Deep phenotype characterization of hypertensive response to exercise: implications on functional capacity and prognosis across the heart failure spectrum
Abstract
Aims
Limited evidence is available regarding the role of hypertensive response to exercise (HRE) in heart failure (HF). We evaluated the systolic blood pressure (SBP) to workload slope during exercise across the HF spectrum, investigating haemodynamic and prognostic correlates of HRE.
Methods and results
We prospectively enrolled 369 patients with HF Stage C (143 had preserved [HFpEF], and 226 reduced [HFrEF] ejection fraction), 201 subjects at risk of developing HF (HF Stages A–B), and 58 healthy controls. We performed a combined cardiopulmonary exercise stress echocardiography testing. We defined HRE as the highest sex-specific SBP/workload slope tertile in each HF stage. Median SBP/workload slope was 0.53 mmHg/W (interquartile range 0.36–0.72); the slope was 39% steeper in women than men (p < 0.0001). After adjusting for age and sex, SBP/workload slope in HFrEF (0.47, 0.30–0.63) was similar to controls (0.43, 0.35–0.57) but significantly lower than Stages A–B (0.61, 0.47–0.75) and HFpEF (0.63, 0.42–0.86). Patients with HRE showed significantly lower peak oxygen consumption and peripheral oxygen extraction. After a median follow-up of 16 months, HRE was independently associated with adverse outcomes (all-cause mortality and hospitalization for cardiovascular reasons: hazard ratio 2.05, 95% confidence interval 1.81–5.18), while rest and peak SBP were not. Kaplan–Meier analysis confirmed a worse survival probability in Stages A–B (p = 0.005) and HFpEF (p < 0.001), but not HFrEF.
Conclusion
A steeper SBP/workload slope is associated with impaired functional capacity across the HF spectrum and could be a more sensitive predictor of adverse events than absolute SBP values, mainly in patients in Stages A–B and HFpEF.
Graphical Abstract
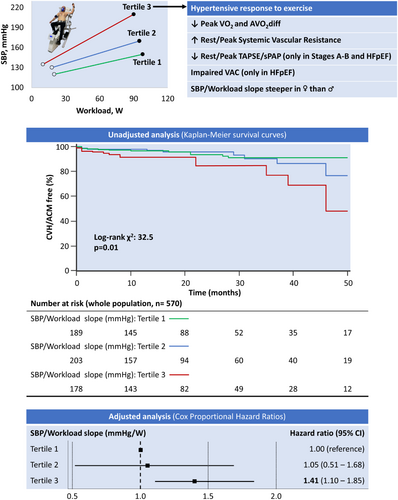
Impact of hypertensive response to exercise on functional capacity and prognosis. Cumulative survival and relative risk of all-cause mortality (ACM) and cardiovascular hospitalizations (CVH) after a median follow-up of 16 months per tertile of systolic blood pressure (SBP)/workload slope. Unadjusted analysis (Kaplan–Meier survival curves) for the primary outcome in the whole patient population. Adjusted analysis (Cox proportional hazard ratios) illustrating higher mortality in the highest tertile of the SBP/workload slope. Hazard ratios are adjusted for age, sex, body mass index, arterial hypertension, diabetes mellitus, previous myocardial infarction, N-terminal pro-B-type natriuretic peptide, rest and peak SBP, rest and peak cardiac output, rest and peak left ventricular ejection fraction, and medications (beta-blockers and calcium channel blockers). AVO2diff, arterial–venous oxygen content difference; CI, confidence interval; HFpEF, heart failure with preserved ejection fraction; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; VAC, ventricular–arterial coupling; VO2, oxygen consumption.
Introduction
Hypertensive response to exercise (HRE) is a poorly understood and often neglected parameter observed in patients undergoing cardiovascular stress testing, commonly defined as peak systolic blood pressure (SBPpeak) >210 mmHg in males and >190 mmHg in females.1, 2 Several studies employing these or other absolute cut-off values showed a significant association of HRE with incident hypertension and cardiovascular events in subjects free of cardiovascular disease, often independent of resting blood pressure (BP) values.3, 4 However, several controversies remain to be addressed. First, there is evidence reporting better outcomes in those with higher SBPpeak, both in patients with low and high cardiovascular risk.5, 6 Second, a greater SBP increase during exercise has been described in healthy women than men for any given workload, despite lower absolute SBPpeak.7, 8 Thus, there is currently no consensus on the definition and significance of an abnormal SBP response to exercise. Noteworthy, SBP rises in proportion to workload during exercise,9 but there are no data about the functional and prognostic impact of sex-specific, workload-indexed HRE in patients at different stages of the heart failure (HF) spectrum. This is an important limitation given that optimal control of resting BP values has important prognostic implications in more advanced cardiovascular diseases, such as overt HF.10 Moreover, an excessive BP increase during exercise may unmask maladaptations of the cardiovascular system in HF, reflecting an early sign of disease progression and/or suboptimal treatment.11 We designed this study to evaluate the cardiovascular, functional, and prognostic correlates of HRE, defined by the SBP/workload slope, across different HF stages.
