Association of left ventricular ejection fraction with worsening renal function in patients with acute heart failure: insights from the RELAX-AHF-2 study
Abstract
Aims
Whether risk of worsening renal function (WRF) during acute heart failure (AHF) hospitalization or the association between in-hospital WRF and post-discharge outcomes vary according to left ventricular ejection fraction (LVEF) is uncertain. We assessed incidence of WRF, factors related to its development and impact of WRF on post-discharge outcomes across the spectrum of LVEF in patients enrolled in RELAX-AHF-2.
Methods and results
A total of 6112 patients who had LVEF measured on admission and renal function determined prospectively during hospitalization were included. WRF, defined as a rise in serum creatinine ≥0.3 mg/dL from baseline through day 5, occurred in 1722 patients (28.2%). Incidence increased progressively from lowest to highest LVEF quartile (P < 0.001). After baseline adjustment, WRF risk in Q4 (LVEF >50%) remained significantly greater than in Q1 (LVEF ≤29%; hazard ratio 1.2, 95% confidence interval 1–1.43; P = 0.050). Age and comorbidity burden including chronic kidney disease increased as LVEF increased. Neither admission haemodynamic abnormalities, extent of diuresis during hospitalization nor residual congestion explained the increased incidence of WRF in patients with higher LVEF. Serelaxin treatment and diuretic responsiveness were associated with reduced risk of WRF in all LVEF quartiles. WRF in patients in the upper three LVEF quartiles increased risk of post-discharge events.
Conclusions
Worsening renal function incidence during AHF hospitalization increases progressively with LVEF. Greater susceptibility of patients with higher LVEF to WRF appears more related to their advanced age and worse underlying kidney function rather than haemodynamic or treatment effects. WRF is associated with increased risk of post-discharge events except in patients in the lowest LVEF quartile.
Introduction
Worsening renal function (WRF) occurs frequently in patients hospitalized for acute heart failure (AHF).1-4 When it occurs, WRF complicates efforts to decongest patients and, in some surveys, has been associated with a less favourable post-discharge outcome.4 Although the association between WRF and potential risk factors has been explored,5-11 the ability to reliably predict WRF has proven to be a difficult task, likely due to the heterogeneity of the population hospitalized for AHF and the involvement of multiple factors that affect renal function during these episodes.
Left ventricular ejection fraction (LVEF) is available in virtually all patients hospitalized with AHF. We have reported that risk of post-discharge events is increased in patients with heart failure (HF) with reduced ejection fraction (HFrEF) who are hospitalized for AHF.12 Moreover, recommendations for long-term management are based on classification of patients according to their LVEF.13 Although the incidence of WRF appears to be similar in patients with chronic HFrEF and those with HF with preserved ejection fraction (HFpEF),14, 15 the association between WRF and LVEF during an AHF hospitalization has been inconsistent.16-19 These discrepancies are likely due to differences between the populations studied and treatment administered during the hospitalization, reliance on historical rather than current values for LVEF and definitions used for WRF.
Patients in the Relaxin in Acute Heart Failure (RELAX-AHF-2) trial underwent measurement of LVEF at the time of hospital admission but entry was not restricted to a specific level. Renal function was measured on a daily basis during hospitalization, in-hospital course was carefully followed and post-discharge events were adjudicated by an expert independent committee.20, 21 Thus, RELAX-AHF-2 provided a unique opportunity to assess the incidence of WRF during AHF hospitalization, factors that predispose to its development and the association between WRF and post-discharge outcomes across the full spectrum of LVEF .
Methods
Study design and participants
As described previously, RELAX-AHF-2 was a multicentre, randomized, double-blind, placebo-controlled phase 3 study of serelaxin, a recombinant form of human relaxin-2 as a treatment for AHF.20, 21 The trial was approved at each study site and written informed consent was obtained from all participants. A total of 6600 who were hospitalized with AHF were recruited between 2 October 2013 and 1 February 2017 at 546 centres in 35 countries. Patients were required to be ≥18 years old with all of the following at entry: dyspnoea; congestion on chest radiograph; brain natriuretic peptide (BNP) ≥500 pg/mL or N-terminal pro BNP (NT-proBNP) ≥2000 pg/mL; systolic blood pressure ≥125 mmHg; mostly mild to moderate renal impairment [estimated glomerular filtration rate (eGFR) 25–75 mL/min/1.73 m2] and persistent HF symptoms after initial intravenous loop diuretic treatment equivalent to ≥40 mg furosemide. Patients received a 48 h infusion of either serelaxin (30 µg/kg/day) or placebo, in addition to standard care. The main study outcome was neutral for either of the co-primary endpoints of time to first event of cardiovascular death at 180 days or occurrence of worsening HF through day 5.20 Serelaxin reduced the incidence of WRF, but this effect was consistent across the spectrum of LVEF (online supplementary Figure S1) and there was no significant interaction between Serelaxin and LVEF on WRF (P-interaction = 0.553). Thus, the treatment groups were pooled for this analysis.
