Any renal SAE or DAE | 4 (0.7%) | 2 (0.7%) | 2 (0.6%) |
| Deaths following COVID-19 diagnosis | |||
| Any death within 30 days of COVID-19 diagnosis | 156 (26.5%) | 70 (25.5%) | 86 (27.4%) |
| Any death after COVID-19 diagnosis | 181 (30.7%) | 81 (29.5%) | 100 (31.8%) |
| Fatal COVID-19 adverse event (investigator-reported) | 131 (22.4%) | 57 (21.0%) | 74 (23.6%) |
| Death definitely or possibly related to COVID-19 (by CEC)a | 142 (24.1%) | 62 (22.5%) | 80 (25.5%) |
- No statistically significant differences by randomized treatment assignment.
- AE, adverse event; CEC, clinical endpoints committee; DAE, adverse events leading to discontinuation of study drug; IP, investigational product; SAE, serious advent event.
- a An additional 13 deaths (for a total of 155 deaths) without an investigator-reported COVID-19 diagnosis were adjudicated by the CEC as definitely or possibly related to COVID-19.
Among patients with COVID-19 during the trial (n = 589), 181 (30.7%) died on or after the date of COVID-19 diagnosis (100/314 participants randomized to dapagliflozin and 81/275 participants randomized to placebo, p = 0.53). The majority (n = 131, 72.4%) of these deaths were linked by investigators to the index COVID-19 diagnosis, of which an independent clinical events committee adjudicated 122 deaths to be definitely or probably related to COVID-19. The clinical events committee determined an additional 33 deaths to be definitely or probably related to COVID-19, for a total of 155 deaths with direct attribution to COVID-19 (15% of total deaths). Deaths within 30 days or at any time following a COVID-19 diagnosis, fatal COVID-19 adverse events, or deaths definitely or possibly related to COVID-19 as determined by the clinical events committee did not differ significantly by treatment assignment (Table 3).
Treatment effects
A previously reported sensitivity analysis demonstrated consistent treatment benefits of dapagliflozin versus placebo when censoring participants at the time of their COVID-19 diagnosis.8 In the overall trial, rates of all-cause death were numerically lower in participants randomized to dapagliflozin versus placebo (hazard ratio [HR] 0.94, 95% CI 0.83–1.07). This observed trend appeared marginally stronger in the pre-specified analysis with censoring participants at the time of COVID-19 diagnosis (HR 0.89, 95% CI 0.78 –1.02). The effects of dapagliflozin versus placebo on the primary endpoint were similar when considering the full trial population (HR 0.82, 95% CI 0.73–0.92) and after censoring at the time of COVID-19 diagnosis (HR 0.81, 95% CI 0.72–0.91). We conducted an additional sensitivity analysis, censoring participants at the time of pandemic onset (11 March 2020). Dapagliflozin consistently reduced the time CV death or worsening HF event (HR 0.72, 95% CI 0.58–0.89) when censoring at pandemic onset.
Risks of death after COVID-19
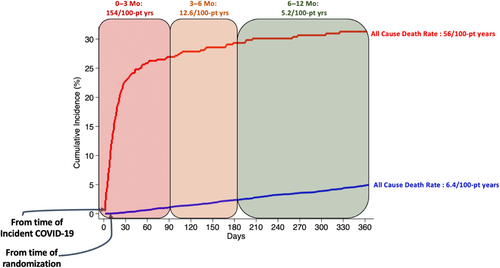
All-cause death rate in the 12 months following diagnosis was 56.1 (95% CI 48.0–65.6) per 100 participant-years compared to 6.4 (95% CI 6.0–6.8) per 100 participant-years among all trial participants censored at the time of COVID-19 (adjusted HR [aHR] 8.22, 95% CI 6.86–9.85). All-cause death was highest in the 3 months following COVID-19 diagnosis (153.5, 95% CI 130.3–180.8 per 100 participant-years) but remained elevated even 3–6 months after COVID-19 diagnosis (12.6, 95% CI 6.6–24.3 per 100 participant-years). Death rates declined 6–12 months from COVID-19 diagnosis (5.2, 95% CI 2.3–11.6 per 100 participant-years (Figure 2). Results were primarily driven by excess non-CV mortality (online supplementary Figure S2). After excluding immediately fatal COVID-19 events, all-cause death rates in the 12 months following diagnosis among COVID-19 survivors (n = 458) remained multifold higher (17.0, 95% CI 12.8–22.6 per 100 participant-years; aHR 2.46, 95% CI 1.83–3.33) than all trial participants from randomization, censoring participants who developed COVID-19 at the time of diagnosis. Results were largely consistent by region (online supplementary Table S5). Rates of the primary endpoint and its components were numerically higher in the year following COVID-19 diagnosis as compared with all participants from randomization with censoring at the time of COVID-19 in both unadjusted and adjusted models (Table 4).
