Improving exercise capacity and quality of life using non-invasive heart failure treatments: evidence from clinical trials
Abstract
Endpoints of large-scale trials in chronic heart failure have mostly been defined to evaluate treatments with regard to hospitalizations and mortality. However, patients with heart failure are also affected by very severe reductions in exercise capacity and quality of life. We aimed to evaluate the effects of heart failure treatments on these endpoints using available evidence from randomized trials. Interventions with evidence for improvements in exercise capacity include physical exercise, intravenous iron supplementation in patients with iron deficiency, and – with less certainty – testosterone in highly selected patients. Erythropoiesis-stimulating agents have been reported to improve exercise capacity in anaemic patients with heart failure. Sinus rhythm may have some advantage when compared with atrial fibrillation, particularly in patients undergoing pulmonary vein isolation. Studies assessing treatments for heart failure co-morbidities such as sleep-disordered breathing, diabetes mellitus, chronic kidney disease and depression have reported improvements of exercise capacity and quality of life; however, the available data are limited and not always consistent. The available evidence for positive effects of pharmacologic interventions using angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and mineralocorticoid receptor antagonists on exercise capacity and quality of life is limited. Studies with ivabradine and with sacubitril/valsartan suggest beneficial effects at improving quality of life; however, the evidence base is limited in particular for exercise capacity. The data for heart failure with preserved ejection fraction are even less positive, only sacubitril/valsartan and spironolactone have shown some effectiveness at improving quality of life. In conclusion, the evidence for state-of-the-art heart failure treatments with regard to exercise capacity and quality of life is limited and appears not robust enough to permit recommendations for heart failure. The treatment of co-morbidities may be important for these patient-related outcomes. Additional studies on functional capacity and quality of life in heart failure are required.
Introduction
Heart failure (HF) affects approximately 26 million people worldwide.1 Its prevalence is increasing and has been estimated at 1–2% of the adult population in Western countries with numbers rising to over 10% in subjects older than 70 years.2 At age 55, the lifetime risk to develop HF is 33% for men and 28% for women.3 HF is the most common reason for hospitalization, the annual increase in hospitalization rates remains high.4 Over the decades, significant progress has been made to reduce high morbidity and mortality in patients with HF. Such progress is the result of major clinical trials that have lead to introducing new treatments into guideline-recommended therapy.2
Large-scale phase III trials since the 1990s have focused on the reduction of morbidity (defined as hospitalization for cardiovascular reasons or for HF) and mortality. Most of these trials have been conducted in patients with HF with reduced ejection fraction (HFrEF), to a lesser extent – and less successful – in patients with HF with preserved ejection fraction (HFpEF). Endpoints that quantitate patients' symptoms such as functional capacity, quality of life (QoL) and HF symptoms [mostly measured as New York Heart Association (NYHA) class], have been evaluated in phase II trials, with less frequency also in phase III trials. However, these patient-oriented outcomes are incompletely studied for many HF interventions even though they may have prognostic value by themselves.5, 6
Recently, attention has been redirected at co-morbidities of HF and at improving QoL and exercise capacity. The proposal of novel endpoints has helped in this undertaking, for example an endpoint such as days alive and out of hospital may better reflect mortality improvement with maintained QoL. Another example is the quality-adjusted life-year, a measure of the value of health outcomes, which reflects health as a function of length of life and QoL.7 Even though not applied routinely, the advent of such endpoints and calculations describes a paradigm shift that calls for symptomatic benefit in addition to reduction in mortality. Herein, we describe the available evidence to improve QoL and exercise capacity in patients with HF using state-of-the-art drugs and treatment of important co-morbidities. The importance of this topic is highlighted by a position statement of the US Food and Drug Administration (FDA) stating that ‘FDA believes there should be further discussion about whether the nature, magnitude, and clinical importance of a symptomatic benefit could justify deferral or omission of outcome studies to assess mortality’. In addition, the statement says that ‘FDA believes there should be discussion about whether and when an increased risk in mortality could be acceptable for a drug with an important symptomatic benefit’.8
Definitions, search strategy, and eligibility criteria
Quality of life can be assessed by questionnaires that have been validated in HF such as the frequently used Minnesota Living with Heart Failure Questionnaire (MLHFQ), the EQ-5D, the Short Form 36 (SF-36), or the Kansas City Cardiomyopathy Questionnaire (KCCQ). Other possible tools include the Chronic Heart Failure Assessment Tool, the Cardiac Health Profile congestive heart failure, the Chronic Heart Failure Questionnaire, the Left Ventricular Disease Questionnaire, and the Quality of Life in Severe Heart Failure Questionnaire.9 Tests of functional improvement include exercise capacity as assessed using spiroergometry testing with the evaluation of peak oxygen consumption (VO2), the 6-min walk test (6MWT), and the assessment of the patient's symptomatic burden by NYHA class. The definition of a minimal important difference (meaningful change) appears to be important in this context, in particular for the endpoint 6MWT distance, because a statistically significant improvement does not necessarily translate into a meaningful difference in daily life. Using data from more than 970 patients with HF, Täger et al.10 have estimated the minimal important difference before and after an intervention at 35–37 m, which reflects a 10% improvement of a severely limited 6MWT distance below 400 m. Interventions that achieve significantly less improvement are most likely not clinically relevant. Similar values have been obtained in patients with idiopathic lung fibrosis (24–45 m),11 chronic obstructive pulmonary disease (COPD 54 m),12 or severe COPD (26 ± 2 m).13 In geriatric patients, a small meaningful change has been estimated as 19–22 m, a large meaningful change as 47–49 m.14
A literature search was performed by two authors (S.v.H., R.E.) independently using MEDLINE and PubMed with the MeSH terms ‘heart failure’, ‘heart failure, systolic’, ‘heart failure, diastolic’, ‘exercise’, ‘exercise test’, and ‘exercise tolerance’. Expert colleagues and study sponsors provided additional information. Studies to be included for guideline-recommended drugs in the present overview required to fulfil the following criteria: (i) English language, (ii) patients aged ≥18 years and diagnosed with HF, (iii) randomized controlled trial design not comparing drugs from the same class of drugs (e.g. ramipril vs. enalapril was not permitted whereas ramipril vs. valsartan was), (iv) inclusion of at least 100 patients in total. The following data were extracted from each study: first author's last name, publication year, number of patients enrolled, inclusion criteria, follow-up duration, outcomes for exercise capacity and, if applicable, QoL evaluated. Due to the scarcity of data, smaller studies with fewer participants were permitted for treatments not currently recommended as state-of-the-art treatments by the European Society of Cardiology (ESC) and also in trials dedicated to investigate the impact of exercise interventions and interventions for co-morbidities.
Guideline-recommended heart failure pharmacotherapy
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers
Angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) decrease hospitalization rates and mortality in patients with HFrEF. Captopril showed a trend towards improvement in NYHA class, but overall the data to support a symptomatic relief are not convincing for ACEi in patients with HFrEF (Table 1 and online supplementary Table S1). QoL was rarely assessed in the trials conducted in the 1980s and 1990s. The available data do not provide convincing evidence for QoL improvement with any ACEi. Improvements in exercise capacity were more likely with shorter trial duration up to 6 months, but most trials achieved, if any, improvements in both comparator groups. The situation for ARBs is similar, even though one trial found improvements in QoL,15 another in exercise capacity with candesartan.16 In the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) trial of candesartan in HF, the number of days alive and out of hospital gained for patients on candesartan was 24.1 days vs. placebo.17 Taken together, however, the evidence base is not convincing to support beneficial effects of ARBs for symptoms, exercise capacity, or QoL with any ARB. The situation in HFpEF is similar. The Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) trial showed improvements in NYHA class and 6MWT in the perindopril group, but missed its primary endpoint.18 None of the other trials in HFpEF using ACEi or ARBs has shown improvements in symptoms, exercise capacity, or QoL (Table 1, Figure 1, and online supplementary Table S1).