Methods
Study population
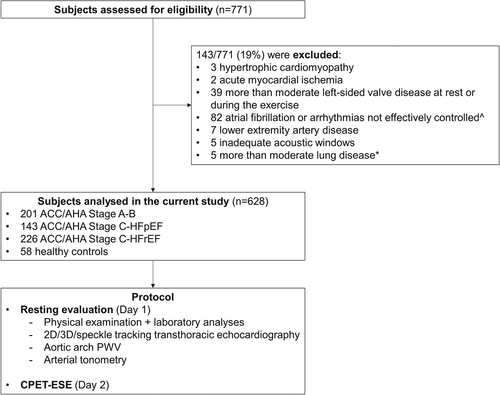
We prospectively enrolled 771 consecutive patients referred for dyspnoea to the University Hospital of Pisa between September 2019 and September 2021. According to the American College of Cardiology/American Heart Association HF staging system,12, 13 patients fell within Stage A (asymptomatic subjects with cardiovascular risk factors), B (structural heart disease without signs or symptoms of HF), or C (clinically overt HF). Physical examination and laboratory analyses were performed on the same day of the enrolment. Resting BP was measured in the supine position after a few minutes of rest; BP values were reported as the mean of three consecutive brachial measurements using a previously validated automatic oscillometric device (Omron M6 Comfort), and arterial hypertension was defined by at least two BP recordings >140/90 mmHg or treatment with antihypertensive medications.14 Stage C patients were stable at the time of recruitment. HF diagnosis was adjudicated by an independent physician blinded to the study protocol. HF diagnosis required at least two typical HF signs or symptoms: third heart sound, pulmonary rales, jugular venous distention, hepatomegaly, peripheral oedema, or lung congestion on lung ultrasound.10 HF with reduced ejection fraction (HFrEF) was defined by a left ventricular ejection fraction (LVEF) <50%, while HF with preserved ejection fraction (HFpEF) required a LVEF ≥50%, N-terminal pro-B-type natriuretic peptide (NT-proBNP) >125 pg/ml and the additional presence of relevant structural heart disease or diastolic dysfunction.10 Exclusion criteria are summarized in Figure 1. The final study population consisted of 201 subjects in Stage A–B HF and 369 patients in Stage C HF (HFrEF, n = 226 and HFpEF, n = 143). A convenience sample of subjects (n = 58) referred for cardiovascular check-up served as a healthy control group after excluding any cardiovascular and respiratory disease. No patient or control subject exercised regularly (more than once per week) at the time of enrolment. The local Ethics Committees approved the protocol (number 19204), and written informed consent was obtained from all patients. The study was conducted following the principles of the Declaration of Helsinki.
Study protocol
The whole study protocol required two working days. All measurements were performed in a quiet room with a stable room temperature. No meal, caffeine, or smoking was allowed 3 h before evaluation. Therapy was left unchanged during the whole protocol to obtain ‘real-world’ evidence. Resting evaluation was conducted on the first day, including baseline echocardiography and arterial tonometry.
Laboratory evaluation
Patients were instructed to fast overnight and not to take any medications before blood sampling on the morning of the tests. Blood samples were drawn after a 30 min supine rest. The estimated glomerular filtration rate was calculated through the Chronic Kidney Disease Epidemiology Collaboration formula.15 Plasma norepinephrine was evaluated using high-performance liquid chromatography with the electrochemical detector CLC 100 (Chromsystems, Munchen, Germany). Direct renin and aldosterone were assayed using a chemiluminescence immunoassay (LIAISON, DiaSorin, Saluggia, Italy). NT-proBNP was measured with the ECLIA monoclonal assay using the Cobas e411 platform (Roche Diagnostics Italia, Monza, Italy).
Baseline echocardiography
All patients underwent a comprehensive transthoracic echocardiography examination (Hitachi Medical Systems LISENDO 880, Tokyo, Japan) according to the international recommendations, including speckle tracking echocardiography (STE).16 In the standard suprasternal view, the single-beat dual-gate Doppler tool was used to simultaneously assess Doppler waveforms in the ascending and descending aorta, as previously described.17 Aortic arch pulse wave velocity (aa-PWV) was defined as the distance between the Doppler recordings divided by the transit time (reproducibility for this parameter has been published previously17). The detailed protocol is summarized in online supplementary Appendix S1.
Arterial tonometry
Arterial tonometry (SphygmoCor, AtCor Medical, Sydney, Australia) of the radial and femoral arteries was performed by highly experienced technicians, according to international recommendations.17, 18 We measured central BP, aortic augmentation index, and carotid–femoral pulse wave velocity (cf-PWV). The detailed protocol is summarized in online supplementary Appendix S1.
Ventricular–arterial coupling
Ventricular–arterial coupling (VAC) was calculated as the ratio of arterial stiffness – measured by either aa-PWV or cf-PWV – to myocardial performance evaluated by STE-derived global longitudinal strain (GLS), that is, aa-PWV/GLS and cf-PWV/GLS.17, 19
Cardiopulmonary exercise stress echocardiography evaluation
A combined cardiopulmonary exercise stress echocardiography (CPET-ESE) protocol was performed in all patients and controls on the first working day after the resting evaluation. Briefly, a symptom-limited graded ramp bicycle exercise test was conducted in the semi-supine position on a tilting cycle ergometer (Ergoline ergoselect 1200 GmbH, Bitz, Germany). ESE was performed concurrently with breath-by-breath gas exchange measurements using the same ultrasound system used at baseline; images were acquired at low load (i.e. after 4 min of exercise) and peak effort. All images were stored for offline analysis. According to the Fick equation, we estimated the arterial–venous oxygen content difference (AVO2diff) as the ratio between oxygen consumption (VO2) and cardiac output (CO). The detailed protocol has been described previously11 and is summarized in online supplementary Appendix S1.
Blood pressure measurement during exercise and calculations
Blood pressure was manually measured in the right arm using standard auscultatory methods with cuff inflation/deflation according to the current guidelines.14 The subject was instructed to let the right arm hang loosely during measurement. SBP was recorded at the appearance of the first Korotkoff sound, while diastolic BP was recorded when Korotkoff sounds disappeared. BP was measured 1–2 min during the warm-up before exercise commenced and every 2 min throughout the test. In all the population, we recorded the first (within the first minute of exercise) and last (peak) BP measurements during the ramp protocol, as well as BP after 4 min of exercise (low load; Figure 2). In a random selection of 40 subjects (10 controls and 10 patients for each HF cohort), we reported two additional BP measurements, that is, between first and low load, as well as between low load and peak. Each BP measurement was recorded with the corresponding test time, heart rate and workload.
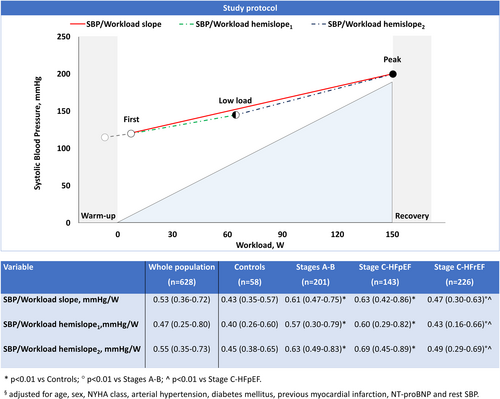
The SBP/workload slope was calculated as the ratio of the difference in SBP from the first to the last SBP measurement during exercise over the increment in watts (W) between these two measurements: (SBPpeak – SBPfirst)/(Wpeak – Wfirst). In each subgroup (i.e. Stages A–B, HFpEF, HFrEF, and controls), we divided patients into SBP/workload slope tertiles, using different cut-off values for women and men; then, we defined the highest tertile as HRE. We also subdivided the SBP/workload slope into two hemislopes to separately describe the first (hemislope1: [SBPlow-load – SBPfirst]/[Wlow-load – Wfirst]) and the final part of the exercise (hemislope2: [SBPpeak – SBPlow-load]/[Wpeak – Wlow-load]). SBP variation during exercise was also plotted against VO2 (expressed in ml/kg/min).
