Global myocardial work index predicts response to biventricular pacing in patients with non-left bundle branch block
Abstract
Aims
Cardiac resynchronization therapy (CRT) improves the prognosis of patients with heart failure (HF) and wide QRS complex. However, patients with non-left bundle branch block (LBBB) show a poor response to CRT. This study evaluated myocardial work estimated by pressure–strain loops on echocardiography for predicting response to CRT in patients with non-LBBB.
Methods and results
Of 267 patients who underwent CRT implantation, 54 patients with non-LBBB (mean age, 62 ± 12 years, 72% males, and 24% with ischemic cardiomyopathy) were retrospectively included. Two-dimensional speckle-tracking echocardiography was performed before and at 6-month follow-up in all patients. Myocardial work was estimated by pressure–strain loop analysis using speckle-tracking echocardiography and non-invasive blood pressure measurement. CRT response was defined as a ≥15% decrease in left ventricular end-systolic volume on echocardiography at the 6-month follow-up. The mean left ventricular ejection fraction (LVEF) before implantation was 27% ± 8% in total. Six months after implantation, 18 patients (33%) responded to CRT. The absolute LVEF improvement for responders and non-responders were 5.5% ± 6.9% and 1.3% ± 7.5%, respectively (P = 0.021). Baseline global work index (GWI), which is the average myocardial work based on the pressure–strain loop, was significantly higher in the responder group than in the non-responder group (590 ± 271 vs. 409 ± 216 mmHg%; P = 0.010). Multivariable analysis showed GWI to be an independent predictor of CRT response (odds ratio, 1.109; 95% confidence interval [CI], 1.013–1.213; P = 0.024). Receiver operating characteristic curve analysis determined the cut-off value of GWI for response as 456 mmHg% (AUC 0.700, 95% CI 0.553–0.840; P = 0.019). During the median 37-month follow-up, all-cause death occurred in 21 patients (39%). On multivariable analysis, GWI ≤ 456 mmHg% was independently associated with an increased risk of all-cause mortality (hazard ratio, 2.882; 95% CI, 1.157–7.176; P = 0.023).
Conclusions
High GWI assessed by speckle-tracking echocardiography and a non-invasively estimated LV pressure curve was independently associated with a favourable response to CRT and improved outcomes in patients with non-LBBB. The use of this non-invasive approach for quantifying myocardial variability and residual contractility can be beneficial for assessing CRT candidates and allow for more accurate patient stratification. Further, large multicentre studies are required to validate these findings.
1 Introduction
Cardiac resynchronization therapy (CRT) is an essential treatment for patients with severe left ventricular (LV) dysfunction and wide QRS complex.1 CRT improves cardiac function and quality of life and reduces mortality and hospitalization due to heart failure (HF). However, approximately one-third of patients do not properly respond to CRT, and some patients experience worsening HF after therapy.2 Patients with HF complicated with left bundle branch block (LBBB) can typically benefit from CRT. In contrast, patients with non-LBBB are less benefitted from CRT than patients with LBBB.3, 4 Current guidelines recommend CRT indications from a careful standpoint in patients with non-LBBB unless the QRS duration is adequately wide.1 More refined and selective criteria are needed to accurately predict the response to CRT in patients with non-LBBB.
Myocardial work is a recently developed non-invasive approach that incorporates LV afterload into global longitudinal strain (GLS) analysis on echocardiography. Myocardial work reflects the contractility of the heart, stroke work, residual myocardial contractility, and myocardial oxygen consumption, as represented by pressure–volume loop analysis. Previous reports demonstrated that baseline myocardial work was associated with the extent of LV reverse remodelling and its outcome in CRT recipients.5-8 However, most studies predominantly included patients with LBBB, and only a few reports specifically focused on patients with non-LBBB.9 We hypothesized that baseline myocardial work may be linked to response following CRT and might be a strong predictor of outcomes in patients with LV dysfunction and non-LBBB.