| Drug | No. of trials | Total no. of patients treated with drug | Inclusion criteria | Trend towards improvement in | Details | ||
|---|---|---|---|---|---|---|---|
| NYHA class | QoL | Exercise capacity | |||||
| ACE inhibitors | |||||||
| Captopril | 3 | 297 | HFrEF | Yes | No data | No | Table S1 |
| Enalapril | 9 | 2229 | HFrEF | No | No | No | Table S1 |
| Lisinopril | 4 | 608 | HFrEF | No | No data | No | Table S1 |
| Perindopril | 1 | 424 | HFpEF | Yes | No data | Yes | Table S1 |
| Ramipril | 6 | 742 | HFrEF | No | No data | Yes | Table S1 |
| Ramipril | 1 | 45 | HFpEF | No data | No | No | Table S1 |
| Trandolapril | 2 | 272 | HFrEF | No | No data | No | Table S1 |
| Angiotensin receptor blockers | |||||||
| Candesartan | 2 | 1370 | HFrEF | Yes | Yes | Yes | Table S1 |
| Candesartan | 1 | (1021) | HFpEF | No data | Yes | No data | Table S1 |
| Irbesartan | 1 | 2123 | HFpEF | No data | Yes | Yes | Table S1 |
| Losartan | 2 | 186 | HFrEF | No | No data | No | Table S1 |
| Valsartan | 2 | 1574 | HFrEF | No | Yes | No | Table S1 |
| Valsartan | 1 | 70 | HFpEF | No data | No | No | Table S1 |
| Beta-blockers | |||||||
| Bisoprolol | 1 | 320 | HFrEF | Yes | No data | No data | Table S2 |
| Carvedilol | 5 | 709 | HFrEF | No | No | No | Table S2 |
| Metoprolol | 4 | 600 | HFrEF | No | No | No | Table S2 |
| Nebivolol | 1 | 57 | HFpEF | No | No | No | Table S2 |
| Ivabradine | |||||||
| Ivabradine | 3 | 3332 | HFrEF | Yes | Yes | Yes | Table S3 |
| Ivabradine | 1 | 95 | HFpEF | No | No | No | Table S3 |
| Angiotensin receptor–neprilysin inhibitors | |||||||
| Sacubitril/valsartan | 1 | 4187 | HFrEF | No data | Yes | No data | Table S4 |
| Sacubitril/valsartan | 2 | 2556 | HFpEF | Yes | Yes | No data | Table S4 |
| Mineralocorticoid receptor antagonists | |||||||
| Spironolactone | 2 | 876 | HFrEF | Yes | No data | No | Table S5 |
| Spironolactone | 3 | 1999 | HFpEF | No data | Yes | No | Table S5 |
- A missing effect in less than half or half of the available studies was summarized as being not effective.
- ACE, angiotensin-converting enzyme; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association; QoL, quality of life.
In summary, the available data do not support ACEi or ARBs to improve QoL or exercise capacity in patients with either HFpEF or HFrEF.
Beta-blockers
The first published experience from the use of beta-blockers in HF dates back to 1966,19 after which beta-blockers remained contraindicated for over 30 years permitting ACEi and ARB trials to be completed earlier.20 Most HF trial with beta-blockers performed in the 1990s and early 2000s did not assess symptomatic burden, exercise capacity, or QoL (Table 1 and online supplementary Table S2). Only one large study in patients with HFpEF was performed, but likewise failed to provide convincing evidence for improvements in any of these endpoints.21
In summary, evidence for an improvement in HF symptoms, exercise capacity, or QoL by beta-blocker therapy is missing.
Ivabradine
The If inhibitor ivabradine reduces heart rate of patients in sinus rhythm without affecting blood pressure.22 The Systolic Heart failure Treatment with the If Inhibitor Ivabradine Trial (SHIFT), which established a reduction of HF hospitalizations and death in patients with HFrEF, indicated a small but significant improvement in NYHA class, 28% (n = 887) of patients on ivabradine improved compared to 24% (n = 776) on placebo (P = 0.001).23 Similar results were seen for patient- and physician-reported assessments as well as for QoL.24 Indeed, the increase in KCCQ overall summary score from baseline to 12 months was higher with ivabradine than with placebo (delta increase 6.7 vs. 4.3 points, P < 0.001). Similar results were found for the KCCQ clinical summary score (5.0 vs. 3.3 points, P = 0.02). A number of smaller studies have thereafter evaluated exercise and QoL changes with ivabradine treatment (Table 1 and online supplementary Table S3). Overall, it appears that there is a trend towards an increase in exercise capacity as assessed by 6MWT and spiroergometry in patients with HFrEF with, for example, an improvement from 347 ± 112 to 475 ± 127 m and from 12.1 ± 2.1 to 15.8 ± 1.9 mL/kg/min, respectively, after 12 weeks of follow-up.25 Improvements in NYHA class and QoL were reported to be more likely compared with beta-blocker therapy; however, study duration was usually short at around 12 weeks. Study results from the Preserved Left Ventricular Ejection Fraction Chronic Heart Failure With Ivabradine Study (EDIFY) in HFpEF did not show an improvement in any of the exercise endpoints.26
In summary, evidence from ivabradine trials points towards improved exercise capacity and QoL, but the study duration was comparatively short in most cases. In HFrEF, this effectiveness is stronger and more reproducible than with beta-blocker treatment; however, no such data are available for HFpEF.
Angiotensin receptor–neprilysin inhibitors
Neprilysin is a protease that degrades a number of peptides including, and most importantly, the natriuretic peptides atrial and B-type natriuretic peptide. Its inhibitor in combination with valsartan (sacubitril/valsartan) showed a reduction of the primary composite endpoint of death from cardiovascular causes or first hospitalization for worsening HF in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial.27
The change in KCCQ clinical summary score was a secondary endpoint in PARADIGM-HF, and it showed a significantly smaller reduction in the least square means (i.e. less deterioration) as compared with enalapril (2.99 vs. 4.63 points, P = 0.001).27 The differential outcome was observed throughout the follow-up period of 36 months. A smaller proportion of angiotensin receptor–neprilysin inhibitor treated patients reported KCCQ deterioration (≥5 point decrease) as compared with the enalapril treated patients (27% vs. 31%; P = 0.01) (Table 1 and online supplementary Table S4). In patients with HFpEF, the recently published Prospective Comparison of ARNI with ARB Global Outcomes in Heart Failure with Preserved Ejection Fraction (PARAGON-HF) trial reported a trend towards less deterioration in QoL with sacubitril/valsartan than with valsartan. The mean change in the KCCQ clinical summary score at 8 months was 1.0 points higher with sacubitril/valsartan than with valsartan only (mean decrease: 1.6 vs. 2.6 points, 95% confidence interval 0.0–2.1). Similar findings were found for symptoms.28
In summary, the number of studies using sacubitril/valsartan that assessed QoL remains limited, but suggests an improvement in this endpoint, both for patients with HFrEF and for patients with HFpEF. The clinical relevance of this effect remains uncertain. Data on exercise capacity are not available.
Mineralocorticoid receptor antagonists
For patients with HFrEF, mineralocorticoid receptor antagonist (MRA) treatment with spironolactone or the newer substance eplerenone is associated with reductions in mortality and hospitalizations.29, 30 Results for patients with HFpEF remain inconsistent, but a second large trial, Spironolactone in the Treatment of Heart Failure (SPIRIT-HF, EudraCT 2017-000697-11), is currently underway. Very few studies in HFrEF have reported changes in QoL with MRA treatment, the one study that assessed peak VO2 failed to find an improvement31 (Table 1 and online supplementary Table S5). Somewhat more studies have been conducted in patients with HFpEF. One study, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial (TOPCAT), reported an improvement in KCCQ whilst another failed to show improvements in MLHFQ and SF-36. Adjusted mean changes in KCCQ were significantly better for patients on spironolactone than for those on placebo in the TOPCAT trial, 1.54 points better at 4 months (P = 0.002), 1.35 points better at 12 months (P = 0.02) and 1.86 points better at 36 months (P = 0.02).32 In the Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) trial, the peak VO2 did not change with spironolactone vs. placebo.33 This trial reported a slight reduction in the distance walked during the 6MWT in the spironolactone vs. the placebo group, with a resulting difference of −15 m, which was confined to the tertile of patients with most severe cardiac fibrosis.34 These results are opposed to a study by Kosmala et al.35 in patients with HFpEF who found an improvement in peak VO2 by 20% after 6 months of treatment with spironolactone. A meta-analysis including 4147 patients with HFpEF from seven randomized controlled trials showed significant improvements for several of the echocardiographic parameters with spironolactone treatment as compared to placebo. However, no significant improvement was found for the distance covered during the 6MWT.36 A second meta-analysis including 755 patients with HFpEF from six trials failed to show significantly different changes of peak exercise oxygen uptake, distance covered in the 6MWT, or QoL questionnaire scores between the MRA treated patients and the control group.37 No such trial data are available for eplerenone. Sexual adverse events including erectile dysfunction are more common with spironolactone than with eplerenone.38
In summary, the available evidence of studies using MRAs in patients with HF does not conclusively support improvements in exercise capacity or QoL. Some data, particularly from the large TOPCAT trial, point towards quantitatively small beneficial effects of spironolactone on QoL, predominantly in patients with HFpEF.
Co-morbidities of heart failure
Atrial fibrillation
Atrial fibrillation is a common co-morbidity in HF. Data from the Framingham Heart Study have shown that 37% of patients with atrial fibrillation have HF and more than half of patients with newly diagnosed HF have atrial fibrillation. Three randomized trials compared a strategy of rhythm control by drug therapy with control of heart rate. In the Atrial Fibrillation and Congestive Heart Failure (AF-CHF) study, 1376 patients with HF were randomized to rhythm or frequency control. There was neither a difference in the primary endpoint (cardiovascular mortality) nor a difference in NYHA class or 6MWT,39 but an improvement in QoL in all domains of the SF-36 questionnaire in patients who were in sinus rhythm for >61% of the time.40 In the Danish Investigations of Arrhythmia and Mortality on Dofetilide in Congestive Heart Failure (DIAMOND-CHF) study, 1518 patients with symptomatic HFrEF were randomized to dofetilide or placebo.41 There was no difference in mortality, the primary endpoint of the study, but a 25% relative risk reduction in HF hospitalizations with dofetilide and an increase in the risk of torsade de pointes tachycardia.