Left ventricular ejection fraction measurement
In RELAX-AHF-2 patients, LVEF was measured by echocardiography during screening or early post-randomization. The method of LVEF quantification was determined at each study site. Patients without LVEF measurement during index hospitalization were excluded (online supplementary Figure S2). Patients were categorized into quartiles according to LVEF: Q1, 7–29%; Q2, 30–38%; Q3, 39–50% and Q4, >50%. These quartiles were similar to guideline classification of HFrEF, Q1 and Q2, HF with mid-range ejection fraction, Q3 and HFpEF, Q4.
Outcome and clinical assessment
According to protocol, serum creatinine was measured during screening (considered the baseline value) and then daily through day 5 or discharge.20 WRF was defined as any rise in creatinine of ≥0.3 mg/dL from baseline through day 5. Patients with missing baseline creatinine were excluded (online supplementary Figure S2). Correlations between LVEF and WRF were assessed analysing LVEF as a categorical variable in quartiles and as a continuous variable.
Physical examination, laboratory tests, and treatment at baseline and daily through day 5 were recorded. Clinical assessment included determination of orthopnoea, oedema, jugular venous pulse, rales and body weight. A composite congestion score ranging from 0 to 8 points was calculated by summing scores for orthopnoea (0–3), peripheral oedema (0–3) and jugular venous pulse (0–2) as described.22 Diuretic response was defined as weight change from baseline through day 5 per 40 mg of intravenous furosemide (or equivalent doses of bumetanide or torsemide) administered during the corresponding period.23 eGFR was calculated using the simplified Modification of Diet in Renal Disease formula.24 Creatinine and other laboratory tests were performed at each study site using validated assays.
Patients enrolled in RELAX-AHF-2 were assessed at pre-defined clinic visits post-discharge. The main outcome for this analysis was time to first event of the composite of cardiovascular death or HF/renal failure rehospitalization through day 180. Other outcomes of interest were the two components of the composite and all-cause mortality. An independent Clinical Event Committee adjudicated all deaths and rehospitalization events according to pre-defined criteria.20, 21
Statistical analysis
Categorical variables are presented as absolute and relative frequencies for baseline characteristics across the LVEF quartiles. Normally and non-normally distributed variables, objectively determined via Shapiro–Wilk testing for normality, are depicted as mean ± standard deviation and as median (25th, 75th), respectively. Means for continuous variables were compared by ANOVA or Kruskal–Wallis test, while categorical variables were compared by Chi-squared test. A two-tailed P-value of ≤0.05 was considered statistically significant.
Kaplan–Meier curves for WRF incidence in the LVEF quartiles through day 5 were compared using log-rank test. For multivariable analysis, Cox regression models were used to adjust for effect of covariates and to calculate hazard ratios (HR). HR between LVEF quartiles were computed using the lowest LVEF (Q1) group as reference. The HRs in multivariable analysis were adjusted using the following variables: study treatment (serelaxin); geographical region; previous hypertension; peripheral arterial disease; atrial fibrillation/flutter; diabetes mellitus; history of HF; systolic blood pressure; pulse rate; respiratory rate; oedema; haemoglobin; baseline eGFR; bilirubin; received intravenous loop diuretic dose in furosemide equivalent at the time of screening; angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB); beta-blockers; mineralocorticoid receptor antagonist (MRA). Selection of variables was based on P-value <0.2 in univariable analysis and from their availability. In multivariable analysis (online supplementary Table S1), the total number was reduced to 4815 patients as participants with missing variables were excluded (online supplementary Figure S3). Smoothing spline functions taking a reference LVEF value of 50% were generated to explore the non-linear relationship between WRF and LVEF as a continuous variable. To determine if the interaction between LVEF and WRF was influenced by in-hospital treatment, changes in creatinine in Q1 and Q4 were compared using repeated mixed effects measures analysis with adjustment for intravenous diuretic, ACEi, ARB, beta-blocker and MRA dose from baseline through day 5 in addition to adjustment for the baseline variables described above.
All analyses were performed using R statistical software version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Of the 6600 patients randomized in RELAX-AHF-2, 6545 were eligible for this analysis (online supplementary Figure S2). Of these, 417 patients without LVEF measurement at index hospitalization and 16 without baseline creatinine measurement were excluded, leaving a study population of 6112 patients.
Table 1 summarizes baseline characteristics of the patients in each LVEF quartile. Age, likelihood of female sex and history of hypertension and other comorbidities (with the exception of coronary artery disease) increased as the LVEF quartile advanced from lowest to highest. Systolic blood pressure and body mass index also increased progressively as LVEF increased. The baseline congestion composite score dropped as LVEF increased. Likelihood of chronic kidney disease increased and eGFR dropped progressively from lowest to highest LVEF quartile. Loop diuretic dose and likelihood of receiving an MRA were less in patients with higher LVEF whereas ACEi/ARB or beta-blocker usage were similar across the quartiles.