| Event rates per 100 participant-years, 95% CI | Full trial population, censored at COVID-19 diagnosis (n = 6263) | All COVID-19 patients in the 12 months after diagnosis (n = 589) | HR, unadjusted | HR, adjusteda | All COVID-19 patients in the 12 months after diagnosis, excluding fatal COVID-19 events (n = 458) | HR, unadjusted | HR, adjusteda |
|---|---|---|---|---|---|---|---|
| Primary endpoint | 8.7 (8.2–9.2) | 8.9 (5.9–13.5) | 1.20 (0.78–1.83) | 1.30 (0.85–1.99) | 8.9 (5.9–13.5) | 1.21 (0.79–1.86) | 1.32 (0.86–2.03) |
| All-cause death | 6.4 (6.0–6.8) | 56.1 (48.0–65.6) | 7.97 (6.69–9.51) | 8.22 (6.86–9.85) | 17.0 (12.8–22.6) | 2.40 (1.79–3.22) | 2.46 (1.83–3.33) |
| CV death | 3.5 (3.2–3.8) | 5.7 (3.5–9.3) | 1.48 (0.89–2.44) | 1.55 (0.93–2.56) | 5.3 (3.2–8.9) | 1.39 (0.83–2.33) | 1.45 (0.86–2.44) |
| Worsening HF events | 6.4 (6.0–6.9) | 5.3 (3.1–9.1) | 1.06 (0.61–1.84) | 1.20 (0.69–2.08) | 5.3 (3.1–9.1) | 1.08 (0.62–1.87) | 1.23 (0.70–2.13) |
- Immediately fatal adverse were determined and reported by the investigators.
- CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio.
- a Adjusted for age, sex, race, geographic region, body mass index, HF duration, N-terminal pro-B-type natriuretic peptide, glycated haemoglobin, estimated glomerular filtration rate, baseline use of loop diuretics and angiotensin receptor–neprilysin inhibitor, and history of type 2 diabetes, chronic obstructive pulmonary disease, sleep apnoea, atherosclerotic vascular disease, prior HF hospitalization, smoking, atrial fibrillation/atrial flutter.
COVID-19 vaccination
Overall, 2722 trial participants received at least one vaccination for COVID-19, representing 47% of the trial population. There were 6456 vaccinations in 2722 participants during the trial; 277 participants received a single COVID-19 vaccination, 1156 received two vaccine doses, and 1289 received three vaccinations. Among participants who developed COVID-19 during the trial period (n = 589), 222 (38%) received at least one dose of COVID-19 vaccination, and 184 (31%) received at least two doses. Of these patients with COVID-19 who completed a primary vaccination series (≥2 doses), 90 (49%) received a third (i.e., booster) vaccine.
Discussion
A substantial proportion of participants with HFmrEF or HFpEF in the DELIVER trial were diagnosed with COVID-19 during the course of the study, with >50% of infections requiring/prolonging hospitalization and many leading to death. The pandemic period was associated with greater all-cause mortality and consequently relatively lower rates of worsening HF events compared to the pre-pandemic period. Participants diagnosed with COVID-19 had higher rates of all-cause death following infection, which remained residually elevated at 3–6 months after diagnosis, after excluding immediately fatal COVID-19 events and after extensive covariate adjustment. Adverse events leading to treatment discontinuation, major hypoglycaemia, DKA, and renal adverse events did not differ by treatment groups, as were all deaths and deaths ascribed to COVID-19. Dapagliflozin reduced clinical HF events as compared to placebo when censoring at the time of COVID-19 diagnosis and pandemic onset. To our knowledge, DELIVER represents one of the most extensive international experiences with COVID-19 of any CV trial during the pandemic. These data provide a global perspective on the immediate to long-term impact of the COVID-19 pandemic in patients with HFmrEF or HFpEF.