Khan et al.42 were the first to show that QoL can be improved by pulmonary vein ablation in patients with HFrEF. Di Biase et al.43 also showed improved QoL and a reduction of hospitalizations and mortality by pulmonary vein ablation in comparison to amiodarone in a randomized trial. Very recently, it was reported that pulmonary vein ablation in patients with HFrEF and atrial fibrillation reduces HF hospitalizations and mortality.44 No data exist for patients with HF and an ejection fraction >35%. Interestingly, pulmonary vein ablation may also induce functional improvement. A recent meta-analysis identified seven randomized trials including a total of 856 patients.45 There was an increase in the distance covered during the 6MWT (+27 m, P < 0.001) and an increase in the patients' peak VO2 value (+3.2 mL/kg/min, P = 0.003). QoL as measured with the MLHFQ score improved by 9.5 points (P < 0.001). However, a major limitation is that patients and physicians were not blinded to study assignment, and a sham-controlled trial is needed to evaluate the unbiased effect of pulmonary vein ablation on QoL. In patients with HFpEF, diagnostic accuracy may be improved using left atrial strain, which has also been shown to help in assessing exercise haemodynamics.46, 47
In summary, the available evidence suggests an improvement in QoL and exercise capacity with pulmonary vein ablation, but the unblinded assessment of endpoints is a major limitation. Antiarrhythmic pharmacotherapy does not improve QoL and exercise capacity.
Diabetes and insulin resistance
Diabetes is a common co-morbidity in HF with a prevalence between 20% and 40%.48 HF patients with diabetes are characterized by markedly higher morbidity and mortality.49 Diabetic patients with HF exhibit an 8.8 times higher mortality.50 Importantly, the underlying pathophysiologic principle of diabetes, i.e. insulin resistance, develops and progresses as an intrinsic aspect of HF itself. In fact, various signals and mechanisms account for the development of insulin resistance in HF independent and on top of any genetic or epigenetic predisposition for diabetes.51 As insulin is a key regulator of cellular energy utilization, insulin resistance was found to be directly and linearly associated with impaired myocardial and skeletal muscle function.52 Indeed, insulin resistance relates to various functional measures in HF of both global (NYHA class, peak VO2)53 or peripheral (quadriceps strength, treadmill walking time) functional testing.52
Limited data are available on functional improvement of metabolic treatments in HF (Table 2).54-60 In a small study, a beneficial effect of metformin on peak VO2 was not confirmed (P = 0.08). However, a significant improvement of the slope of the ratio of minute ventilation to carbon dioxide production was present (from 32.9 ± 15.9 to 28.1 ± 8.8, P = 0.034), which reflects cellular metabolism independent of oxygen uptake and has a strong prognostic power in HF.55 Another small unblinded study using the novel glucometabolic compound sodium–glucose co-transporter 2 inhibitor empagliflozin showed promising results in that it improved functional capacity in HF as measured by the 6MWT and peak VO2 (delta change +1.21, range 0.66–1.76 mL/kg/min, P < 0.001).59 Effects on QoL or exercise capacity were not reported in the recent Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME)61 and Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58)62 trials using empagliflozin and dapagliflozin, respectively, but both trials suggest lower hospitalization rates for HF. The recently published DAPA-HF (Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure) trial using dapagliflozin in symptomatic patients with HFrEF suggests an improvement in QoL both in patients with and without diabetes.60
| Reference | Study design | n (in treatment group) | Treatment (mean dose achieved) | Main inclusion criteria | Duration | NYHA class baseline (study end) | Change in QoL | 6MWT baseline (study end) (m) | Peak VO2 baseline (study end) (mL/kg/min) |
|---|---|---|---|---|---|---|---|---|---|
| Giles54, 2010 | Double-blind, randomized, controlled | 151 149 | Pioglitazone Glyburide | Diabetes mellitus, mild cardiac disease (NYHA I) | 1 year | No data | No data | No difference between groups | No data |
| Wong55, 2012 | Double-blind, randomized, placebo-controlled | 62 (39) | Metformin 1000 mg/day | Non-diabetic HFrEF | 4 months | I/II/III/IV, 11/45/6/0 (12/25/1/0) | No significant change (MLHFQ) | No difference between groups | No difference between groups |
| Nielsen56, 2016 | Open-label, randomized controlled | 40 (20) | Optimized diabetes therapy with HbA1c target <7.5% vs. standard of care | HFrEF LVEF <45% NYHA II/III | 4 months | No data | No significant change (SF-12) | No difference between groups | No difference between groups in peak VO2, but handgrip strength improved with optimized therapy |
| Margulies57, 2016 | Double-blind, randomized, placebo-controlled | 300 (154) | Liraglutide Placebo | Advanced HFrEF after hospitalization for acute HF | 180 days | 0/49/93/8 0/36/96/6 | No difference between groups (KCCQ) | No difference between groups | No data |
| Lepore58, 2016 | Double-blind, randomized, placebo-controlled | 82 (52) | Abliglutide 30/15/3.75 mg | NYHA II–III, LVEF <40% | 13 weeks | No data | No difference between groups (MLHFQ) | 411 ± 23 (429 ± 20) only in 30 mg group. No significant change | 16.2 ± 0.9 (17.1 ± 1.0) only in 30 mg group. No significant change |
| Núñez59, 2018 | Open-label, uncontrolled | 19 | Empagliflozin 10 mg/day | HFrEF or HFpEF NYHA ≥II | 30 days | No data | Significant improvement by 40% compared to baseline | Significant increase by 8.7% compared to baseline | Significant increase in peak VO2 +1.21 (0.66–1.76) |
| McMurray60, 2019 | Double-blind, randomized, placebo-controlled | 4744 (2373) | Dapagliflozin 10 mg/day | Symptomatic HF, LVEF ≤40%, elevated NT-proBNP | 18.2 months | 0/68/31/1% | Significant improvement favouring dapagliflozin (KCCQ) | No data | No data |
- 6MWT, 6-min walk test; HbA1c, glycated haemoglobin; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; QoL, quality of life; SF-12, Short Form 12; VO2, oxygen consumption.
In summary, treatment of diabetic HF patients with insulin, metformin, dipeptidyl peptidase 4 inhibitors, thiazolidinediones, or sulfonylureas does not improve exercise capacity or QoL, but the database for this notion remains very limited. The novel concept of a metabolic treatment of HF to improve energy efficacy of the myocardium and skeletal muscle is intriguing and warrants future studies. The sodium–glucose co-transporter 2 inhibitors reduce hospitalization rates for HF, but no data are available with regard to their effectiveness on QoL or exercise capacity.
Sleep-disordered breathing
Almost 50% of all patients with clinically stable HF may have detectable sleep-disordered breathing (SDB).63 SDB is even more prevalent in patients with acute HF.64 Of those patients with HF and SDB, approximately 30% have central sleep apnoea (CSA), another 30% have obstructive sleep apnoea (OSA), and about 40% have coexisting OSA and CSA, i.e. mixed sleep apnoea.65 Patients with HF with co-existing SDB have a higher symptom burden that includes fatigue, unintentional sleep, xerostomia (dryness in the mouth), nocturia, and nocturnal dyspnoea compared to HF patients without SDB.66 Among patients with HF, both OSA and CSA are associated with increased prevalence of malignant ventricular arrhythmias and increased mortality rates.64, 67, 68
In patients with HF, continuous positive airway pressure (CPAP) therapy has been shown to reduce the number of apnoeas and hypopnoeas during sleep, nocturnal hypoxia, and sleep fragmentation. CPAP is the standard treatment for OSA.69, 70 CPAP may alleviate symptom burden (e.g. excessive daytime sleepiness) and increases left ventricular ejection fraction in patients with HFrEF and OSA (Table 3).71-82 The effects of positive airway pressure on exercise capacity and QoL in patients with OSA and HF are unknown.83, 84 The results of randomized trials addressing these outcomes are pending.83 Some studies have addressed treatment of co-existing OSA and CSA in patients with HF using adaptive servo-ventilation (ASV) (Table 3).74-77 Findings with respect to changes in functional capacity are not consistent (Table 3).74-77 Treatment of CSA by use of CPAP in patients with HFrEF modestly increases left ventricular ejection fraction and the distance covered in the 6MWT compared to control (20.0 ± 55 vs. 0.8 ± 64.8 m, P = 0.016), but has no effect on survival or hospital admissions (Table 3).78 The Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure (SERVE-HF) trial has shown that ASV, an advanced mode of positive airway pressure support, did not improve left ventricular ejection fraction but increased all-cause and cardiovascular mortality.79, 85 Therefore, ASV is contraindicated in patients with HFrEF.