The present study aimed to investigate the usefulness of a non-invasive method for estimating myocardial work on echocardiography for the response to CRT in patients with non-LBBB and to evaluate the predictive value of outcomes and prognosis independently among the existing parameters in those populations.
2 Methods
2.1 Study population
Consecutive HF patients with a decreased LV ejection fraction (LVEF) (≤35%) and wide QRS complex (≥120 ms) who underwent CRT device implantation at Nagoya University Hospital from February 2007 to March 2022 were initially assessed retrospectively. Patients with LBBB, those who were upgraded from right ventricular pacing, and those with advanced atrioventricular block who required continuous pacing were excluded. Patients with recent (<3 months) acute coronary syndrome, atrial fibrillation with rapid ventricular response, and poor echocardiography quality at the time of evaluation and those who were lost to follow-up <6 months after implantation were also excluded. The remaining patients with non-LBBB were included in the analysis. The indications for CRT implantation were in accordance with the most recent guidelines available at the time of device implantation.10-12 LBBB was in accordance with Strauss criteria.13 The non-LBBB involved right bundle branch block (RBBB)14 and non-specific intraventricular conduction delay (NICD). NICD cohort composed of patients whose electrocardiogram showed QRS ≥ 120 ms and who did not meet the above criteria for LBBB or RBBB. This study was approved by the local ethics committee and complied with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients before the procedure.
2.2 Echocardiography assessment
All patients underwent standard transthoracic two-dimensional echocardiography at baseline and 6 months after implantation using the Vivid 7 E95 ultrasound imaging system (GE Healthcare). Standard M-mode, greyscale two-dimensional colour Doppler, and tissue Doppler images were obtained.15 The LVEF was assessed using the modified Simpson method. Septal-posterior wall motion delay (SPWMD),16 the standard deviation of time-to-peak systolic velocity for 12 LV segments (Ts-SD),17 and the absolute difference in time between the septal and lateral walls on tissue Doppler imaging (Ts sep-late) were calculated before and after CRT.18 Septal flash and apical rocking were evaluated in the short-axis or long-axis parasternal views and apical four-chamber views, respectively.19, 20
For speckle-tracking echocardiography analysis, standard two-dimensional greyscale images were acquired in the apical chamber and LV short-axis views at the papillary muscle level. The recordings were analysed using the software for speckle-tracking strain echocardiography (EchoPAC v203; GE Healthcare). Automatic tracking of the endocardial cavity was carefully checked, and the region of interest was manually adjusted to ensure optimal tracking and cover the entire thickness of the LV myocardium. Radial strain (RS) analysis was applied to the LV short-axis images at the papillary muscle level. Time–RS curves were obtained from six segments (septal, anteroseptal, anterior, posterior, lateral, and inferior) of the LV short-axis plane. Peak RS and time from the QRS onset to the peak RS for the LV segments were obtained, and the absolute difference time between the septal and posterior segments (RS sep-post) was calculated.21 Standard deviation of time-to-peak systolic RS of six LV segments (RS-SD), mean RS of six LV segments (RS mean), and the i-index, which is the product of RS-SD and RS mean, were also calculated.22 The LV GLS was automatically calculated from the strains in the three apical views (long-axis, two-chamber, and four-chamber).23 All echocardiography examinations were performed by experienced echocardiographers who were completely blinded to outcomes in this study.