| LVEF category | Q1 [7,29] (n = 1543) | Q2 [30,38] (n = 1555) | Q3 [39,50] (n = 1812) | Q4 [51,87] (n = 1202) | P-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 69.0 [61.0;77.0] | 74.0 [65.0;80.0] | 76.0 [68.0;82.0] | 78.0 [70.0;84.0] | <0.001 |
| Male sex | 1190 (77.1) | 1029 (66.2) | 965 (53.3) | 471 (39.2) | <0.001 |
| White race | 1329 (86.1) | 1465 (94.2) | 1726 (95.3) | 1104 (91.8) | <0.001 |
| Geographical region | <0.001 | ||||
| America/other | 471 (30.5) | 279 (17.9) | 260 (14.3) | 287 (23.9) | |
| Eastern Europe | 609 (39.5) | 786 (50.5) | 954 (52.6) | 431 (35.9) | |
| Western Europe | 463 (30.0) | 490 (31.5) | 598 (33.0) | 484 (40.3) | |
| Medical history | |||||
| Hypertension | 1293 (84.0) | 1374 (88.5) | 1684 (93.0) | 1143 (95.1) | <0.001 |
| Diabetes mellitus | 684 (44.3) | 696 (44.8) | 851 (47.0) | 573 (47.7) | 0.199 |
| AF/AFL | 672 (43.7) | 787 (50.7) | 1030 (57.0) | 729 (60.7) | <0.001 |
| Peripheral arterial disease | 190 (12.5) | 208 (13.5) | 255 (14.2) | 155 (13.0) | 0.512 |
| CKD (baseline eGFR <60 mL/min/1.73 m2) | 977 (63.4) | 1028 (66.1) | 1311 (72.4) | 879 (73.2) | <0.001 |
| COPD | 234 (15.3) | 212 (13.7) | 317 (17.6) | 198 (16.5) | 0.017 |
| Depression | 143 (9.31) | 96 (6.19) | 144 (7.99) | 174 (14.5) | <0.001 |
| Prior history of HF | 1201 (77.9) | 1203 (77.4) | 1314 (72.5) | 818 (68.1) | <0.001 |
| Ischaemic cause of HF | 679 (56.7) | 740 (61.6) | 735 (56.0) | 293 (35.8) | <0.001 |
| NYHA FC 1-month prior to index admission | <0.001 | ||||
| I | 34 (2.88) | 57 (4.82) | 56 (4.34) | 51 (6.37) | |
| II | 405 (34.3) | 442 (37.4) | 530 (41.1) | 359 (44.8) | |
| III | 581 (49.2) | 570 (48.2) | 586 (45.4) | 324 (40.4) | |
| IV | 160 (13.6) | 114 (9.64) | 118 (9.15) | 67 (8.36) | |
| Physical examination | |||||
| BMI (kg/m2) | 28.1 [24.8;32.1] | 28.7 [25.2;32.6] | 29.1 [25.6;33.5] | 30.1 [25.9;34.9] | <0.001 |
| Pulse rate (bpm) | 84.0 [73.0;95.0] | 81.0 [70.0;93.0] | 78.0 [68.0;90.0] | 75.0 [65.0;88.0] | <0.001 |
| Respiratory rate (breaths/min) | 21.0 [18.0;24.0] | 21.0 [18.0;24.0] | 21.0 [18.0;24.0] | 20.0 [18.0;24.0] | 0.015 |
| Systolic blood pressure (mmHg) | 134 [128;143] | 138 [130;148] | 140 [132;152] | 144 [134;158] | <0.001 |
| Diastolic blood pressure (mmHg) | 81.0 [73.0;91.0] | 80.0 [72.0;89.0] | 79.0 [70.0;87.0] | 76.0 [67.0;86.0] | <0.001 |
| Oedema | 0.004 | ||||
| 0 | 255 (17.3) | 199 (13.5) | 236 (14.0) | 174 (15.4) | |
| 1+ | 417 (28.3) | 436 (29.7) | 542 (32.2) | 330 (29.3) | |
| 2+ | 472 (32.1) | 542 (36.9) | 562 (33.4) | 413 (36.6) | |
| 3+ | 328 (22.3) | 292 (19.9) | 341 (20.3) | 211 (18.7) | |
| Jugular venous pulse | 0.001 | ||||
| <6 cm | 334 (24.4) | 353 (26.2) | 445 (29.0) | 308 (30.0) | |
| 6–10 cm | 630 (46.1) | 666 (49.4) | 715 (46.6) | 485 (47.3) | |
| >10 cm | 403 (29.5) | 330 (24.5) | 373 (24.3) | 233 (22.7) | |
| Orthopnoea | <0.001 | ||||
| None | 58 (3.94) | 44 (3.00) | 69 (4.10) | 40 (3.55) | |
| 1 Pillow (10 cm) | 205 (13.9) | 230 (15.7) | 323 (19.2) | 193 (17.1) | |
| 2 Pillows (20 cm) | 692 (47.0) | 728 (49.6) | 767 (45.6) | 586 (52.0) | |
| >30° | 517 (35.1) | 467 (31.8) | 522 (31.1) | 308 (27.3) | |
| Rales (yes) | 1379 (93.7) | 1388 (94.5) | 1589 (94.6) | 1058 (93.8) | 0.668 |
| Composite congestion score (mean ± SD) | 4.76 (1.81) | 4.72 (1.74) | 4.57 (1.80) | 4.54 (1.76) | 0.002 |
| Laboratory measurements | |||||
| BNP (pg/mL) | 1548 [956;2604] | 1207 [817;1872] | 998 [680;1487] | 882 [594;1320] | <0.001 |
| NT-proBNP (pg/mL) | 7843 [4663;13 501] | 6877 [4092;12 850] | 5460 [3377;9000] | 4339 [2790;7475] | <0.001 |
| BUN(mg/dL) | 24.3 [18.5;31.9] | 24.1 [18.7;31.3] | 24.2 [18.6;32.2] | 24.3 [18.7;33.0] | 0.722 |