| Reference | Study design | n (in treatment group) | Intervention | Main inclusion criteria | Duration | NYHA (n) baseline (study end) | QoL Sleepiness baseline (study end) | 6MWT baseline (study end) (m) | Peak VO2 baseline (study end) (mL/kg/min) |
|---|---|---|---|---|---|---|---|---|---|
| Mansfield71, 2004 | Open-label, randomized controlled | 28 (19) | CPAP (vs. no CPAP) | OSA, LVEF ≤55%, NYHA II–IV | 3 months |
2.2 ± 0.2 (2.3 ± 0.2) No significant change |
Significant improvement in 4/8 domains (SF-36) | No data |
16.4 ± 0.7 (16.3 ± 0.7) No significant change |
| Smith72, 2007 | Double-blind, randomized sham-controlled cross-over | 26 (11) | Baseline auto-titrating CPAP (vs. sham CPAP) | OSA, LVEF <45%, NYHA II–IV | 6 weeks | No data | No significant change (SF-36 and MLHFQ) | No significant change | No significant change |
| Hall73, 2014 | Open-label, parallel randomized controlled | 45 (22) | CPAP (vs. no CPAP) | OSA, LVEF <45%, NYHA II–IV | 6–8 weeks | No data | No data | No data | No significant change |
| Kasai74, 2010 | Open-label, randomized controlled | 31 (16) | ASV vs. CPAP | OSA + CSA, LVEF <50%, NYHA II/III | 3 months | No data | Significant improvement in 4/8 domains favouring ASV (SF-36) | ASV: 393 ± 62 (428 ± 65) CPAP: 409 ± 58 (401 ± 56) | No data |
| Arzt75, 2013 Hetzenecker76, 2016 | Open label, parallel, randomized controlled | 72 (37) | ASV vs. no ASV | OSA, OSA + CSA and CSA, LVEF ≤40%, NYHA II/III | 3 months | Not assessed NYHA II 24% NYHA III 26% |
Significant improvement in 2/8 domains favouring ASV (SF-36) No significant change (MLHFQ) |
No data | No data |
| Randerath77, 2012 | Open-label, randomized controlled | 70 (36) | ASV (vs. CPAP) | OSA + CSA, LVEF ≥20%, NYHA II/III | 12 months | No data | No significant change (MLHFQ) | No data | No significant change |
| Bradley78, 2005 | Open-label, randomized controlled | 152 (128) | CPAP (vs. no CPAP) | CSA, LVEF ≤40%, NYHA II–IV | 24 months | No data | No significant change | Improvement by 20.0 ± 55 vs. 0.8 ± 64.8 but not sustained until study end | No data |
| Cowie79, 2018 | Open-label, randomized controlled | 312 (159) | ASV (vs. no ASV) | CSA, LVEF ≤45%, NYHA II–IV | 3 months | No significant change | No significant change | No data | No data |
- 6MWT, 6-min walk test; ASV, adaptive servo-ventilation; CPAP, continuous positive airway pressure; CSA, central sleep apnoea; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; OSA, obstructive sleep apnoea; QoL, quality of life; SF-36, Short Form 36; VO2, oxygen consumption.
In summary, SDB is highly prevalent in patients with HF and associated with symptoms such as fatigue, unintentional sleep, xerostomia, nocturia, and nocturnal dyspnoea. However, there is no evidence for positive effects of the available therapies on QoL or functional capacity.
Skeletal muscle wasting
The impact of HF on skeletal muscle wasting is well documented and contributes to the state of frailty in patients with advanced HF. Myopathy in HF affects both cardiac and skeletal muscle disturbing both structure and function with loss of muscle size, strength, and oxidative capacity. Patients with HF frequently develop skeletal muscle atrophy secondary to muscle fiber atrophy contributing to reduced peak VO2.86 Approximately 20–50% of all patients with HF develop sarcopenia, i.e. skeletal muscle wasting that exceeds a set cut-off of skeletal muscle mass in the limbs beyond two standard deviations of the mean of healthy control cohort.87, 88 Since weight loss is not a prerequisite of sarcopenia, it is pathophysiologically different from cachexia, but both can be associated with reduced muscle strength and function.89 Muscle weakness90 relates to muscle atrophy and reduced intrinsic skeletal muscle contractile function.91 Patients with sarcopenia are thus prone to exhibit lower distance on the 6MWT, lower gait speed, lower handgrip strength, and lower quadriceps strength as compared to their non-sarcopenic counterparts. Finally, patients with HF exhibit abnormal skeletal muscle metabolism with a shift towards glycolytic pathways, changes in mitochondrial function92, 93 and structure,94 and decreased oxidative enzyme activity,93 reduced capillary density94-96 and inflammation.97 Further, impaired anabolic signalling with insulin resistance and impaired growth factor action contribute to skeletal muscle dysfunction and atrophy. This results in a shift from fatigue-resistant, oxidative type I fibers towards oxidative, type II fibers.98 Altogether, these abnormalities in skeletal muscle structure, function, and cell viability are linked to each other and contribute to the abnormal exercise response, enhanced fatigability and progressive symptom complex of patients with HF, even though skeletal muscle myopathy of HF may partly be also due to physical inactivity/deconditioning.99 Exercise training (section below) and a number of substances have been used to increase muscle strength and muscle function in patients with HF. Testosterone is among the substances that has been shown to significantly improve exercise capacity as assessed using 6MWT and peak VO2 assessment (online supplementary Table S6).
In summary, skeletal muscle wasting and weakness have strong impact on patients' QoL and exercise capacity. Few studies have addressed the improvement of skeletal muscle mass and function in patients with HF. Treatment with anabolic substances like testosterone requires further scrutiny.
Iron deficiency
Iron deficiency is highly prevalent in patients with HF, affecting up to 50% of all patients.100 Prevalence is increasing with increasing NYHA class. Iron deficiency increases morbidity and mortality of HFrEF patients. Importantly, iron deficiency is associated with reduced exercise tolerance.101 Many patients with HF have iron deficiency without being anaemic. The ESC guidelines for HF recommend to screen all patients with HF for the presence of iron deficiency using serum ferritin and transferrin saturation values independent of their haemoglobin levels. Iron deficiency is defined as serum ferritin <100 μg/L or as serum ferritin 100–299 μg/L with transferrin saturation <20%.2 Seven larger studies have evaluated iron administration, highlighting significant benefit in terms of improved exercise capacity, improved NYHA class, and improved QoL (Table 4 and Figure 2).102-107 For example, in the FAIR-HF trial, 6MWT increased from 274 ± 6 to 313 ± 7 m after 24 weeks, mean KCCQ increased from 52 ± 1 to 66 ± 1 points.104 Oral administration does not sufficiently replete iron stores, and the ESC guidelines thus recommend intravenous iron supplementation for patients who have iron deficiency and HF.