2.3 Calculations of myocardial work
Myocardial work was calculated using EchoPAC v203 (GE Healthcare) from the speckle-tracking echocardiographic strain data and non-invasive blood pressure values. The opening and closing times of the aortic and mitral valves were determined in the parasternal long-axis view. Non-invasive blood pressure values were obtained using a brachial artery sphygmomanometer at the time of transthoracic echocardiography in the examination room, and non-invasive systolic blood pressure was assumed to be the LV peak pressure. The LV pressure–strain loop was then constructed from the LV longitudinal strain data of the entire cardiac cycle, mitral and aortic valve opening and closing timing, and non-invasive blood pressure values. Cardiac work was calculated automatically per myocardial segment by differentiating the strain values over time to yield the segmental shortening rate, which was then multiplied by the instantaneous LV pressure. The product, that is, instantaneous power, was subsequently integrated over time, providing values for LV segmental and total LV work as a time function.24 The myocardial work index was defined as work within the area of the LV pressure–strain loop calculated from mitral valve closure to mitral valve opening. Constructive work was defined as the cardiac work performed during the shortening of a myocardial segment in systole or during lengthening in isovolumic relaxation, whereas wasted work was defined as the work performed by a segment during lengthening in systole or during shortening in isovolumic relaxation. The myocardial, constructive, and wasted work were calculated for each LV segment. The mean myocardial, constructive, and wasted work were subsequently calculated at the level of the lateral and septal walls, which were further used to evaluate the global LV (global work index [GWI], global constructive work [GCW], and global wasted work [GWW], respectively) (Figure 1). The absolute differences of myocardial work, constructive work, and wasted work between the lateral and septal walls (late-sep work difference) were calculated.25 Myocardial work was evaluated by experienced investigators who were blinded to the volumetric measurements and outcomes.
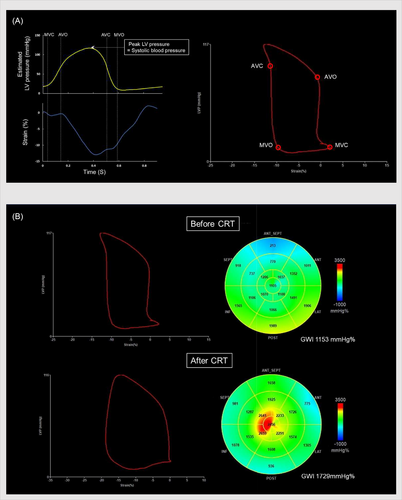
2.4 Cardiac resynchronization therapy implantation
A CRT device was implanted using the standard transvenous approach. The right ventricular and atrial leads were implanted in the low- and mid-septum and right atrial appendages, respectively. The non-apical site of the lateral or posterolateral vein of the coronary sinus was primarily selected for fixation of the LV lead. The final locations of the leads were evaluated with an adequate distance between the right ventricular and LV leads as the opposite site using fluoroscopic images. Device programming was performed at the discretion of the attending physicians and clinical engineers. After the procedure, device performance and lead parameters were assessed at the outpatient clinic of our institution at 1 week, 1 month, and 6 months after implantation and every 6 months thereafter.
2.5 Clinical outcomes
Response to CRT was defined as ≥15% decrease in LV end-systolic volume on echocardiography assessment at 6-month follow-up after the implantation from baseline.18 All-cause death, cardiac death, and composite events (death or HF hospitalization) were assessed in all patients after implantation. Baseline characteristics, examination results, and clinical events during the follow-up period were assessed using patient medical records from the hospital or by interviews with the patients' primary care physicians or relatives.
2.6 Statistical analyses
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as numbers (percentages). Comparison of the parameters between the two groups was performed using Student's t-tests for continuous values and χ2 tests or Fisher's exact test for categorical values. Univariable logistic regression analysis was performed to assess the predictive value of response to CRT. Variables with a P value <0.1 in univariable analysis were entered in the multivariable analysis (forward stepwise method) to determine an independent predictor for outcomes. Receiver operating characteristic (ROC) curve analysis was performed, and the area under the curve (AUC) was calculated. The optimal cut-off value for each parameter was determined from the ROC curve. Event-free survival rates were calculated using Kaplan–Meier survival curve analysis, and differences between the curves were compared using the log-rank test. Univariable and multivariable analyses of prognostic outcomes were performed to assess the predictive value of time-dependent factors using Cox proportional hazard models. Intra-observer and inter-observer variability was evaluated using interclass correlation with 95% confidence interval (CI). To test the universal ability and performance of parameters for predicting outcomes in this limited dataset, the K-fold cross-validation method was used to divide the data into two subsets, namely, training data for learning and data testing for validation or evaluation in ‘K’ subsets with the same amount of data. The K-value was set to five-fold in this limited sample, along with the balance between the verification of precision and improvement of the validation model. Performance evaluation was analysed with the confusion matrix which consisted of accuracy, precision, recall, and F-score. The results of this analysis were compared with those of the original analysis. SPSS version 25.0 (IBM Corp.) and R (version 4.3.3) were used for the statistical analysis. Statistical significance was set at P < 0.05.