Clinical follow-up
Follow-up events were adjudicated by an independent trained investigator blinded to clinical data. The primary outcome was a composite of all-cause mortality and hospitalization for cardiovascular reasons (acute myocardial infarction, HF hospitalization, transient ischaemic attack/stroke, or new-onset atrial fibrillation). When multiple events occurred, patients were censored at the time of the first event.
Statistical analysis
Categorical variables were presented as percentages and were compared using Pearson's Chi-square test or the Fisher's exact test. Continuous measures were expressed as the mean value ± standard deviation or median and interquartile range (IQR) for normally or skewed distributed variables, respectively. ANOVA or Kruskal–Wallis test was used to test the differential distribution of data among groups, with post-hoc corrections for interactions (Tukey or Conover test, respectively). We measured the adjusted R2 of different regression lines (i.e. straight line, logarithmic curve, exponential curve, quadratic and cubic regression) to assess the best-fitting equation describing the relationship between SBP and workload during exercise. We used multivariable linear regression analysis in the study population to determine the relationship between the SBP/workload slope and peak VO2. We performed Kaplan–Meier analyses with log-rank statistics to investigate the relationship between SBP/workload slope values and the outcome in the patient population (n = 570). Multivariable Cox regression analysis was also performed in the patient population (n = 570) to analyse the impact of HRE on the primary outcome. All the variables included in the models were selected based on prior knowledge and pathophysiology.20, 21 All tests were two-sided, with a p-value of <0.05 considered statistically significant. Data were analysed with SPSS version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Clinical evaluation
Demographic, clinical and laboratory characteristics of controls and patients according to HF staging are shown in online supplementary Table S1, while the distribution of the patient population according to the presence or absence of HRE as defined by the SBP/workload slope is presented in Table 1. There were no missing data. HFpEF and HFrEF patients with HRE had significantly higher serum levels of high-sensitivity C-reactive protein (hs-CRP), uric acid, and NT-proBNP. HFrEF patients with HRE showed substantially higher levels of norepinephrine and renin at rest. Demographic and clinical characteristics of the patient population using absolute SBPpeak >190 mmHg in women and >210 mmHg in men to define HRE are presented in online supplementary Table S2.
| Variable | Stages A–B (n = 201) | Stage C HFpEF (n = 143) | Stage C HFrEF (n = 226) | |||
|---|---|---|---|---|---|---|
| No HRE (n = 136) | HRE (n = 65) | No HRE (n = 94) | HRE (n = 49) | No HRE (n = 162) | HRE (n = 64) | |
| Demographics | ||||||
| Age, years | 62 ± 10 | 64 ± 10 | 72 ± 11 | 75 ± 8 | 66 ± 12 | 66 ± 12 |
| Male sex | 108 (79) | 52 (80) | 52 (55) | 28 (57) | 138 (85) | 55 (86) |
| BMI, kg/m2 | 28.3 ± 4.4 | 28.2 ± 4.4 | 26.9 ± 4.2 | 27.9 ± 4.7 | 26.7 ± 4.3 | 27.6 ± 4.5 |
| Obesity | 41 (30) | 22 (34) | 16 (17) | 14 (29) | 33 (20) | 17 (27) |
| Waist circumference, cm | 96 (90–121) | 108 (95–124) | 99 (87–108) | 112 (97–126) | 99 (86–106) | 93 (90–101) |
| Current smoker | 33 (24) | 9 (14) | 18 (19) | 12 (25) | 47 (29) | 21 (33) |
| Clinical evaluation | ||||||
| NYHA class II–III | 17 (13) | 14 (22) | 60 (64) | 30 (61) | 95 (59) | 38 (59) |
| KCCQ score, % | 83 (74–88) | 78 (70–87) | 66 (56–80) | 65 (55–83) | 67 (59–84) | 69 (61–82) |
| Arterial hypertension | 101 (74) | 51 (79) | 71 (76) | 36 (74) | 88 (54) | 41 (64) |
| Dyslipidaemiaa | 105 (77) | 56 (86) | 49 (52) | 26 (53) | 98 (61) | 39 (62) |
| Diabetes mellitus | 83 (61) | 47 (72) | 28 (30) | 21 (43) | 42 (26) | 21 (33) |
| CKD | 15 (11) | 7 (10) | 30 (32) | 22 (46) | 54 (34) | 23 (38) |
| CAD | 21 (15) | 11 (17) | 15 (16) | 9 (18) | 72 (44) | 33 (52) |
| Previous MI | 9 (7) | 9 (14) | 7 (7) | 7 (14) | 63 (39) | 28 (44) |
| Pacemaker/ICD | 2 (1) | 3 (5) | 18 (20) | 7 (14) | 108 (67) | 36 (57) |
| Laboratory tests | ||||||
| Haemoglobin, g/dl | 14.4 ± 1.2 | 13.9 ± 1.5* | 12.7 ± 2.3 | 12.8 ± 1.6 | 13.5 ± 1.6 | 13.5 ± 1.5 |