| Reference | Study design | n (in treatment group) | Treatment | Main inclusion criteria | Definition iron deficiency | Duration | NYHA baseline (study end) | QoL baseline (study end) | 6MWT baseline (study end) (m) | Peak VO2 baseline (study end) (mL/kg/min) |
|---|---|---|---|---|---|---|---|---|---|---|
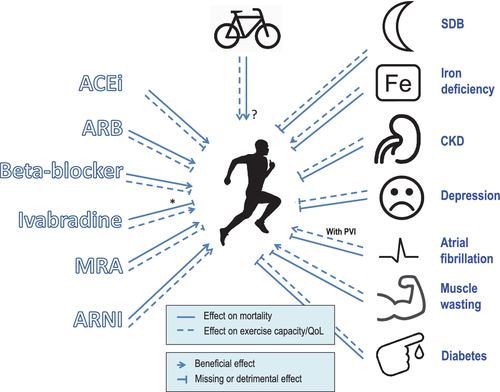
| Toblli102, 2007 | Double-blind, randomized, placebo-controlled | 40 (20) | Iron sucrose 200 mg weekly for 5 weeks (vs. placebo) | NYHA II–III, LVEF ≤35%, | TSAT <20%, ferritin <100 ng/mL | 6 months | 2.9 ± 0.7 (2.0 ± 0.2) Significant improvement only in the IV iron group | Significant improvement (MLHFQ) | 192.3 ± 60.9 (240.1 ± 51.2) | No data |
| Okonko103, 2008 (FERRIC-HF) | Double-blind, randomized, placebo-controlled | 35 (24) | Iron sucrose 200 mg weekly until ferritin 500 ng/mL (vs. placebo) | NYHA II–III, LVEF ≤45% | Serum ferritin <100 ng/mL, or serum ferritin 100–299 ng/mL with TSAT <20% | 16 weeks | 2.5 ± 0.5 (2.1 ± 0.5) Significant improvement only in the IV iron group | Significant improvement only in the IV iron group (MLHFQ and Patient Global Assessment) | No data | 13.9 ± 2.7 (15.4 ± 3.5) Significant improvement only in the IV iron group |
| Anker104, 2009 (FAIR-HF) | Double-blind, randomized, placebo-controlled | 459 (304) | 200 mg ferric carboxymaltose (vs. placebo) | NYHA II–III, LVEF ≤40% | Serum ferritin <100 ng/mL, or serum ferritin 100–299 ng/mL with TSAT <20% | 24 weeks | 0/17/83/0% (6/41/50/1%) | Significant improvement (KCCQ, EQ-5D, Patient Global Assessment) | 274 ± 105 (313 ± 7) Significant improvement only in the IV iron group | No data |
| Beck da Silva105, 2013 (IRON-HF) | Double-blind, randomized, placebo-controlled | 18 | Iron sucrose 200 mg weekly for 5 weeks (vs. placebo) | NYHA II–IV, LVEF ≤40% | TSAT < 20% and ferritin <500 μg/L | 3 months | No data | Significant improvement (MLHFQ, Patient Global Assessment) | No data | 14 ± 3.4 (+3.5 mL/kg/min) |
| Ponikowski106, 2015 (CONFIRM-HF) | Double-blind, randomized, placebo-controlled | 304 (152) | 500 or 1000 mg ferric carboxymaltose (vs. placebo) | NYHA II–III, LVEF ≤45% | Serum ferritin <100 ng/mL, or serum ferritin 100–299 ng/mL with TSAT <20% | 52 weeks | 0/53/47/0% Significant improvement in IV iron group | Significant improvement (KCCQ, EQ-5D, Patient Global Assessment) | 288 ± 98 (324 ± 98) Significant improvement in IV iron group | No data |
| van Veldhuisen107, 2017 (EFFECT-HF) | Open-label, randomized, placebo-controlled | 172 (86) | 1000 mg ferric carboxymaltose (vs. placebo) | NYHA II–III, LVEF ≤45% | Serum ferritin <100 ng/mL, or serum ferritin 100–299 ng/mL with TSAT <20% | 24 weeks | 0/71/29/0% Significant improvement in IV iron group | Significant improvement in Patient Global Assessment | No data | Improvement only in the last observation carried forward-analysis |
- CONFIRM-HF, Ferric Carboxymaltose Evaluation on Performance in Patients with Iron Deficiency in Combination with Chronic Heart Failure; EFFECT-HF, Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Iron Deficiency and Chronic Heart Failure; FAIR-HF, Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure; FERRIC-HF, Ferric Iron Sucrose in Heart Failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; IRON-HF, Iron Supplementation in Heart Failure Patients With Anemia; IV, intravenous; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; QoL, quality of life; TSAT, transferrin saturation; VO2, oxygen consumption.
In summary, it is recommended to screen all patients with HF for the presence of iron deficiency. Intravenous iron administration has been shown to improve 6MWT distance at a level deemed to be clinically meaningful. Likewise, QoL can be improved.
Depression
Depression is prevalent in approximately 20% of patients with HF (ranging from 11% in NYHA class I to 42% in NYHA class IV). Depression is independently related to increased morbidity and mortality108 as well as to substantially reduced QoL109 and moderately reduced exercise capacity. Clinical severity of HF as assessed by cardiac function parameters is assumed to interact with depression in a bidirectional way, leading to a vicious circle.110 Together with frailty and anxiety, depression is among the factors associated with higher rates of recurrent events after an episode of hospitalization for HF.111 Recommended treatments for depression in patients with HF include exercise training (see below), cognitive behavioural therapy and – despite negative results of the two largest trials [Sertraline Against Depression and Heart Disease in Chronic Heart Failure (SADHART-CHF),112 Effects of Selective Serotonin Re-Uptake Inhibition on Morbidity, Mortality and Mood in Depressed Heart Failure Patients (MOOD-HF113)] – selective serotonin reuptake inhibitors (Table 5).2, 112-117 In support of the recommendations, a recent meta-analysis shows that cognitive behavioural therapy, i.e. individual or group psychotherapy mainly addressing dysfunctional thoughts and related behaviours, significantly improves QoL in depressed HF patients by 0.3 standard deviations.118 However, QoL is typically reduced by more than 1 standard deviation in depressed vs. non-depressed HF patients.109 Additional treatments are therefore needed. In the Heart Failure-A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, exercise intervention modestly but significantly improved depressive symptoms116 and this improvement was associated with a significant though small improvement in overall QoL on the KCCQ (Cohen's d < 0.1 denoting a small effect size) and peak VO2 (d < 0.2). SADHART-CHF112 and MOOD HF113 were unable to show superiority of the selective serotonin reuptake inhibitors sertraline and escitalopram over placebo and active disease management. However, in both trials, depressive symptoms were reduced substantially, and in MOOD-HF this reduction went along with improvements in overall QoL on the KCCQ of similar magnitude (d = 0.6 at 6 months, denoting a moderate effect). Changes in the 6MWT distance were rarely assessed, but patients who achieved depression remission in SADHART-CHF improved the 6MWT distance more than the non-remission group (difference from baseline: 63.5 ± 238.8 vs. 16.2 ± 115.7 m, P = 0.03).114
| Reference | Study design | n (in treatment group) | Treatment (mean dose achieved) | Main inclusion criteria | Duration | NYHA baseline (study end) | QoL or depression rating baseline (study end) | 6MWT baseline (study end) (m) | Peak VO2 baseline (study end) (mL/kg/min) |
|---|---|---|---|---|---|---|---|---|---|
| Antidepressant medication | |||||||||
| O'Connor112, 2010 | Double-blind, randomized, placebo-controlled |
469 (234) |
Sertraline (S, 65 mg/day) vs. placebo (P) plus enhanced nurse support in both groups |
LVEF ≤45%, NYHA II-IV, MDD |
12 weeks | S: 34.9% worse P: 41% worse | HDRS: S: 18.3 ± 5.5 (−7.1) P: 18.3 ± 5.4 (−6.8) | – | – |
| Xiong114, 2012 | Secondary analysis of O'Connor 2010 | 153 depression remitters (R) vs. 132 non-remitters (NR) | Adjusted in analyses |
KCCQ total: R + 24.7; NR + 10.4 SF-36 PF: R + 16.6; NR +2.5 SF-36 MH: R + 20.2; NR +8.9 |
R + 63.5 m NR + 16.2 m |
– | |||
| Angermann113, 2016 | Double-blind, randomized, placebo-controlled |
372 (185) |
Escitalopram (E, 15.8 mg) vs. placebo (P) plus enhanced support in both groups | LVEF <45%, NYHA II–IV, MDD (SCID) | 18 months |
Improved at 12 months E: 20% P: 24% Worsened at 12 months E: 8% P: 9% |
MADRS: E: 20.2 ± 8.6 (12 weeks: 11.2 ± 8.1) P: 21.4 ± 8.8 (12 weeks: 12.5 ± 7.6) PHQ-9: E: 12.1 ± 5.1 (12 mo: −3.5) P: 12.7 ± 4.9 (12 mo: −3.3) KCCQ total: E: 47.9 ± 18.8 (12 mo: +8.6) P: 46.8 ± 18.2 (12 mo: +12.7) |
E: 356 ± 125 (12 mo: +17) P: 342 ± 125 (12 mo: +6) |
– |
| Cognitive behavioural therapy | |||||||||
| Freedland115, 2015 | Randomized controlled |
158 (79) |
CBT (11 sessions, 1 h each) vs. enhanced standard of care | HF diagnosis, current MDE | 12 months | – |
BDI: CBT: 30.7 ± 10.2 (12 mo: 11.2 ± 10.7) standard: 29.6 ± 10.2 (12 mo: 16.0 ± 10.8) KCCQ total CBT: 45.2 ± 23.3 (12 mo: 64.2 ± 25.2) standard: 46.1 ± 23.3 (12 mo: 56.0 ± 24.0) |
CBT: 981 ± 410 ft (6 mo: 1017 ± 430 ft) Standard: 1026 ± 411 ft (6 mo: 1027 ± 431 ft) |
– |
| Other treatments | |||||||||
| Blumenthal116, 2012 | Open-label, randomized controlled |
2322 (1159) |
Aerobic exercise training (Ex) vs. standard of care (Std) |
LVEF ≤35%, NYHA II–IV |
12 months | – |
Delta KCCQ total: (3 mo) Ex: + 5.2 Std: +3.3 (P < 0.001; unchanged until end of study) BDI: Ex (3 mo): 9.0 Std (3 mo): 9.7 (P = 0.002) Ex (12 mo): 8.9 Std (12 mo): 9.5 (P = 0.01) Depressed subgroup Ex (12 mo): 15.8 Std (12 mo): 17.3 (P = 0.02) |
No difference between groups | Improvement in exercise group |
| Scherer117, 2013 | Secondary analysis from double-blind, randomized, controlled trial | 589 (589) | Carvedilol vs. bisoprolol |
NYHA ≥II, LVEF ≤45% |
12 weeks | – |
SF-36 PFa38.2 ± 9.5 (40.4 ± 8.7) SF-36 MHa 45.2 ± 12.0 (48.3 ± 10.8) |
– | – |
- 6MWT, 6-min walk test; BDI, Beck Depression Inventory; CBT, cognitive behavioural therapy; HDRS, Hamilton Depression Rating Scale; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MADRS, Montgomery Åsberg Depression Rating Scale; MDD, major depression disorder; MDE, major depression episode; MH, Mental Health subscale; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; PF, Physical Function subscale; PHQ-9, Patient Health Questionnaire, 9-item depression scale; QoL, quality of life; SCID, Structured Clinical Interview for DSM-IV Disorders; SF-36, Short Form 36; VO2, oxygen consumption.