| Creatinine (µmol/L) | 116 [100;141] | 113 [97.2;137] | 113 [94.9;140] | 110 [92.0;138] | <0.001 |
| eGFR (mL/min/1.73 m2) | 53.0 [42.0;64.0] | 52.4 [41.0;64.0] | 50.0 [39.0;61.0] | 48.9 [38.0;60.0] | <0.001 |
| BUN:creatinine ratio | 73.8 [58.7;90.6] | 75.2 [60.9;94.2] | 76.8 [62.3;94.2] | 78.0 [63.8;96.7] | <0.001 |
| Total bilirubin (µmol/L) | 17.0 [11.8;24.5] | 14.6 [9.58;21.4] | 12.4 [8.55;18.8] | 12.0 [8.55;17.2] | <0.001 |
| Haemoglobin (g/L) | 133 [119;145] | 130 [116;143] | 124 [110;138] | 119 [106;132] | <0.001 |
| Baseline HF medications | |||||
| Oral loop diuretic daily dose, mg of furosemide equivalent, mean ± (SD) | 61.6 (57.6) | 53.6 (57.0) | 53.1 (58.9) | 50.8 (56.8) | <0.001 |
| ACEi or ARB | 973 (68.3) | 1029 (70.7) | 1207 (69.6) | 804 (69.1) | 0.570 |
| Beta-blockers | 1058 (74.2) | 1103 (75.8) | 1297 (74.8) | 878 (75.5) | 0.782 |
| MRA | 590 (41.4) | 506 (34.8) | 461 (26.6) | 201 (17.3) | <0.001 |
| Calcium channel blockers | 157 (11.0) | 263 (18.1) | 486 (28.0) | 431 (37.1) | <0.001 |
| Digoxin | 230 (16.1) | 210 (14.4) | 214 (12.3) | 125 (10.7) | <0.001 |
- Categorical variables are presented as n (%), continuous data as median [25th–75th percentile] unless specified.
- ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AFL, atrial flutter; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate (by simplified Modification of Diet in Renal Disease formula); HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro brain natriuretic peptide; NYHA FC, New York Heart Association functional class; SD, standard deviation.
In-hospital treatment and outcomes
Table 2 summarizes in-hospital treatments and outcomes through hospital day 5 according to LVEF. The total dose of both intravenous and oral loop diuretic decreased progressively as LVEF increased. Neither use of intravenous vasoactive therapy nor mechanical circulatory support differed significantly between quartiles. Weight loss and diuretic responsiveness decreased as the LVEF increased while residual congestion was slightly more likely in Q4 patients than in the other quartiles. Systolic blood pressure reduction was greatest in Q1 patients. Creatinine and blood urea nitrogen increased (and eGFR decreased) in stepwise manner from Q1 to Q4.
| LVEF category | P-value | ||||
|---|---|---|---|---|---|
| Q1 [7,29] (n = 1543) | Q2 [30,38] (n = 1555) | Q3 [39,50] (n = 1812) | Q4 [51,87] (n = 1202) | ||
| Total IV loop diuretics dose through day 5 (mg) | 160 [80.0;300] | 160 [80.0;280] | 140 [60.0;260] | 140 [40.0;260] | <0.001 |
| Total oral loop diuretics through day 5 (mg) | 300 [200;460] | 280 [180;440] | 260 [180;400] | 260 [160;400] | <0.001 |
| IV vasoactive therapy or MCS, n (%) | 89 (5.75) | 100 (6.43) | 129 (7.11) | 90 (7.44) | 0.263 |
| Congestion status at day 5 | |||||
| Weight change at day 5 (% change) | −3.20[−5.80;-1.20] | −3.00 [−5.00;-1.20] | −3.00 [−5.00;-1.20] | −2.50 [−5.00;-1.00] | <0.001 |
| Diuretic response through day 5 (kg of weight change per 40 mg of furosemide) | −0.67 [−1.50;-0.23] | −0.60[−1.33;-0.24] | −0.67 [−1.33;-0.25] | −0.55 [−1.33;-0.17] | 0.009 |
| Haemoconcentration (increase of Hb) at day 5, n (%) | 563 (45.5) | 596 (45.1) | 747 (48.2) | 494 (49.5) | 0.093 |
| Oedema 2+ or 3+ at day 5, n (%) | 496 (32.3) | 474 (30.6) | 571 (31.7) | 423 (35.1) | 0.079 |
| Jugular venous pulse >10 cm at day 5, n (%) | 98 (6.79) | 119 (8.24) | 113 (6.72) | 101 (8.98) | 0.068 |
| Orthopnoea (2 pillows or >30°) at day 5, n (%) | 203 (16.8) | 209 (11.6) | 214 (13.8) | 233 (15.2) | <0.001 |