| Total cholesterol, mg/dl | 164 ± 37 | 161 ± 41 | 161 ± 37 | 166 ± 36 | 152 ± 36 | 150 ± 50 |
| eGFR, ml/min/1.73 m2 | 83 (72–93) | 82 (74–104) | 68 (57–83) | 65 (47–77) | 68 (56–82) | 65 (54–79) |
| HbA1c, mmol/mol | 52 ± 12 | 53 ± 11 | 42 ± 7 | 45 ± 10 | 43 ± 11 | 47 ± 11 |
| Uric acid, mg/dl | 5.4 ± 1.4 | 5.3 ± 1.6 | 5.8 ± 1.6 | 6.6 ± 1.9* | 6.2 ± 1.7 | 7.3 ± 2.4*** |
| hs-CRP, mg/dl | 0.18 (0.10–0.35) | 0.27 (0.11–0.48) | 0.32 (0.21–0.49) | 0.45 (0.24–0.75)** | 0.30 (0.20–0.41) | 0.37 (0.28–0.53)* |
| NT-proBNP, pg/ml | 86 (47–152) | 104 (33–169) | 681 (244–1202) | 824 (423–1719)* | 760 (311–1789) | 1217 (546–2757)* |
| Norepinephrine, pg/ml | 243 (96–391) | 240 (157–303) | 229 (120–329) | 270 (140–411) | 283 (153–470) | 356 (230–590)* |
| Renin, mIU/L | 11 (5–36) | 18 (6–70) | 15 (6–53) | 23 (6–77) | 14 (6–52) | 33 (10–103)* |
| Aldosterone, ng/dl | 9 (7–16) | 11 (7–15) | 12 (7–16) | 14 (8–16) | 12 (7–15) | 13 (7–18) |
| Therapy | ||||||
| Beta-blockers | 40 (29) | 18 (28) | 75 (80) | 32 (65) | 140 (86) | 54 (84) |
| DHP CCBs | 35 (26) | 23 (35) | 23 (25) | 11 (22) | 12 (7) | 2 (3) |
| Non-DHP CCBs | 2 (2) | 1 (2) | 0 | 1 (2) | 0 | 0 |
| ACEi or ARBs | 81 (60) | 41 (63) | 65 (69) | 34 (69) | 94 (58) | 32 (50) |
| MRAs | 9 (7) | 5 (8) | 23 (25) | 16 (33) | 92 (57) | 34 (53) |
| ARNI | 0 | 0 | 6 (6) | 3 (6) | 64 (40) | 24 (38) |
| Statins | 95 (70) | 50 (77) | 41 (44) | 26 (53) | 88 (54) | 35 (57) |
| Furosemide | 7 (5) | 5 (8) | 49 (52) | 21 (43) | 112 (69) | 47 (73) |
| Oral hypoglycaemic medications | 54 (40) | 36 (55) | 20 (21) | 12 (25) | 31 (19) | 10 (16) |
| Insulin | 10 (7) | 10 (15) | 8 (9) | 2 (4) | 9 (6) | 6 (10) |
- Values are mean ± standard deviation, n (%), or median (25th–75th quartile).
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease (eGFR <60 ml/min/1.73 m2); DHP CCB, dihydropyridine calcium channel blocker; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin (available only in patients with diabetes mellitus); HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HRE, hypertensive response to exercise; hs-CRP, high-sensitivity C-reactive protein; ICD, implantable cardioverter defibrillator; KCCQ, Kansas City Cardiomyopathy Questionnaire; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
- a Total cholesterol ≥200 mg/dl or low-density lipoprotein cholesterol LDL ≥116 mg/dl or on lipid-lowering therapy.
- * p < 0.05 vs. no HRE.
- ** p < 0.01 vs. no HRE.
- *** p < 0.001 vs. no HRE.
Cardiovascular structure and function at rest
All patients performed a maximal CPET (peak respiratory exchange ratio ≥1.00); there were no missing data. In Stages A–B, subjects with HRE had significantly higher systolic pulmonary artery pressure (sPAP), worse right ventricular–pulmonary arterial (RV-PA) coupling (i.e. lower tricuspid annular plane systolic excursion [TAPSE]/sPAP ratio; all p < 0.01), and higher cf-PWV compared to those without HRE (p < 0.05). In HFpEF patients, HRE was associated with higher cf-PWV and aa-PWV, while both indices of VAC (i.e. aa-PWV/GLS and cf-PWV/GLS) were more impaired (all p < 0.05) than in those without HRE. In HFrEF, patients with HRE showed higher systemic vascular resistance (SVR), with lower total arterial compliance than those without HRE (Table 2). Cardiac and arterial properties at rest were similar in healthy controls with and without HRE (online supplementary Table S3).
| Variable | Stages A–B (n = 201) | Stage C HFpEF (n = 143) | Stage C HFrEF (n = 226) | |||
|---|---|---|---|---|---|---|
| No HRE (n = 136) | HRE (n = 65) | No HRE (n = 94) | HRE (n = 49) | No HRE (n = 162) | HRE (n = 64) | |
| Cardiac structure and function at rest | ||||||
| HR, bpm | 78 ± 14 | 79 ± 15 | 76 ± 12 | 78 ± 16 | 75 ± 12 | 77 ± 14 |
| LVMi, g/m2.7 | 96 ± 25 | 97 ± 22 | 112 ± 29 | 113 ± 29 | 136 ± 38 | 132 ± 36 |
| LVEDVi, ml/m2 | 57 ± 15 | 57 ± 15 | 68 ± 18 | 65 ± 19 | 107 ± 31 | 102 ± 36 |
| SV, ml/beat | 69 ± 20 | 70 ± 21 | 62 ± 20 | 61 ± 20 | 59 ± 18 | 60 ± 22 |
| CO, L/min | 5.4 ± 1.5 | 5.5 ± 1.6 | 4.7 ± 1.5 | 4.8 ± 1.5 | 4.3 ± 1.4 | 4.5 ± 1.8 |
| LVEF, % | 60 ± 6 | 61 ± 6 | 59 ± 7 | 60 ± 7 | 34 ± 9 | 35 ± 9 |
| GLS, % | 16.5 ± 3.0 | 17.1 ± 2.9 | 14.6 ± 3.5 | 14.7 ± 3.8 | 8.6 ± 3.5 | 8.7 ± 3.2 |
| Mitral E wave, cm/s | 67 ± 17 | 68 ± 16 | 86 ± 40 | 96 ± 30 | 79 ± 35 | 75 ± 36 |