- a QoL not predicted by type or dosage of beta-blocker, Δ heart rate or Δ LVEF.
- b Most studies used MLHFQ with higher scores indicating more impairment.
In summary, a number of interventions have shown beneficial effects in the reduction of depressive symptoms and improvement in QoL in patients with HF, but only few data suggest improvements in exercise capacity.
Chronic kidney disease
Heart failure and kidney dysfunction are common co-morbidities with overlapping and synergizing mechanisms. Co-incidence of both pathologies aggravates disease progression rates, symptoms (i.e. dyspnoea and oedema driven by fluid retention), and outcomes (hospitalization, cardiovascular death). Online supplementary Table S7 summarizes milestone studies in HFrEF with respect to the renal situation. Online supplementary Table S8 highlights the relative levels of strength of evidence for goal-directed medical therapies in HFrEF across varying stages of non-dialytic chronic kidney disease (CKD).119 It should be noted, however, that the benefit observed in most of these studies was similar in patients with estimated glomerular filtration rate <60 (or even <45) mL/min compared to >60 mL/min, and even greater in some studies.
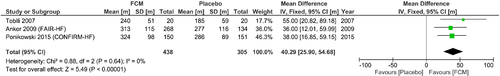
The most significant data on CKD and HF exists for the administration of erythropoiesis-stimulating agents (ESAs). Here, a Cochrane meta-analysis of 11 studies120 in anaemic patients with HF demonstrated an association between the administration of ESAs and an improvement in exercise tolerance [exercise duration +96.8 s; 6MWT +69.3 m; peakVO2 + 2.29 mL/kg/min; anaerobic threshold +2.92 (all P < 0.04)] and QoL [NYHA class −0.73; KCCQ +4.60; Patient Global Assessment +1.16; rate of HF-related hospitalizations (risk ratio 0.62, all P < 0.04)]. These results held true both in patients with and without CKD. The data are summarized in Table 6 and Figure 3.121-129
| Reference | Study design | n (in treatment group) | Kidney function | Treatment (target Hb or Hct) | Main inclusion criteria | Duration | NYHA baseline (change in NYHA class) | QoL baseline (study end) | 6MWT baseline (study end) (m) | Peak VO2 baseline (study end) (mL/kg/min) |
|---|---|---|---|---|---|---|---|---|---|---|
| Swedberg121, 2013 |
Randomized, double-blind, placebo-controlled, multicentre study |
2278 (1136) |
S-creatinine 1.4 mg/dL |
Darbepoetin alfa (Hb 13.0 g/dL) |
LVEF ≤40% Hb 9.0–12.0 g/dL |
6 months |
65% NYHA class III or IV |
KCCQ (LS mean change ± SD) 6.68 ± 1.33 |
No data | No data |
|
Parissis122, 2008 |
Randomized, single-blind, placebo-controlled, single-centre study |
32 (21) |
S-creatinine 1.7 mg/dL |
Darbepoetin alfa (Hb 14.0 g/dL) |
LVEF <40% Hb < 12.5 g/dL |
3 months |
NYHA II–III (−1.1) |
No data |
227 ± 105 (296 ± 87) |
No data |
|
Ghali123, 2008 |
Randomized, double-blind, placebo-controlled, multicentre study |
319 (162) |
S-creatinine 1.5 mg/dL |
Darbepoetin alfa (Hb 14.0 g/dL) |
LVEF<40% Hb < 12.5 g/dL |
1 year |
NYHA II–IV (−0.06) |
MLHFQ (mean change ± SD) −9.3 ± 1.6 |
No data | No data |
| Kourea124, 2008 |
Randomized, single-blind, placebo-controlled, single-centre study |
41 (21) |
S-creatinine 1.7 mg/dL |
Darbepoetin alfa (Hb 12.5–14.0 g/dL) |
LVEF<40% Hb < 12.5 g/dL |
3 months |
NYHA II–III (−0.50) |
KCCQ Functional 57 ± 24 (78 ± 14) Summary 47 ± 22 (68 ± 20) |
201 ± 113 (274 ± 97) |
No data |
|
van Veldhuisen125, 2007 |
Randomized, double-blind, placebo-controlled, multicentre study |
165 (110) |
S-creatinine 1.4 mg/dL |
Darbepoetin alfa (Hb 14.0 g/dL) |
LVEF <40% Hb 9.0–12.5 g/dL |
26 weeks |
Symptomatic HF (−0.07) |
KCCQ 57.9 ± 19.2 (65.9 ± 20) MLHFQ 40.5 ± 21.5 (30.5 ± 20) |
287 ± 124 (321 ± 80) |
No data |
|
Ponikowski126, 2007 |
Randomized, double-blind, placebo-controlled, multicentre study |
41 (19) |
S-creatinine 1.32 mg/dL |
Darbepoetin alfa (Hb 13.0– 15.0 g/dL) |
LVEF <40% Hb 9–12 g/dL |
26 weeks | Symptomatic HF |
KCCQ (mean change ± SD) 12.4 ± 17 MLHFQ −10.8 ± 22.1 |
No data |
[mean (95% CI) change] 0.5 (−0.6 to 1.7) |
|
Palazzuoli127, 2006 |
Randomized, double-blind, placebo-controlled, single-centre study |
38 (20) |
S-creatinine 2.5 mg/dL |
Erythropoietin beta (Hb 11.5–12.5 g/dL) |
Hb ≤11 g/dL | 3 months |
NYHA III/IV (−0.80) |
No data | No data |
9.2 ± 2.0 (13.2 ± 3.6) |
|
Mancini128, 2003 |
Randomized, single-blind, placebo-controlled, single-centre study |
23 (15) |
S-creatinine 1.6 mg/dL |
Erythropoietin alfa (Hct >45%) |
Hct <35% | 3 months |
NYHA III/IV |
No data |
Feet: 1187 ± 279 (1328 ± 254) |
11.0 ± 1.8 (12.7 ± 2.8) |
|
Silverberg129, 2001 |
Randomized, open-label, single-centre study |
32 (16) |
S-creatinine 1.7 mg/dL |
Erythropoietin (Hb 12.5 g/dL) |
LVEF <40% Hb 10.5–11.5 g/dL |
8.2 months |
NYHA III/IV (−1.70) |
No data | No data | No data |
- 6MWT, 6-min walk test; Hb, haemoglobin; Hct, haematocrit; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LS, least square; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; QoL, quality of life; S, serum; SD, standard deviation; VO2, oxygen consumption.
In summary, data on treatment of CKD and its effects on HF are very sparse. There is currently no evidence that improving CKD contributes to improving QoL or exercise capacity in patients with HF, whereas anaemic patients seem to benefit from ESA administration, but this beneficial effect may be outweighed by an increase in the likelihood of adverse events.