| Rales at day 5, n (%) | 359 (23.4) | 314 (20.3) | 417 (23.1) | 327 (27.2) | <0.001 |
| Any persisting congestion at day 5, n (%) | 675 (45.7) | 598 (40.6) | 751 (43.7) | 563 (48.8) | <0.001 |
| Change in haemodynamics at day 5 (% change from baseline values) | |||||
| Systolic blood pressure | −12.16 [−18.90;-5.11] | −10.16 [−17.30;-3.70] | −10.00 [−17.29;-3.12] | −10.89 [−18.63;-2.12] | <0.001 |
| Diastolic blood pressure | −11.39 [−21.79;0.00] | −9.09 [−19.54;1.29] | −7.14 [−17.78;3.01] | −6.98 [−18.60;5.35] | <0.001 |
| Respiration rate | −17.65 [−29.52;-5.56] | −16.67 [−28.00;-7.14] | −18.18 [−30.00;-5.88] | −16.67 [−27.27;0.00] | 0.003 |
| Change in renal function through day 5 (% change from baseline values) | |||||
| eGFR | 0.00 [−15.22;17.7] | −2.28 [−17.56;15.6] | −4.45 [−19.08;12.2] | −5.08 [−21.61;10.0] | <0.001 |
| Serum creatinine | 0.00 [−12.89;15.3] | 1.98 [−12.14;18.1] | 4.02 [−9.02;20.0] | 4.48 [−8.05;24.1] | <0.001 |
| Blood urea nitrogen | 11.4 [−11.94;45.9] | 16.3 [−9.81;52.1] | 19.2 [−6.88;51.7] | 21.8 [−6.74;57.1] | <0.001 |
| In-hospital outcomes | |||||
| Length of stay (days) | 6.67 [4.96;9.84] | 6.86 [5.06;9.93] | 6.87 [5.12;9.97] | 6.85 [5.06;10.6] | 0.005 |
| Worsening HF through day 5, n (%) | 101 (6.52) | 115 (7.39) | 122 (6.72) | 100 (8.27) | 0.279 |
| Death through day 5, n (%) | 7 (0.45) | 7 (0.45) | 14 (0.77) | 4 (0.33) | 0.344 |
| WRF through day 5, n (%) | 356 (23.1) | 419 (27.1) | 532 (29.4) | 415 (34.4) | <0.001 |
- Continuous data are presented as median [25th–75th percentile]. Doses of loop diuretic are furosemide equivalent.
- eGFR, estimated glomerular filtration rate (by simplified Modification of Diet in Renal Disease formula); Hb, haemoglobin; IV, intravenous; LVEF, left ventricular ejection fraction; MCS, mechanical circulatory support; WRF, worsening renal function.
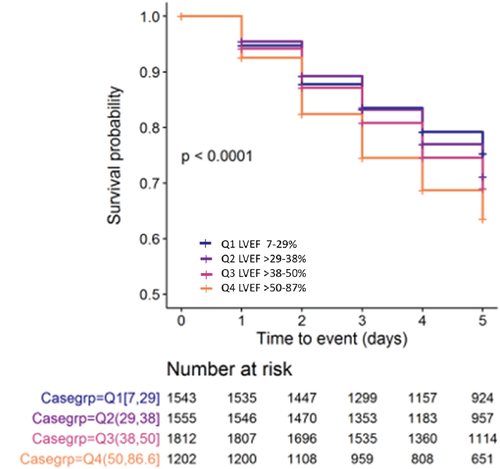
While neither worsening HF nor death during the first 5 days of hospitalization differed significantly between the LVEF quartiles, incidence of WRF increased progressively going from 23.1% in Q1 to 34.4% in Q4 (P < 0.001).
Association between worsening renal function and left ventricular ejection fraction
To further assess the association between LVEF and WRF, Kaplan–Meier curves for patients in the four quartiles over the first 5 days of hospitalization were compared (Figure 1). The risk of WRF between quartiles differed significantly (P < 0.0001 by log-rank test) with Q4 patients experiencing the highest risk. To account for baseline differences between the patients in the quartiles that might have influenced this outcome, the HR for WRF was determined without adjustment (Model 1), after adjustment for age and sex (Model 2) and after adjustment for multiple baseline differences identified during univariate analysis (Model 3). As summarized in online supplementary Table S2, in comparison to Q1, the risk of developing WRF was significantly higher in Q4 in Model 1 [HR 1.57, 95% confidence interval (CI) 1.34–1.84; P < 0.001], Model 2 (HR 1.39, 95% CI 1.17–1.64, P < 0.001) and Model 3 (HR 1.2, 95% CI 1–1.43, P = 0.050). Smooth spline modelling with log-transformed HR for the correlation between LVEF and WRF (depicted in online supplementary Figure S4) demonstrates increased risk for WRF as LVEF increases, although the slope of the association flattens after baseline adjustment. As shown online supplementary Figure S5, change in serum creatine over the 5-day period seen in Q4 patients remained significantly greater than in Q1 patients after adjustment for baseline characteristics and for intravenous diuretic and neurohormonal agent dose.