| Average e′, cm/s | 8.6 ± 2.0 | 8.4 ± 1.5 | 8.1 ± 2.6 | 8.0 ± 2.0 | 6.7 ± 2.1 | 6.4 ± 2.3 |
| Average E/e′ | 8.0 ± 2.1 | 8.4 ± 2.6 | 11.6 ± 5.6 | 12.9 ± 6.7 | 13.0 ± 7.1 | 12.3 ± 6.2 |
| LAVi, ml/m2 | 26 ± 8 | 28 ± 9 | 42 ± 20 | 41 ± 16 | 45 ± 18 | 43 ± 25 |
| LA reservoir strain, % | 36 ± 7 | 36 ± 7 | 21 ± 11 | 20 ± 10 | 17 ± 8 | 15 ± 6 |
| TAPSE, mm | 21 ± 3 | 21 ± 3 | 20 ± 3 | 19 ± 3 | 19 ± 3 | 19 ± 4 |
| sPAP, mmHg | 24 ± 5 | 26 ± 7** | 35 ± 12 | 39 ± 18 | 32 ± 14 | 33 ± 12 |
| TAPSE/sPAP, mm/mmHg | 0.90 ± 0.17 | 0.83 ± 0.18** | 0.64 ± 0.21 | 0.59 ± 0.25 | 0.67 ± 0.26 | 0.66 ± 0.27 |
| B-lines | 1 (0–3) | 1 (0–4) | 3 (1–7) | 3 (1–8) | 4 (1–10) | 5 (1–8) |
| Arterial properties at rest | ||||||
| Brachial SBP, mmHg | 136 ± 20 | 131 ± 15 | 135 ± 20 | 131 ± 17 | 123 ± 18 | 117 ± 18 |
| Brachial DBP, mmHg | 82 ± 12 | 80 ± 11 | 78 ± 12 | 74 ± 12 | 77 ± 11 | 75 ± 12 |
| Brachial PP, mmHg | 56 ± 16 | 64 ± 17 | 59 ± 17 | 66 ± 25 | 51 ± 14 | 48 ± 18 |
| Central SBP, mmHg | 131 ± 16 | 142 ± 22 | 130 ± 21 | 134 ± 27 | 122 ± 18 | 119 ± 20 |
| Central PP, mmHg | 42 ± 13 | 50 ± 18 | 48 ± 16 | 56 ± 26 | 42 ± 14 | 41 ± 16 |
| SVR, dyne⋅s/cm5 | 1609 ± 444 | 1534 ± 384 | 1819 ± 591 | 1707 ± 557 | 1620 ± 566 | 1876 ± 631** |
| TAC, ml/mmHg | 1.4 ± 0.7 | 1.4 ± 0.6 | 1.4 ± 0.8 | 1.2 ± 0.6 | 1.9 ± 0.9 | 1.5 ± 0.6* |
| AIx at 75, % | 26 ± 11 | 25 ± 10 | 28 ± 11 | 26 ± 10 | 25 ± 11 | 25 ± 8 |
| Carotid–femoral PWV (m/s) | 10.4 ± 1.6 | 11.5 ± 1.5** | 13.5 ± 1.9 | 14.6 ± 1.8* | 9.1 ± 2.0 | 9.7 ± 1.9 |
| Carotid–femoral PWV/GLS | 0.62 ± 0.17 | 0.67 ± 0.13 | 0.93 ± 0.21 | 1.02 ± 0.24* | 1.06 ± 0.32 | 1.11 ± 0.30 |
| Aortic arch PWV (m/s) | 7.6 ± 2.3 | 8.1 ± 2.8 | 8.6 ± 2.2 | 9.9 ± 2.8* | 5.1 ± 2.1 | 5.3 ± 2.9 |
| Aortic arch PWV/GLS | 0.46 ± 0.15 | 0.48 ± 0.17 | 0.58 ± 0.25 | 0.69 ± 0.30* | 0.57 ± 0.24 | 0.61 ± 0.29 |
| Cardiopulmonary function during exercise | ||||||
| Workload, W at peak | 130 (110–159) | 100 (90–125)*** | 80 (60–110) | 70 (53–88) | 90 (65–115) | 80 (60–100) |
| HR, bpm at peak | 133 ± 18 | 134 ± 18 | 118 ± 21 | 117 ± 25 | 113 ± 23 | 114 ± 22 |
| Chronotropic incompetencea | 80 (59) | 34 (52) | 56 (60) | 31 (63) | 102 (63) | 50 (78)* |
| SBP, mmHg at peak | 201 ± 21 | 220 ± 21*** | 171 ± 25 | 200 ± 27*** | 155 ± 25 | 179 ± 28*** |
| Peak SBP >190 mmHg in ♀/ >210 mmHg in ♂ | 65 (48) | 52 (80)*** | 14 (15) | 22 (45)*** | 3 (2) | 12 (19)** |
| SBP/workload slope (males) | 0.52 (0.39–0.59) | 0.77 (0.73–0.88)*** | 0.43 (0.23–0.55) | 0.79 (0.66–0.99)*** | 0.33 (0.25–0.42) | 0.67 (0.61–0.79)*** |
| SBP/workload slope (females) | 0.64 (0.48–0.71) | 1.00 (0.84–1.16)*** | 0.56 (0.41–0.80) | 1.14 (1.00–1.32)*** | 0.56 (0.41–0.73) | 1.10 (1.07–1.85)*** |
| DBP, mmHg at peak | 95 ± 15 | 97 ± 16 | 84 ± 16 | 86 ± 18 | 82 ± 14 | 89 ± 23** |
| PP, mmHg at peak | 106 ± 20 | 123 ± 22*** | 87 ± 21 | 115 ± 30*** | 73 ± 22 | 89 ± 28*** |
| SVR dyne⋅s/cm5 at peak | 885 ± 230 | 958 ± 248* | 1086 ± 410 | 1245 ± 513* | 1051 ± 421 | 1210 ± 509* |
| SV, ml/beat at peak | 102 ± 26 | 108 ± 44 | 83 ± 28 | 81 ± 29 | 72 ± 27 | 73 ± 35 |
| Flow reserveb | 84 (63) | 42 (65) | 42 (45) | 20 (42) | 75 (47) | 31 (48) |
| CO, L/min at peak | 13.6 ± 4.0 | 14.6 ± 6.6 | 9.7 ± 3.4 | 9.4 ± 4.1 | 8.8 ± 3.9 | 8.1 ± 4.6 |
| AVO2diff, ml/dl at peak | 12.2 (10.4–14.8) | 10.6 (8.4–13.0)** | 11.9 (9.5–13.4) | 10.3 (8.4–12.8)* | 12.9 (10.1–15.4) | 11.2 (8.8–12.8)** |
| RER at peak | 1.09 ± 0.05 | 1.09 ± 0.07 | 1.08 ± 0.07 | 1.07 ± 0.06 | 1.07 ± 0.06 | 1.08 ± 0.07 |
| VO2, ml/kg/min at peak | 19.3 (17.0–22.8) | 17.8 (16.2–20.2)** | 14.3 (12.6–16.5) | 13.1 (11.1–14.9)** | 14.8 (11.8–18.4) | 13.1 (11.4–15.6)** |
| VE/VCO2 slope | 27.5 (25.0–30.2) | 27.7 (25.2–30.6) | 34.0 (29.5–38.6) | 37.5 (32.2–41.7)* | 33.9 (27.8–39.7) | 33.7 (29.4–40.1) |
| LVEF, % at peak | 69 ± 7 | 70 ± 7 | 68 ± 9 | 68 ± 7 | 41 ± 13 | 41 ± 10 |
| Mitral E wave, cm/s at peak | 142 ± 31 | 138 ± 26 | 143 ± 38 | 153 ± 48 | 136 ± 35 | 133 ± 35 |
| Average e′, cm/s at peak | 16.3 ± 3.6 | 16.2 ± 3.7 | 13.5 ± 4.6 | 12.8 ± 3.5 | 11.5 ± 4.0 | 10.8 ± 3.3 |
| Average E/e′ at peak | 8.8 ± 4.8 | 8.8 ± 2.2 | 11.8 ± 5.4 | 12.6 ± 5.6 | 13.4 ± 6.8 | 13.2 ± 5.2 |
| TAPSE, mm at peak | 28 ± 4 | 28 ± 4 | 25 ± 4 | 24 ± 4 | 24 ± 4 | 24 ± 5 |
| sPAP, mmHg at peak | 35 ± 11 | 40 ± 15** | 52 ± 16 | 59 ± 19* | 47 ± 17 | 50 ± 16 |