Exercise and endothelial function
Exercise intervention
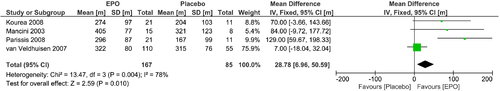
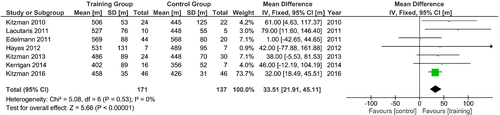
A hallmark of the impaired QoL in patients with HF is exercise intolerance. The underlying mechanisms are complex and involve central (cardiac output), peripheral muscular factors (e.g. impaired skeletal muscle blood flow, abnormal skeletal morphology, and function) and vascular factors, especially impaired endothelial function. In both, HFrEF and HFpEF, physical inactivity (exercise intolerance) is independently associated with worse outcome. Therefore, an exercise training intervention is thought to be one of the most promising and effective approaches to improve exercise ability and QoL. In fact, in HFrEF a large number of randomized controlled studies with a sufficient number of patients demonstrate a significant improvement of exercise capacity (peak VO2, 6MWT distance) and QoL (both physical and total scores) (Table 7).130-147 As shown in the ACTION-HF trial, the long-term application of exercise training was effective, feasible and safe in a large number of patients with clinically stable HFrEF.148 Assessment of KCCQ overall summary score showed greater improvement with usual care plus exercise training than with usual care alone at 3 months (mean improvement: 5.2 points, 95% confidence interval 4.4–6.0).149 These findings were confirmed by a recent meta-analysis of trials in cardiac rehabilitation; however, the analysis – just like another large meta-analysis – unfortunately failed to show an effective improvement in mortality or hospitalization.150, 151 Another limiting factor in the effectiveness of exercise training is long-term acceptance, adherence, and the overall poor implementation of exercise interventions into HF treatment programmes,152 leading to only modest effects on prognosis, which needs to be addressed in the future.
| Reference | Study design | n (in treatment group) | Treatment (intervention planned) | Main inclusion criteria | Duration | NYHA baseline (study end) | QoL baseline (study end) | 6MWT baseline (study end) (m) | Peak VO2 baseline (study end) (mL/kg/min) |
|---|---|---|---|---|---|---|---|---|---|
| Coats130, 1992 | Controlled cross-over trial | 17 |
Incremental (4 min, 25 W stages) upright bicycle exercise test to exhaustion, with 1-min average measurements of respiratory gas exchange |
Stable moderate to severe CHF (LVEF 19.6 ± 2.3%) |
8 weeks |
No data |
No data | No data |
13.2 ± 0.9 (15.6 ± 1.0) |
|
Meyer131, 1996 |
Controlled cross-over trial | 18 |
Training programme consisted of interval exercise with bicycle ergometer (15 min) 5 x weekly, interval treadmill walking (10 min), and exercises (20 min), each 3 times weekly |
Severe HF due to dilated cardiomyopathy (n = 9) or coronary artery disease (n = 9) |
3 weeks | No data | No data | No data |
12.2 ± 0.7 (training: 14.6 ± 0.7; activity restriction: 12.6 ± 0.7) |
|
Kavanagh132, 1996 |
Non-randomized, parallel-group, controlled |
30 (21) |
Progressive, supervised aerobic walking programme |
Stable HF LVEF 22 ± 7% NYHA II–III |
52 weeks |
No data |
CHFQ: small trend to improve emotional function at 12 weeks |
81 ± 13 m/s (95 ± 13 m/s) |
15.1 ± 0.5 (15.8 ± 0.5) |
|
Dubach133, 1997 |
Open-label, randomized, controlled |
25 (12) |
2 h of walking daily and 4 weekly sessions of high intensity monitored stationary cycling (40 min at 70% to 80% peak capacity) at a residential rehabilitation centre |
Stable HF LVEF <40% (LVEF 31.5 ± 6.7%) NYHA II–III after myocardial infarction or CABG surgery |
2 months | No data | No data | No data |
19.4 ± 3.0 (25.1 ± 4.8) |
| Belardinelli134, 1999 | Open-label, randomized, controlled |
99 (50) |
ET at 60% of peak VO2, initially 3 times a week for 8 weeks, then twice a week for 1 year |
HFrEF LVEF ≤40% (LVEF 28.4 ± 6) |
14 months | No data |
MLHFQ 52 ± 22 (39 ± 20) |
No data |
15.7 ± 2 (19.9 ± 1) |
| Hambrecht135, 2000 | Open-label, randomized, controlled |
73 (36) |
Two weeks of in-hospital ergometer exercise for 10 min 4 to 6 times per day, followed by 6 months of home-based ergometer exercise training for 20 min per day at 70% of peak VO2 |
HFrEF LVEF <40% NYHA I–III |
2 weeks in hospital, 6 months at home |
No data | No data | No data |
18.2 ± 3.9 (23.0 ± 4.7) |
|
Wisløff136, 2007 |
Open-label, randomized, controlled |
27 (MCT n = 9, AIT n = 9) |
MCT (70% of peak heart rate) or AIT (95% of peak heart rate) 3 times per week or to a control group that received standard advice regarding physical activity |
Stable HF post-myocardial infarction | 12 weeks | No data |
The MacNew global score for QoL increased after MCT and after AIT |
No data |
MCT: 13.0 ± 1.1 (14.9 ± 0.9) AIT: 13.0 ± 1.6 (19.0 ± 2.1) |
| Erbs137, 2010 | Open-label, randomized, controlled |
37 (18) |
3 weeks of 3 to 6 times of daily exercises for 5 to 20 min on a bicycle ergometer adjusted to the work load at which 50% of peak VO2 was reached. Afterwards 20 to 30 min exercise once daily |
Advanced HF (NYHA IIIb) (LVEF 24.3 ± 5.4%) |
12 weeks | No data | No data | No data |
15.3 ± 3.3 (17.8 ± 3.2) |
| Fu138, 2013 | Open-label, randomized, controlled | 45 | AIT (3-min intervals at 40% and 80% peak VO2 or MCT (sustained 60% peak VO2 for 30 min/day, 3 days/week |
Stable HF NYHA II-III, LVEF ≤40% |
12 weeks | No data |
AIT increased the SF-36 physical/mental component scores and decreased the MLHFQ score |
No data |
AIT: 16.0 ± 1.0 (19.6 ± 1.2) MCT: 15.9 ± 0.7 (16.0 ± 1.5) |
|
Ellingsen139, 2017 |
Open-label, randomized, controlled | 261 (HIIT n = 90, MCT n = 85) |
During the first 12 weeks: 3 supervised sessions of HIIT at 90% to 95% of maximal heart rate, MCT at 60% to 70% of maximal heart rate, or RRE |
Stable patients with CHF (NYHA II–III, LVEF ≤ 35%) (LVEF 24.3 ± 5.4%) |
12 weeks of supervised training (afterwards patient were encouraged to continue exercising on their own with a concluding follow-up at 12 months) |
No data |
There were no within-group or between-group differences in the QoL measures KCCQ, HADS, Global Mood Scale, or Type D Scale 14 at baseline, 12 weeks, or 52 weeks |
No data |
RRE (n = 73) 18.4 (16.8–19.6) 17.4 (15.7–19.8) 18.2 (15.8–20.0) MCT (n = 65) 16.2 (15.3–18.7) |
|
17.0 (15.7–19.6) 16.4 (15.0–18.6) HIT (n = 77) 16.8 (15.8–17.8) 18.2 (16.3–20.0) 17.1 (15.5–18.6) |
|||||||||
|
Laoutaris140, 2011 |
Open-label, randomized, controlled |
15 (10) |
3-5x 45 min/week biking and 2-3x/week high-intensity inspiratory muscle training |
Clinically stable condition post-LVAD implantation | 10 weeks | No data |
MLHFQ: 48.9 ± 12.8 (38.2 ± 11.6) |
462 ± 88 (527 ± 76) | 16.8 ± 3.7 (19.3 ± 4.5) |
|
Hayes141, 2012 |
Open-label, randomized, controlled, assessor blinded, |
14 (7) |
3x/week 60 min biking/treadmill and strength exercises for 2 sets of 10 repetitions of 6 muscle groups |
LVAD insertion as a bridge to heart transplantation, age > 18 years |
8 weeks | No data |
SF-36 Total Score: 30.4 ± 10.7 (59.6 ± 24.2) |
351 ± 77 (531 ± 131) | 10.5 ± 2.3 (14.8 ± 4.9) |
|
Kerrigan142, 2014 |
Open-label, randomized, controlled |
26 (18) |
3x/week 30 min ergometer training (biking, treadmill, etc.) | Recently implanted LVAD (i.e. 1 to 6 months from surgery date), age > 18 years | 6 weeks | No data |
KCCQ: difference vs. baseline +14.4 |
350 ± 65 (402 ± 89) | 13.6 ± 3.3 (15.3 ± 4.4) |
|
Kitzman143, 2010 |
Single-blind, randomized, attention-controlled |
46 (24) |
Aerobic training programme including walking on a track and lower extremity cycling 3xweekly, (20 min each, 60–70% intensity) with additionally warm up and cool down |
HFpEF LVEF ≥50% |
16 weeks | No data |
MLHFQ: 16 ± 10 (11 ± 11) Total sum 32 ± 20 (25 ± 24) |
Feet: 1494 ± 224 (1659 ± 173) |
13.8 ± 2.5 (16.1 ± 2.6) |
| Edelmann144, 2011 |
Open-label, randomized, controlled, assessor blinded |
64 (44) |
Supervised aerobic and resistance training programme (3x/week, 60 min) consisting of cycling on an ergometer (up to 70% intensity) with additional warm up and cool down and strength training |
NYHA II–III, LVEF ≥50%, diastolic dysfunction |
12 weeks |
0/35/9/0 (10/29/5/0) |
SF-36 PF: 569 ± 88 (79 ± 19) MLHFQ: 25 ± 20 (17 ± 17) |
545 ± 86 (569 ± 88) |
16.1 ± 4.9 (18.7 ± 5.4) |
|
Smart145, 2012 |
Open-label, randomized, controlled |
30 (16) |
3x/week, 30 min supervised, outpatient, cycle ergometer training with an intensity of 60% to 70% peak VO2 | HFpEF LVEF >45% delayed relaxation or pseudonormal filling pattern | 16 weeks |
No data |
No change in MLHFQ |
No data |
12.2 ± 3.6 (15 ± 4.9) |
| Kitzman146, 2013 |
Single-blind randomized, attention-controlled |
63 (32) |
Aerobic training programme consisting of walking on a track and lower extremity and arm cycling 3 times weekly (70% intensity) with additionally warm up and cool down for 60 min |
HFpEF LVEF ≥50% |
16 weeks | No data |
SF-36 PF: 48 ± 18 (63 ± 20) MLHFQ: 36 ± 19 (26 ± 19) |
447 ± 107 (486 ± 89) | 14.2 ± 2.8 (15.8 ± 3.3) |
|
Kitzman147, 2016 |
Randomized, attention-controlled, 2x2 factorial trial (exercise/control; diet/control), assessor blinded |
100 (51) |
Progressive aerobic walking programme for 3x/week (60 min). Out of 51 patients in the exercise group, 25 were also allocated to diet |
Age ≥ 60 years BMI ≥30 kg/m2 Stable HF LVEF ≥50% |
20 weeks | Significant improvement |
KCCQ: 62 ± 16 (75; CI 71–79) SF-36 PF: 37 ± 9 (42; CI 40–44) |
Feet: 1337 ± 270 (1503; CI 1470–1536) |
14.5 ± 2.9 (16; CI 15.6–16.4) |
- 6MWT, 6-min walk test; AIT, aerobic interval training; BMI, body mass index; CABG, coronary artery bypass graft; CHF, chronic heart failure; CHFQ, Chronic Heart Failure Questionnaire; CI, confidence interval; ET, exercise threshold; HADS, Hospital Anxiety and Depression Scale; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HIIT, high-intensity interval training; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MCT, moderate continuous training; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; PF, Physical Function subscale; QoL, quality of life; RRE, recommendation of regular exercise; SF-36, Short Form 36; VO2, oxygen consumption.