In this large, international trial of patients with HFmrEF or HFpEF, COVID-19 was reported in nearly 10% of trial participants during the study period, with over half of infections requiring or prolonging hospitalization and nearly one in five resulting in a fatal outcome. These data highlight the burden and disease severity associated with COVID-19 in a large and growing segment of the HF population. Trial participants with cardiometabolic (but not pulmonary) comorbidities were more likely to have COVID-19; these data substantiate early reports identifying existent CV conditions as associated with increased susceptibility to viral infection and potential to more severe disease.1, 9, 10 Participants with severe forms of COVID-19 in DELIVER were older and had higher NP levels at baseline compared with those with non-severe disease. Prior observational data from a large, claims database11 and a clinical registry12 found NP levels at hospital admission to be prognostically important in indicating severity of COVID-19 disease; our data potentially extend the prognostic potential of NP levels to ambulatory settings prior to COVID-19 diagnosis. Existent endothelial, microvascular, and myocardial abnormalities, and reduced immune responses in more severe HF phenotypes characterized by higher NP levels may contribute to the higher incidence of more severe forms of COVID-19.13-16 Overall, among patients with HFmrEF or HFpEF, risks of COVID-19 are high and often associated with severe disease phenotypes. Innovations in prevention, rapid detection and early mitigation of COVID-19 should be prioritized in this high-risk patient population.
The pandemic period was associated with a rise in all-cause death and a decline in the proportion of primary outcome events attributable to worsening HF (vs. CV death) compared with the pre-pandemic period in DELIVER. Deaths attributable to or influenced by COVID-19 likely contributed significantly to the observed rise in all-cause death. A smaller trial assessing haemodynamic monitoring of HF patients found no difference in death rates between pre-pandemic and pandemic period, though was limited to North American participants only and was operational for a shorter period of time during the pandemic.7 Concurrent observational studied during the early pandemic reported declines in hospitalizations for acute CV conditions, including HF hospitalizations.17, 18 Potential reasons include the effects of stay-at-home messaging, concerns about the safety and accessibility of hospital care, and the potential effects of environmental factors (e.g. reduced pollution, lower exposure to other viral pathogens [e.g. influenza], and availability of sodium and high caloric foods), though direct evidence for the latter is limited. Concurrently, reports indicated increased out-of-hospital cardiac arrests19 and lower survival/return of spontaneous circulation rates during the pandemic.20 Implantation of primary and secondary prevention defibrillators also declined.21 These trends may contribute to the observed shift between worsening HF events and CV death observed during the pandemic.5, 6 These findings may have important implications for the design and conduct of cardiovacular trials, particularly as COVID-19 remains endemic; the use of adaptative clinical trial designs may help to deal with uncertainty in event rate accrual caused by unexpected trial disruptions such as COVID-19. However, COVID-19 had little impact on treatment effect estimates of dapagliflozin versus placebo on clinical outcomes. These data come in contrast to prior trials in which the pandemic period was associated with general attenuation treatment effects.5, 7 Reasons for these discrepant findings are unclear but may be related to DELIVER remaining active in later timepoints during the pandemic. Importantly, given the post-randomization nature of the exposure (the COVID-19 pandemic), natural evolution of fatal and non-fatal events in a time-to-event design with a composite endpoint may have also contributed, as non-fatal events among high-risk participants are generally accrued early (i.e. before the pandemic) while fatal events generally occur later (i.e. during the pandemic)22; conclusions about the causes of the changing epidemiology of clinical events during later trial periods should therefore be interpreted with caution.
Study drug interruptions or withdrawals following COVID-19 diagnosis were largely temporary and occurred at similar rates among those randomized to dapagliflozin or placebo. Guidance to sites largely mirrored clinical practice and included consideration of study drug interruption among participants with COVID-19 who were at high risk for or with prior history of DKA. There was no reported major hypoglycaemia or DKA events and adverse renal events were rare in the 30 days following COVID-19 diagnosis; precise reasons for interruption or withdrawal were not systematically available. Poor nutritional intake and volume depletion during acute COVID-19 infection raised concern that sodium–glucose cotransporter 2 inhibitor (SGLT2i) use might further increase risks of euglycaemic DKA.23 However, a retrospective cohort of patients with diabetes and COVID-19 found no evidence of increased risk of DKA among patients receiving SGLT2i. This study observed potential favourable effects on endothelial function and reductions in oxidative stress with this therapy, which may have implications in attenuating the severity of acute viral illness.24 The Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) trial randomized 1250 patients with cardiometabolic risk factors who were hospitalized with COVID-19 to dapagliflozin versus placebo and also found no increase in the risks of acute kidney injury or DKA among participants randomized to dapagliflozin25, 26; however, only 90 participants (7.2%) had a history of HF in DARE-19. Data from DELIVER extend the available safety data of dapagliflozin with standard of care guidance to a large cohort of patients with HFmrEF or HFpEF; in fact, similar safety profiles were observed in clinical trials of patients with HFrEF conducted prior to the COVID-19 pandemic. Deaths following a COVID-19 diagnosis did not statistically differ by treatment groups. Collectively, the data from this DELIVER analysis and DARE-19 are reassuring in regard to the tolerability of SGLT2i in patients that develop COVID-19, and support consideration of the continuation of SGLT2i (with usual care guidance) in stable HF with COVID-19. Of note, additional studies including from two large platform trials (Randomised Evaluation of COVID-19 Therapy [RECOVERY] and Accelerating COVID-19 Therapeutic Interventions and Vaccines 4 ACUTE [ACTIV-4A]) have assessed the safety and efficacy of SGLT2i in patients hospitalized with COVID-19, independent of HF status. These data, in aggregate, will further clarify the role of SGLT2i in patients with COVID-19.