| TAPSE/sPAP, mm/mmHg at peak | 0.87 ± 0.25 | 0.79 ± 0.27* | 0.54 ± 0.22 | 0.44 ± 23* | 0.58 ± 0.26 | 0.57 ± 0.27 |
| B-lines at peak | 3 (1–7) | 4 (2–9) | 9 (4–18) | 11 (5–22) | 12 (5–21) | 15 (6–20) |
| ΔB-lines | 2 (0–5) | 3 (1–5) | 6 (2–11) | 7 (1–11) | 7 (2–12) | 10 (2–15) |
- Values are mean ± standard deviation, n (%), or median (25th–75th quartile).
- AIx, augmentation index; AVO2diff, arterio–venous oxygen difference; CO, cardiac output; DBP, diastolic blood pressure; EAT, epicardial adipose tissue; EDVi, end-diastolic volume index; GLS, global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; HRE, hypertensive response to exercise; LA, left atrium; LAVi, left atrial volume; LVEF, left ventricular ejection fraction; LVMi, left ventricular mass index; PP, pulse pressure; PWV, pulse wave velocity; RER, respiratory exchange ratio; RWT, relative wall thickness; SBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure; SV, stroke volume; SVR, systemic vascular resistance; TAC, total arterial compliance; TAPSE, tricuspid annular plane systolic excursion; VE/VCO2, minute ventilation to carbon dioxide production; VO2, oxygen consumption.
- a Failure to achieve ≥80% of the difference between age-predicted maximal HR and resting HR (HR reserve) during exercise.
- b Increase in SV ≥40% during exercise.
- * p < 0.05 vs. no HRE.
- ** p < 0.01 vs. no HRE.
- *** p < 0.001 vs. no HRE.
Cardiopulmonary function during exercise
The main reasons for test interruption in the patient population were dyspnoea (72%) and leg discomfort (28%); no subject experienced chest pain and/or syncope. There were no missing data. In all HF stages, patients with HRE displayed reduced peak VO2 and AVO2diff. Also, controls with HRE exhibited lower peak VO2 (online supplementary Table S3). At peak effort, patients in Stages A–B and HFpEF with HRE expressed higher SVR and sPAP, as well as reduced TAPSE/sPAP. HFpEF patients with HRE also showed steeper minute ventilation to carbon dioxide production (VE/VCO2) slope, while HFrEF patients with HRE had higher SVR with a greater prevalence of chronotropic incompetence than those without HRE (Table 2).
Cardiovascular evaluation at rest and during exercise using absolute SBPpeak >190 mmHg in women and >210 mmHg in men to define HRE is presented in online supplementary Table S4.
Systolic blood pressure/workload slope and hemislopes
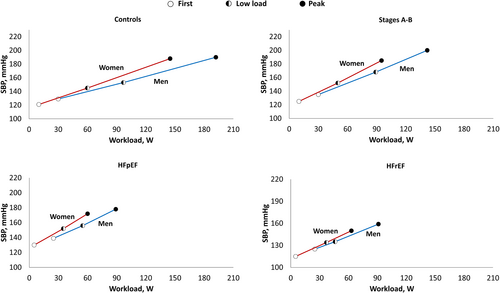
We tested different regression lines in a random selection of 40 subjects in which we reported five SBP measurements during exercise. The linear regression resulted the equation with the highest goodness of fit (adjusted R2: 0.78; online supplementary Table S5). In the same patients, there was no difference between the best-fit regression line and SBP/workload slope assessed using only the first and last (peak) SBP measurements. Figure 2 shows SBP/workload slopes and hemislopes in the overall population and each subgroup. The median workload-indexed SBP increase of the whole study population was approximately 0.5 mmHg/W (IQR 0.36–0.72). After adjusting for age, sex, New York Heart Association class, arterial hypertension, diabetes mellitus, previous myocardial infarction, NT-proBNP, and rest SBP, the SBP/workload slopes and hemislopes were significantly steeper in Stages A–B and HFpEF than HFrEF, which had values similar to controls. Overall, SBP increased approximately steadily during exercise, as the two SBP/workload hemislopes were similar in all subgroups. Online supplementary Table S6 shows the sex-related differences in workload-indexed SBP variation during exercise. Overall, SBP/workload slope was 39% steeper in women than in men (0.49, IQR 0.34–0.64 vs. 0.68, 0.46–0.91 mmHg/W; p < 0.0001). This trend was confirmed in all subgroups, also when considering hemislopes (all p < 0.05), with the highest sex-related difference in HFpEF and HFrEF (Figure 3). We also plotted the SBP variation during exercise over VO2 (SBP/VO2 slope: 4.51, IQR 2.97–6.16 mmHg/ml/kg/min in the whole population). Like the SBP/workload slope, the SBP/VO2 slopes were significantly steeper in Stages A–B and HFpEF (5.16, 3.95–6.29 and 5.33, 3.29–7.89, respectively) than HFrEF (3.80, 2.66–5.40) and controls (2.96, 2.23–3.73; all p < 0.01).