Factors associated with worsening renal function
Baseline haemodynamic abnormalities, in-hospital diuretic treatment and post-treatment residual congestion have been identified as potential contributors to the development of WRF. Consequently, the association between selected variables reflecting these factors and WRF in patients in the highest and lowest LVEF quartiles were compared to determine whether their impact on WRF differed according to LVEF. As noted in Table 3 and online supplementary Table S1, treatment with serelaxin and greater diuretic responsiveness were associated with reduced risk of WRF but the effects of each were seen across the spectrum of LVEF and there was no interaction of either variable with LVEF. In patients with the lowest LVEF (Q1), rales >1/3 way up the lung fields on admission were associated with reduced risk of developing WRF while residual 3+ peripheral oedema and higher clinical congestion score at day 5 were associated with increased risk of WRF. In patients with the highest LVEF (Q4), only higher respiratory rate on admission was associated with increased risk of WRF. When the impact of selected variables on the risk WRF was compared between LVEF quartiles, the only significant interaction was for baseline rales, which was associated with reduced risk of WRF in Q1 and nominally higher risk in Q4. Modest interactions towards higher baseline respiratory rate (P = 0.051) associated with higher risk of WRF in Q4 than in Q1 and greater risk of WRF for residual congestion in Q1 than Q4 were noted. Neither baseline natriuretic peptide levels nor haemoconcentration (as evidenced by change in haemoglobin) was associated with WRF risk in either quartile and there was no significant interaction of the impact of these variables on WRF and LVEF. As shown in online supplementary Figure S5, increases in creatinine were greater in patients in Q4 than in Q4 at all time points. A significant interaction between WRF and LVEF persisted after repeated mixed effects measures analysis which incorporated dose of intravenous loop diuretics and neurohormonal agents as well as the other covariates described previously.
| LVEF Q1 | LVEF Q4 | P-value for interaction | |||
|---|---|---|---|---|---|
| HR [95% CI] | P-value | HR [95% CI] | P-value | ||
| Baseline JVP >10 cm | 1.08 [0.85;1.38] | 0.510 | 1.00 [0.78;1.29] | 0.980 | 0.721 |
| Rales ≥1/3 at baseline | 0.56 [0.39;0.81] | 0.002 | 1.34 [0.83;2.15] | 0.226 | 0.008 |
| Respiration rate (breaths/min) | 0.99 [0.97;1.01] | 0.365 | 1.02 [1.00;1.05] | 0.038 | 0.051 |
| Baseline NT-proBNP (pmol/L) | 1.00 [1.00;1.00] | 0.384 | 1.00 [1.00;1.00] | 0.547 | 0.325 |
| Baseline eGFR ≥60 mL/min/1.73 m2 | 1.00 [0.81;1.25] | 0.972 | 0.79 [0.63;1.00] | 0.047 | 0.135 |
| Serelaxin treatment | 0.69 [0.56:0.86] | 0.001 | 0.80[0.66;0.97] | 0.022 | 0.426 |
| IV loop diuretics through day 5 (total furosemide dose equivalent) (mg) |
1.00 [1.00;1.00] |
0.034 |
1.00 [1.00;1.00] |
<0.001 | 0.551 |
| Diuretic response at day 5 (kg of body weight change per 40 mg furosemide) | 1.09 [1.02;1.18] | 0.015 | 1.08 [1.03;1.14] | 0.002 | 0.952 |
| No change or decrease of Hb at day 5 | 1.02 [0.81;1.28] | 0.895 | 1.11 [0.91;1.37] | 0.304 | 0.665 |
| Oedema at day 5: 3+ | 1.59 [1.11;2.28] | 0.011 | 1.08 [0.77;1.52] | 0.652 | 0.104 |
| Residual clinical congestion composite score at day 5 | 1.08 [1.02;1.13] | 0.003 | 1.01 [0.97;1.06] | 0.603 | 0.078 |
- CI, confidence interval; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; HR, hazard ratio; IV, intravenous; JVP, jugular venous pulse; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro brain natriuretic peptide.