Exercise training has also been proposed for patients with an implanted left ventricular assist device (LVAD) to further stimulate the recovery of exercise capacity and QoL.153 However, the existing evidence in support of regular exercise training in LVAD patients remains very limited. Table 7 and Figure 4 are summarizing the current evidence of randomized trials in this field. Of particular interest, no trial was able to demonstrate a significant difference between the intervention and the control group with regard to peak VO2, distance covered during the 6MWT, or QoL, and only within-group effects were detected. The first multicentre trial in this field (Ex-VAD) tries to answer the question whether exercise training will be superior in comparison to usual care in improving exercise capacity and QoL in patients with LVAD.154
The function of the peripheral muscles and the endothelium seem to be very important determinators of exercise tolerance in HFpEF and are sensitive to exercise training.155 In current guidelines, aerobic exercise training is recommended for patients with HFpEF.2 The evidence is derived from a few randomized controlled trials (only one multicentre trial) with an intermediate follow-up of 12 to 20 weeks. These trials demonstrated an increase in peak VO2 of 10–20% after exercise training (Table 7). Furthermore, the distance covered during the 6MWT (24 m) and physical dimensions of QoL (SF-36: +14 points, MLHFQ: −5 points) also improved by aerobic exercise training. However, the long-term effects of exercise training on exercise capacity and QoL as well as the most effective type of exercise intervention are still unknown.156, 157
In summary, exercise training is recommended for patients with HF by all major guidelines. Besides its positive effect on physical QoL and maximal and submaximal exercise capacity, exercise training is safe and effective in patients with HFrEF, HFpEF, and even in those with implanted LVAD. Still, more studies and particularly those with longer follow-up duration are required.158
Conclusions
Previous large-scale clinical studies in HF have focused primarily on improvements in morbidity and mortality. Morbidity in this context was mostly defined as less hospital admissions for cardiovascular reasons or for HF. Since HF tends to worsen over time and no definitive cure is available, maintaining QoL and exercise capacity are primary treatment goals. State-of-the-art HF treatments like ACEi, ARBs, beta-blockers, and MRAs have been disappointing in this regard. The available results with regard to exercise capacity and QoL are mixed at best. The data on the use of spironolactone in HFpEF are somewhat more promising, but definitive answers are still missing. Ivabradine was associated with improvements in exercise capacity and QoL; however, their quantitative clinical relevance is uncertain. Sacubitril/valsartan suggests beneficial effects on improving exercise capacity; however, the evidence base for this class of drugs remains limited. Likewise, the recently published data of the DAPA-HF trial suggest beneficial effects for dapagliflozin on QoL both in patients with HF with or without diabetes, but the evidence remains limited, and it is not clear if this effect is a class effect or restricted to dapagliflozin. Physical exercise training has been shown to be safe and effective at improving exercise capacity and QoL. This seems to be true for HFrEF and for HFpEF. The general recommendation is aerobic exercise training; however, its long-term effectiveness and the most effective type of intervention remain to be determined. Other interventions with a convincing evidence base with regard to improvements in exercise capacity include intravenous iron supplementation in patients with HF and iron deficiency and potentially anabolics like testosterone in highly selected patients. Sinus rhythm may have some advantage when compared with atrial fibrillation, but a definitive evidence base is missing here as well. Treating the co-morbidities SDB, diabetes mellitus, depression, or CKD does not have a strong evidence base with regard to improving exercise capacity in patients with HF.
The main conclusion from this review of the evidence is the great unmet need in clinical cardiology for effectively powered trials of adequate duration to study exercise capacity and QoL changes. In addition, the review shows that addressing the co-morbidities of HF may be key to develop effective strategies to improve exercise capacity, symptom burden, and QoL. Targeting improvement in functional capacity needs to become a major target in clinical trials in HF in order to improve patients' QoL and global disease burden.
Acknowledgements
The present manuscript is the result of a cooperation under the umbrella of the Study Group 10 (heart failure) of the German Cardiac Society. The authors are members of the German Cardiac Society and assembled twice at the University of Göttingen in Winter 2017 and Spring 2018 to critically discuss the current literature on measures to improve exercise capacity and QoL in patients with HF to address a current gap in evidence. The focus was primarily on standard, i.e. guideline-recommended HF treatments as well as on the treatment of major co-morbidities. These meetings and thus the preparation of this manuscript were kindly supported by unrestricted grants from Boehringer Ingelheim, Novartis, Servier, and Vifor. Expert secretarial assistance was provided by Mrs. Anja Janssen.
Conflict of interest: S.v.H. has been a paid consultant for and/or received honoraria payments from Bayer, Boehringer Ingelheim, BRAHMS, Chugai, Grünenthal, Helsinn, Hexal, Novartis, Respicardia, Roche, Sorin, and Vifor; owns shares in Actimed. SvH; and reports research support from IMI and the German Center for Cardiovascular Research (DZHK). M.A. received research support from ResMed, ResMed Foundation and Philips Respironics as well as lecture fees from ResMed and Philips Respironics. W.D. reports speaker fees and advisory honoraria from Aimediq, Bayer, Boehringer Ingelheim, Medtronic, Pfizer, Sanofi-Aventis, Sphingotec, and Vifor Pharma; also reports research support from EU (Horizon2020), the German Ministry of Education and Research, German Center for Cardiovascular Research, Vifor Pharma, and ZS Pharma. F.E. has received advisory board and lecture honoraria from Boehringer Ingelheim, Actelion, Amgen, Bayer Healthcare, Abbott, Novartis, Merck Sharp and Dohme, Vifor Pharma, Berlin Chemie and Servier; received grants from the German Research Foundation (DFG), German Ministry of Education and Research (BMBF), German Centre for Cardiovascular Research (DZHK), Thermo Fisher, and Servier. During the last 2 years C.H.L. has received lecture honoraria from Novartis and royalties from Hogrefe Huber publishers. M.K. has been a paid consultant for and/or received honoraria payments from CVRx, Novartis, Pfizer and Servier. R.W. received advisory board and/or lecture honoraria from Bayer, Berlin Chemie, BMS, Boehringer Ingelheim, CVRx, Daiichi-Sankyo, Medeeonic, Novartis, Sanofi, Servier; received research support from Bundesministerium Bildung und Forschung, Boehringer Ingelheim and European Union. M.N. has received honoraria payments from Abbott, Abiomed, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Novartis, Pfizer and Roche. G.H. has received honoraria for lectures and/or consulting from AstraZeneca, Corvia, Impulse Dynamics, Novartis, Servier, Springer and Vifor. U.L. or his institution have received fees for lectures from Amgen, AstraZeneca, Bayer, Berlin-Chemie, Boehringer, Daiichi-Sankyo, Medtronik, Novartis, Sanofi, Servier. The other authors have nothing to disclose.