A steeper SBP/workload slope was independently associated with lower peak VO2 in the whole population without interaction with atrioventricular blocking agents (i.e. beta-blockers and calcium channel blockers). There was no association between peak VO2 and absolute SBP values at rest or peak effort (Table 3).
| Variable | SRC | p-value |
|---|---|---|
| SBP/workload slope | −0.234 | 0.025 |
| SBP/workload slope*AVN blocking agents | −0.041 | 0.13 |
| SBP, mmHg at rest | 0.028 | 0.42 |
| SBP, mmHg at peak | −0.053 | 0.28 |
| Age, years | −0.002 | 0.95 |
| Sex category (male) | 0.158 | <0.001 |
| Workload, W | 0.910 | <0.001 |
| CO, L/min at rest | −0.081 | 0.326 |
| CO, L/min at peak | 0.108 | <0.001 |
| LVEF, % at rest | 0.111 | 0.37 |
| LVEF, % at peak | 0.079 | 0.15 |
| p-value | <0.001 | |
| Adjusted R2 | 0.76 |
- AVN, atrioventricular node; CO, cardiac output; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; SRC, standardized regression coefficient.
Hypertensive response to exercise and prognosis
During a median follow-up of 16 (8–31) months, 45 events were reported: 38 hospitalizations for cardiovascular reasons and seven deaths from any cause (online supplementary Table S7). Kaplan–Meier analyses for the composite endpoint showed that the highest SBP/workload tertile (i.e. HRE) was associated with adverse outcomes in the whole population (log-rank χ2: 32.5, p = 0.01; Graphical Abstract). HRE retained prognostic significance in Stages A–B and HFpEF but not in HFrEF (online supplementary Figure S1). After adjusting for age, sex, body mass index, arterial hypertension, diabetes mellitus, previous myocardial infarction, NT-proBNP, rest and peak SBP, rest and peak CO, rest and peak LVEF, and medications (beta-blockers and calcium channel blockers), patients with the highest SBP/workload slope had a 41% (hazard ratio 1.41, 95% confidence interval 1.10–1.85) higher risk of the primary outcome than subjects in the lowest tertile (Graphical Abstract and online supplementary Table S8), while HRE defined as SBPpeak >190 mmHg in women and >210 mmHg in men did not enter in the model (online supplementary Table S9). In both survival models, age and peak CO were independent predictors of adverse events.
Discussion
We thoroughly evaluated the cardiovascular, functional, and prognostic correlates of HRE in subjects with different baseline cardiovascular risk profiles, including, for the first time, patients with overt HF across the LVEF spectrum. We demonstrated that: (i) HRE is associated with distinct pathophysiological features, especially in patients with established cardiovascular risk factors/structural cardiac disease (HF Stages A–B) and in those with a definite diagnosis of HFpEF; (ii) women have a steeper SBP/workload slope than men across the HF spectrum; (iii) SBP/workload slope could be a more sensitive predictor of impaired functional capacity and adverse events compared to absolute SBPpeak.
Blood pressure evaluation during exercise: the systolic blood pressure/workload slope
The importance of interpreting the SBP/workload slope has recently been analyzed by Hedman et al.7 in a large population of subjects free from underlying cardiovascular disease. Indeed, workload-indexed SBP can express cardiovascular (patho) physiology during exercise more clearly7, 22 and is a better predictor of all-cause mortality in patients with low and high cardiovascular risk compared to absolute BP values.23 Current guidelines on exercise testing suggest the analysis of SBP response to effort in relation to workload, but this has had limited use in clinical practice as yet.1 On average, the increase in SBP during exercise is linear in healthy subjects.7 SBP rises in proportion to the increment in workload as a result of the interplay between CO and the peripheral vascular system. Conversely, the failure of SBP to increase during exercise can result from myocardial ischaemia or severe heart valve disease (e.g. aortic stenosis).1 We confirmed a linear relation in our control group, with values in line with those reported by Hedman et al.7 We demonstrated for the first time a steady increase of SBP in response to exercise also across the HF spectrum, with peculiar differences in SBP/workload and SBP/VO2 slopes. Indeed, both slopes were significantly steeper in Stages A–B and HFpEF than in controls and HFrEF after adjusting for age and sex. These findings could be explained by the impaired arterial compliance, resistance, and elastance reserve at rest and during exercise in these populations.24-26 Increased vasoconstrictor sympathetic tone27 and impaired endothelial function due to a chronic inflammatory state28 could also play a role. Overall, these abnormalities lead to elevated left ventricular filling pressure, impaired CO reserve, and ventricular–arterial uncoupling, especially in patients with HFpEF.17, 26 On the other hand, it is conceivable that the inadequate CO increment during exercise in patients with HFrEF could prevent a relevant BP increase at any given workload.29, 30
We consistently observed steeper SBP/workload slopes and hemislopes in women than in men in all subgroups. This sex-related difference has already been highlighted in healthy subjects,7, 8 but we now show that such gender-specific differences in the haemodynamic adaptations to exercise persist across the HF spectrum. A more significant SBP increase in female subjects has been attributed to the need for a greater CO increase for any given workload due to a lower total and lean body mass in women.31 Indeed, when workload-indexed SBP increment is adjusted for body mass, this sex-specific difference was reported to disappear.32 Consistently, the adjusted SBP/VO2 slope was similar in men and women when considering controls and subjects in Stages A–B in our study. However, women with HF displayed a steeper SBP/VO2 slope than men, regardless of LVEF. This different behaviour of SBP in men and women with HF could represent a further step towards a more sex-specific characterization. Indeed, women with HF seem to be characterized by heightened inflammation, more severe microvascular dysfunction, exaggerated arterial wave reflection, lower systemic vascular compliance, and poorer VAC than men, irrespective of LVEF.33
Haemodynamic and prognostic implications of hypertensive response to exercise in different heart failure stages
Across all HF stages, subjects with HRE displayed significantly impaired functional capacity and peripheral oxygen extraction during exercise. Reduced peak AVO2diff has been observed in subjects with cardiovascular risk factors24, 34 and those with overt HF, mainly in HFpEF.25, 26 Impaired skeletal muscle vasodilatory capacity can represent a pivotal factor limiting exercise tolerance.25, 35 Indeed, subjects with HRE in Stages A–B and Stage C HFpEF displayed blunted exercise-induced vasodilatation (higher peak SVR), with greater total aortic stiffness and pulsatile load during exercise. HFpEF patients with HRE also showed greater proximal aortic stiffness and impaired VAC. Augmented total and proximal aortic stiffness enhances the systolic pulsatile load on the left ventricle,36 significantly increasing the risk of developing or worsening HF.37 We recently demonstrated VAC was inversely related to AVO2diff and independently predicted peak VO2 in patients with or at risk of developing HF,17 suggesting that the supply of blood and oxygen to exercising muscles may be hindered in the presence of impaired VAC. The deregulation of microcirculatory blood flow by an underlying proinflammatory state can have an additional role in functional and haemodynamic limitation,30 and low-grade, chronic inflammation has been previously associated with increased vascular stiffness and SBPpeak.17, 38-40 Indeed, we observed more elevated serum levels of hs-CRP and uric acid in HF patients with HRE, with higher NT-proBNP levels likely reflecting increased left ventricular filling pressures and neuro-hormonal activation.