Participants in DELIVER who developed COVID-19 during the trial faced increased risks of all-cause death following diagnosis, compared to those who did not. Importantly, elevated all-cause death risk persisted 3–6 months after diagnosis, after adjustment for clinically relevant confounders, and when assessing only the population surviving their COVID-19 event. Rates of the primary endpoint and its components were numerically higher in the year following COVID-19 as compared to participants without COVID-19 in both unadjusted and adjusted models. A prior claims-based analysis found that among patients with HF, COVID-19 hospitalization carried a significantly elevated prognostic risk of death compared to similar patients hospitalized with acute HF; however, follow-up was limited to only the in-hospital setting.1 Recent evidence from >500 000 registry participants found an increased incidence of new HF diagnoses in patients in the year following COVID-19 diagnosis; however, the registry findings were limited to those without prior known HF.2 Relatively small sample sizes and limited post-COVID-19 follow-up in DELIVER affected statistical power to detect excess CV death risk among participants diagnosed with COVID-19. Our data from DELIVER extend the potential downstream risks of COVID-19 to patients with existing HF in ambulatory and hospitalized settings. Similar trends have been observed with influenza infection; a recent secondary analysis of two large HF trials found that trial participants faced >4× greater risk of death following influenza diagnosis compared with participants without influenza infection, though the excess risks after COVID-19 diagnosis in DELIVER were larger.27 Extended surveillance programmes among HF patients with COVID-19 (and other viral disease) are needed to fully understand and attempt to mitigate possible longer-term collateral effects of COVID-19 illness.28
Limitations
The results of our analysis should be considered in the context of several potential limitations. First, COVID-19 diagnosis was determined by adverse event reporting from site investigators; local regulations, access to and availability of testing may have varied over time and by site or region, which may have led to ascertainment bias and undercounting of true COVID-19 cases. The inclusion of possible COVID-19 cases without polymerase chain reaction confirmation may have also affected the results, though all deaths were centrally adjudicated for relatedness to COVID-19. Second, unmeasured confounding and natural changes during a trial may in part explain the observed findings. Differences in trial event rates before and during the pandemic should be interpreted in the context of time-to-first endpoints, in which early accrual of non-fatal events may have affected the observed findings. Third, data on in-hospital resource use (e.g. mechanical ventilation and haemodynamic monitoring) and COVID-19 specific treatment (e.g. corticosteroids) were not available, nor was specific detailed assessment of potential cardiopulmonary sequelae of COVID-19 disease. Fourth, drug interruption and withdrawal rates were obtained from adverse event reporting; the specific reasons for study drug interruption and timing of interruption/resumption were unavailable and underreporting during acute illness (e.g. during COVID-19 hospitalization) may be possible. Fifth, event reporting may have been impacted by operational challenges during the pandemic. Sixth, data on COVID-19 variants were not available; time-dependent variation in the risk of COVID-19 infection influenced by circulating variants may have impacted the observed findings. Sixth, availability, eligibility, and access to COVID-19 vaccination may have varied significantly across sites and regions during the trial period; reasons for not obtaining COVID-19 vaccine were not systematically captured. Finally, most patients who developed COVID-19 did so substantially after randomization, so post-randomization treatment comparisons are only hypothesis generating and should be interpreted with caution.