Association between worsening renal function and post-discharge events
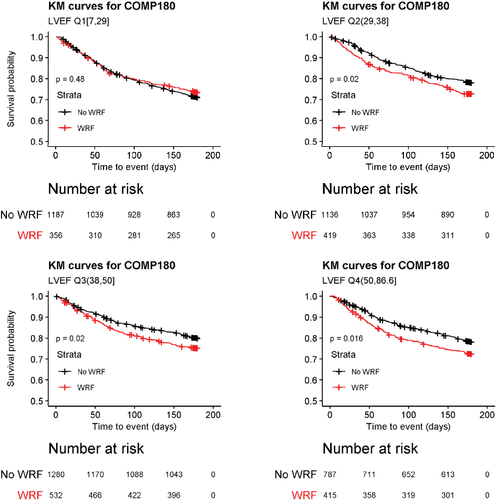
As shown in online supplementary Table S3, patients who experienced WRF during index hospitalization were at increased risk for the composite of cardiovascular mortality or HF/renal failure rehospitalization through day 180 (HR 1.18, 95% CI 1.05–1.31; P = 0.004). This was driven predominantly by increased risk for cardiovascular death (HR 1.25, 95% CI 1.04–1.50; P = 0.018). WRF during the first 5 days of hospitalization was also associated with increased risk for all-cause mortality at 180 days (HR 1.29, CI 1.10–1.51; P = 0.002). Occurrence of the 180 composite endpoint according to incident WRF during the index hospitalization is shown for each quartile in Figure 2. While occurrence of WRF did not significantly affect this outcome in patients in the lowest LVEF quartile, patients in Q2–4 who developed WRF in hospital were at higher risk for events that patients without WRF. As shown in Table 4, there was a significant interaction between WRF and LVEF for the composite outcome, with similar directional trends for both components.
| WRF status: WRF (no WRF as reference) | HR Q1 (95% CI) | P-value | HR Q4 (95% CI) | P-value | P-value for interaction (Q1 vs. Q4) |
|---|---|---|---|---|---|
| Composite endpoint of CV death or HF/RF rehospitalization through day 180 | 0.92 [0.73;1.16] | 0.481 | 1.34 [1.05;1.70] | 0.016 | 0.027 |
| Rehospitalization due to HF/RF through day 180 | 0.85 [0.65;1.11] | 0.228 | 1.21 [0.92;1.60] | 0.167 | 0.079 |
| CV death through day 180 | 0.97 [0.66;1.42] | 0.867 | 1.54 [1.01;2.33] | 0.043 | 0.099 |
| All death through day 180 | 0.98 [0.69;1.38] | 0.894 | 1.28 [0.91;1.80] | 0.149 | 0.253 |
- CI, confidence interval; CV, cardiovascular; HF, heart failure, HR, hazard ratio; RF, renal failure; WRF, worsening renal function.
Discussion
In this analysis of RELAX-AHF-2 results, WRF occurred in over a quarter of patients within the first 5 days of an AHF hospitalization, with the incidence increasing progressively from 23.1% of patients in the lowest quartile to 34.4% in the highest. Differences in the incidence of WRF between patients in the lowest and highest LVEF quartile persisted after adjustment for baseline characteristics and treatment during hospitalization. Our results suggest that more advanced age and worse underlying kidney function on hospital admission rather than more severe baseline haemodynamic abnormalities, more extensive diuresis or greater blood pressure reduction during hospitalization or residual congestion after 5 days of treatment may explain the greater susceptibility of patients with higher LVEF to develop WRF during an AHF hospitalization. Although serelaxin treatment and greater diuretic responsiveness were associated with reduced WRF incidence, their effects were consistent across the entire spectrum of LVEF. Our results also show that WRF during AHF hospitalization is associated with increased risk for post-discharge events except in patients in the lowest LVEF quartile.
Previous reports assessing the association between LVEF and risk of WRF in patients with HF have had discrepant results. In patients with chronic HF, those with HFrEF have a greater likelihood of developing WRF over time than do patients with HFpEF.25, 26 In hospitalized patients with AHF, however, the relationship between LVEF and WRF has been inconsistent. A retrospective study of 363 patients with AHF reported that the incidence of WRF was increased in patients with higher baseline LVEF values.18 An analysis of 105 388 patients in the ADHERE registry also reported that WRF incidence during HF hospitalization was significantly higher in patients with preserved LVEF than in patients with severely reduced LVEF16 but it is difficult to compare these findings to results of the present study due to differences in the definition of WRF. Results from the Korean AHF Registry suggested that WRF was more frequent in patients with HFrEF than in those with HFpEF (56.9% vs. 50.3%).19 However, after multivariable analysis which took into account differences in baseline creatinine and treatment with inotropic agents, there was a trend towards lower risk for WRF (odds ratio 0.84, P = 0.077) in HFrEF compared to HFpEF patients. In contrast to these results, a prospective study from a tertiary HF referral centre in Italy reported that reduced LVEF was independently related to higher risk of WRF,17 but this population may have been skewed towards inclusion of more advanced HFrEF as nearly a third of the patients required inotrope support. Although the present analysis shows that risk of WRF increases as baseline LVEF increases, it is important to recognize that RELAX-AHF-2 excluded patients with baseline systolic blood pressures <125 mmHg21 who might have been at higher risk of developing WRF. This possibility is supported by an analysis of RELAX-AHF-eligible patients in the ADHERE registry showing that they had better renal function than non-eligible patients.27
Examination of patient baseline characteristics and hospital course provide potential clues why risk of developing WRF increased with higher LVEF. The age of the patients and burden of comorbidities, including chronic kidney disease, of patients enrolled in RELAX-AHF-2 increased as LVEF increased while responsiveness to diuretics, another indicator of renal function, decreased. Patients with higher LVEF, however, were not more severely congested at the time of index hospitalization nor did not have lower blood pressures on admission. These patients were not more extensively diuresed nor did they experience either greater depletion of intravascular volume or reduction in blood pressure than did patients with lower LVEF levels as evidenced by the fact that total diuretic dose, weight loss in hospital and reduction in systolic or diastolic blood pressures were less in Q4 than in Q1 patients while haemoconcentration did not differ significantly between quartiles. Although residual congestion was more common in Q4 than in Q1 patients, it was not a significant predictor of WRF in the higher LVEF patients. Furthermore, the greater increase in creatinine seen in Q4 than in Q1 patients persisted after accounting for intravenous diuretic and neurohormonal agent dose during the 5-day period. These findings argue against the possibility that the increased incidence of WRF in patients with higher LVEF was related to baseline haemodynamic abnormalities, use of higher doses of diuretics, greater depletion of intravascular volume, reduced renal perfusion pressure, or more severe residual congestion. While both serelaxin treatment and diuretic responsiveness were associated with reduced likelihood of developing WRF, both were equally protective across the spectrum of LVEF and there was no interaction between LVEF and either variable for WRF risk. Our results are most consistent with the possibility that the increased vulnerability of patients with higher LVEF for developing WRF was due to their increased age and more severely impaired baseline renal function.