Patients in Stages A–B and HFpEF with HRE also showed worse RV-PA uncoupling at peak exercise, a marker of poor prognosis in HF.21, 41 Exercise can significantly increase left ventricular filling and pulmonary arterial pressures in patients with HF or more at risk of developing HF, thus hampering right ventricular function.24, 42 Patients with HRE may be at increased risk of progression towards overt HF or to more advanced stages of the disease. In a cohort of US Veterans at relatively low cardiovascular risk, workload-indexed SBP response to exercise was superior to SBPpeak in predicting all-cause mortality.23 We confirmed that a steeper SBP/workload slope is independently associated with adverse outcomes, mainly in patients falling into Stages A–B and those with a definite diagnosis of HFpEF, while absolute SBPpeak did not predict an unfavourable prognosis. Since the patients who developed HRE had normal resting BP values, haemodynamic abnormalities could be overlooked during a conventional ambulatory visit.
The role of a steeper SBP/workload slope is more challenging to interpret in HFrEF. In this cohort, HRE is associated with multiple signs of sympathetic overactivity (i.e. greater levels of norepinephrine and renin at rest, higher prevalence of chronotropic incompetence, and increased systemic vasoconstriction throughout the exercise), leading to worse functional capacity.43 At the same time, indices of arterial stiffness and VAC were similar between those with and without HRE, probably because low values of CO and SBP at rest and during effort impose a relatively smaller degree of mechanical damage on arterial walls compared to HFpEF.17, 44 Finally, the presence of HRE failed the survival analysis, likely due to the pivotal role of reduced CO throughout the exercise in affecting the prognosis of HFrEF patients.45 Thus, the role of HRE in HFrEF should be further analysed in larger studies.
Clinical perspectives
Across the HF spectrum and irrespective of LVEF, patients with HRE defined by the highest tertile of the SBP/workload slope displayed impaired AVO2diff and reduced functional capacity. In subjects in Stages A–B and Stage C HFpEF, HRE is associated with increased arterial stiffness, impaired exercise-induced vasodilatation, worse VAC, and RV-PA uncoupling. Therefore, patients with HRE in Stages A–B may be at increased risk of progression towards overt HF, while patients with definite HFpEF may progress more rapidly to more advanced stages of the disease. Interestingly, women showed steeper SBP/workload slope and hemislopes than men, with sex-related differences becoming more extreme in HF patients and Stages A–B than in healthy controls. Whether these sex-specific differences affect the risk of HF development and progression is unknown and should be investigated. Noteworthy, absolute SBPpeak was not associated with any specific pathophysiological features. Finally, the role of HRE in patients with HFrEF is less clear; albeit HRE was associated with a worse functional capacity and sympathetic overactivity, survival analysis was inconclusive in this subgroup.
Limitations
This is a single-centre study from a tertiary referral hospital and thus has inherent flaws associated with selection and referral bias. As an observational study, we cannot deduce causality. We defined HRE as the highest sex-specific SBP/workload slope tertile in each HF stage, albeit age- and gender-specific reference values for workload-indexed SBP response during exercise have been proposed in healthy subjects.7 However, this approach does not consider the heterogeneity of the different HF stages in demographics (e.g. body mass index and comorbidities) and treatment that can significantly affect BP.10, 14 We evaluated all HF patients with LVEF <50% as HFrEF, but 81 subjects had mildly reduced LVEF (41–49%).10 However, a sensitivity analysis of these patients revealed similar arterial properties and CPET-ESE-derived values to those observed in subjects with LVEF ≤40%. We included in the same group patients in Stage A (n = 78) and B (n = 123); however, we performed a sensitivity analysis revealing similar arterial properties and CPET-ESE-derived values (online supplementary Table S10). Cycle ergometry testing has been associated with a steeper SBP/VO2 slope than treadmill testing46; however, it is conceivable that the between-group difference we described across the HF spectrum would be maintained with other exercise testing modalities. The accuracy of manual auscultatory methods during exercise for BP measurement remains questionable due to several factors, including noise and movement artefacts. Nonetheless, manual auscultation remains the routine clinical method for BP measurement during exercise, and the results reported in this study remain applicable for clinical use. Our findings could be affected by drug-related chronotropic incompetence, albeit we tested drug interaction with SBP/workload slope in predicting peak VO2. The number of adverse clinical events during our follow-up is relatively low; thus, we cannot exclude overfitting in the model. Larger studies should investigate the precise relationship between HRE and arterial stiffness, VAC, cardiopulmonary function and prognosis in patients at different HF stages before considering a steep SBP/workload slope as a target for therapy.
Conclusions
Hypertensive response to exercise, as evaluated by the SBP/workload slope, is associated with distinct pathophysiological features and impaired functional capacity across the HF spectrum, especially in the earliest stages of cardiovascular disease (Stages A–B) and in patients with HFpEF. Patients with HRE in these subgroups may be at increased risk of adverse cardiovascular outcomes.
Conflict of interest: none declared.