Conclusion
In DELIVER, COVID-19 was common, with >50% of diagnoses requiring/prolonging hospitalization and a high proportion leading to death. Serious adverse events, adverse events leading to treatment discontinuation, DKA, renal adverse events, hypoglycaemia, and deaths following COVID-19 diagnosis did not differ between dapagliflozin and placebo arms and permanent treatment withdrawals were rare. Dapagliflozin improved clinical outcomes when censoring at the time of COVID-19 diagnosis and at the time of pandemic onset. Patients surviving a COVID-19 diagnosis face residual elevations in clinical risk in the months following this event.
Conflict of interest: M.N.K. has received research grant support from AstraZeneca and Boehringer Ingelheim; has served as a consultant or on an advisory board for Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma; has received other research support from AstraZeneca; and has received honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. B.L.C. has received consulting fees from Amgen, Cardurion, Corvia, and Novartis. M.V. has received research grant support, served on advisory boards, or had speaker engagements with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, and participates in clinical trial committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics. C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Bayer and Roche Diagnostics; has served as consultant or on the Advisory Board/ Steering Committee/Executive Committee for Actelion, Alleviant Medical, Allysta Pharma, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., EchoNous Inc, Eli Lilly, Impulse Dynamics, Ionis Pharmaceutical, Janssen Research & Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Prosciento Inc, Radcliffe Group Ltd., Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics and Us2.ai; and serves as co-founder and non-executive director of Us2.ai. A.F.H. has received research grants from American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Somologic and Verily; and has served as a consultant or on the Advisory Board for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cytokinetics, Eidos, Intercept, Merck, and Novartis. F.A.M. has received consultation fees and research grants from AstraZeneca, Baliarda, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Gador, Milestone, Novartis, Pfizer, and St Lukes University. S.E.I. has served on clinical trial committees or as a consultant to AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Lexicon, Merck, Pfizer, vTv Therapeutics, Abbott, and Esperion; and has given lectures sponsored by AstraZeneca and Boehringer Ingelheim. S.J.S. has received research grants from the National Institutes of Health (U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, R01 HL149423), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer, and has received consulting fees from and consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, GSK, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Sardocor, Shifamed, Tenax, Tenaya, and United Therapeutics. R.A.d.B. has received research grants and/or fees from AstraZeneca, Abbott, Boehringer Ingelheim, Cardior Pharmaceuticals GmbH, Ionis Pharmaceuticals, Inc., Novo Nordisk, and Roche; and has had speaker engagements with Abbott, AstraZeneca, Bayer, Bristol Myers Squibb, Novartis, and Roche. P.S.J. reports speakers' fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, Sun Pharmaceuticals; advisory board fees from AstraZeneca, Boehringer Ingelheim, Novartis; research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices Inc. P.S.J.'s employer the University of Glasgow has been remunerated for clinical trial work from AstraZeneca, Bayer AG, Novartis and NovoNordisk. Director, Global Clinical Trial Partners (GCTP). A.S.D. reports institutional grant support from Abbott, Alnylam, AstraZeneca, Bayer, Novartis, and consulting fees from Abbott, Alnylam, AstraZeneca, Avidity, Axon Therapeutics, Bayer, Biofourmis, Boston Scientific, Cytokinetics, GlaxoSmithKline, Merck, Novartis, Parxel, Regeneron, Roche, and Verily. J.C.F. has received research grants from the National Institutes of Health, has consulted with Novartis, Amgen, AstraZeneca, Boehringer Ingelheim/Lilly, Abbott, Capricor, Windtree, LabCorp, and has provided support to AHA, NIH, HFSA, HRS. J.C.C. has received research grants from Novartis, Orion Pharma, AstraZeneca, Vifor Pharma, Bristol Myers Squibb, and has consulted for Novartis, Orion Pharma, AstraZeneca, Vifor Pharma, Bayer, Boehringer Ingelheim, Gilead, Menarini and Pfizer. J.D. has received research grants from AstraZeneca, Servier Poland, and has received lecture fee from Bayer Healthcare, Boehringer Ingelheim, Novartis. O.V. has received institutional research support for DELIVER from AstraZeneca and has received institutional research support from Bayer. D.L. reports being a former employee of AstraZeneca. M.P. and A.M.L. are employees and shareholders of AstraZeneca. J.J.V.M. has received payments through Glasgow University for work on clinical trials, consulting, and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal-Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, Theracos Personal lecture fees: the Corpus, Abbott, Hikma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Servier Director, Global Clinical Trial Partners (GCTP). S.D.S. has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, US2.AI and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros. All other authors have nothing to disclose.