The association between WRF during an AHF hospitalization and clinical events post-discharge is controversial with some studies reporting that patients who experience WRF are at increased risk28-30 while others suggest that this association depends on the clinical context. Our results are consistent with a previous registry-based study which demonstrated that WRF is a stronger predictor of 1-year mortality in patients with HFpEF than in those with HFrEF.19 Although the reason for the significant association between WRF and 180-day outcome in the three highest LVEF quartiles but not the lowest is uncertain, it could be due to different mechanisms involved in the development of WRF between patients in these groups.31-34 It is possible that the rise in creatinine in patients in Q1 patients was related to a reduction in preload that in the setting of severely reduced LVEF may have had effects on cardiac function that translated to long-term clinical benefits. Further studies to sort out the underlying mechanisms of WRF in patients during an AHF hospitalization are needed to better understand the pathophysiology involved. Interestingly, treatment with serelaxin reduced the incidence of WRF, an effect that was seen in patients across the spectrum of LVEF. This result is consistent with a previous study that reported renal protective effects of serelaxin in patients hospitalized with AHF35 but unfortunately this effect did not translate to long-term clinical benefit as the results of RELAX-AHF-2 were neutral.20
Strengths of this analysis include the fact that RELAX-AHF-2 enrolled patients according to strict criteria for AHF, including elevated natriuretic peptide levels. LVEF was measured on admission to hospital, thus capturing the state of the patient at the time of index event. Renal function and other variables were prospectively collected daily through day 5. The association between LVEF and WRF was assessed using LVEF as both a categorical and a continuous variable to minimize bias due to the use of arbitrary LVEF cut-points. Finally, post-discharge events were pre-defined and adjudicated by an independent committee that was blinded to study treatment.
Study limitations
In this post-hoc analysis of RELAX-AHF-2 data, confounding variables that were not identified or considered may have affected the results. Entry criteria which excluded patients with systolic blood pressure <125 mmHg or receiving inotrope treatment may have reduced the number of patients with WRF, particularly in the lower LVEF quartiles. Since patients were required to have an eGFR 25–75 mL/min/1.73 m2, our findings are most applicable to patients who have evidence of mild to moderate chronic kidney disease during AHF hospitalization. LVEF measurements and serum creatinine values were accessed at local study sites rather than at a central core laboratory. The evaluation of some clinical signs is subjective and considerable interobserver variation has been noted. A change in the absolute creatinine value was used to define WRF and this approach might have failed to detect WRF in elderly cachectic patients with lower baseline creatinine level. Absence of variables from multivariable analysis resulted in a substantial reduction in patient numbers and some important in-hospital variables including inotrope use19, 36 and changes in neurohormonal blocking medications were not included due to incompleteness of data.
Conclusion
In patients hospitalized for AHF in RELAX-AHF-2, WRF occurred commonly, with incidence rising from lowest to highest LVEF quartile. There was no evidence, however, that factors commonly recognized as influencing kidney function in this setting (e.g. baseline haemodynamic abnormalities, in-hospital diuretic treatment, post-treatment residual congestion) were more prominent in patients in the higher LVEF quartiles. Rather, the increased susceptibility of these patients for developing WRF appears to be related to their more advanced age and worse baseline kidney function. While WRF in hospital was not associated with post-discharge outcomes in patients in the lowest LVEF quartile, it did portend a less favourable course in patients in the upper quartiles. How WRF impacts the post-discharge course in these patients and whether interventions can both mitigate the increased risk for development of WRF and improve long-term outcomes in this vulnerable population requires further study.
Conflict of interest: B.G. served on the Executive Committee for the RELAX-AHF-2 study and consulted for the following companies: ACI, Actelion, Akcea, Amgen, Bayer, EBR systems, Ionis, Janssen, Merck, Myokardia, Relypsa, Rocket, Sanofi, Vifor, Viking, Zensun. All other authors have nothing to disclose.