3 Results
Among the 267 patients who underwent CRT device implantation during the study period, 203 patients with LBBB, upgrading from right ventricular pacing, and advanced atrioventricular block were excluded; seven and three patients were further excluded because of incomplete echocardiography and insufficient follow-up data, respectively. The final study cohort consisted of 54 patients with non-LBBB (16 patients with RBBB and 38 patients with NICD) (Figure 2). The clinical characteristics, echocardiographic results, and myocardial work data of the overall population are shown in Table 1. The mean patient age was 62 ± 12 years, and 39 patients (72%) were men. Thirteen patients (24%) had ischemic cardiomyopathy. The mean LVEF before implantation was 27% ± 8%. Fifty-one patients (94%) were implanted with CRT-defibrillator and 3 (6%) with CRT-pacemaker. The guidelines used as reference for patients and the recommended classes across the study period are shown in Table S1.
| Entire population (n = 54) | Non-responder (n = 36; 67%) | Responder (n = 18; 33%) | P value | |
|---|---|---|---|---|
| Clinical data | ||||
| Age (years) | 62 ± 12 | 61 ± 11 | 64 ± 13 | 0.433 |
| Male sex | 39 (72) | 29 (81) | 10 (56) | 0.053 |
| Body weight (kg) | 58 ± 14 | 59 ± 16 | 55 ± 10 | 0.309 |
| Ischaemic cardiomyopathy | 13 (24) | 11 (31) | 2 (11) | 0.115 |
| Systolic blood pressure (mmHg) | 101 ± 13 | 100 ± 14 | 102 ± 11 | 0.534 |
| Diastolic blood pressure (mmHg) | 60 ± 10 | 61 ± 11 | 60 ± 7 | 0.766 |
| NYHA functional class | ||||
| II | 14 (26) | 9 (25) | 5 (28) | 1.000 |
| III | 21 (39) | 16 (44) | 5 (28) | 0.236 |
| IV | 19 (35) | 11 (31) | 8 (44) | 0.314 |
| CRT-defibrillator | 51 (94) | 35 (97) | 16 (89) | 0.255 |
| Medications | ||||
| Beta-blockers | 44 (81) | 29 (81) | 15 (83) | 1.000 |
| ACE-I/ARB | 35 (65) | 22 (61) | 13 (72) | 0.495 |
| ARNI | 1 (2) | 1 (3) | 0 (0) | 1.000 |
| MRA | 38 (70) | 25 (69) | 13 (72) | 0.833 |
| SGLT2 inhibitors | 7 (13) | 5 (14) | 2 (11) | 1.000 |
| Laboratory data | ||||
| BNP levels (pg/mL) | 708 ± 997 | 651 ± 983 | 813 ± 1,043 | 0.569 |
| Creatinine levels (μmol/L) | 1.3 ± 0.9 | 1.4 ± 0.9 | 1.2 ± 0.7 | 0.418 |
| eGFR (mL/min/1.73 m2) | 53 ± 22 | 52 ± 23 | 54 ± 21 | 0.840 |
| Electrocardiographic data | ||||
| QRS duration (ms) | 146 ± 21 | 144 ± 23 | 149 ± 23 | 0.472 |
| RBBB | 16 (30) | 8 (22) | 8 (50) | 0.092 |
| NICD | 38 (70) | 28 (78) | 8 (50) | 0.092 |
| Atrial fibrillation | 12 (22) | 6 (17) | 6 (33) | 0.184 |
| LV lead position | ||||
| Short axis | ||||
| Anterior | 2 (4) | 2 (6) | 0 (0) | 0.308 |
| Anterior-lateral | 16 (30) | 9 (25) | 7 (39) | 0.292 |
| Lateral | 27 (50) | 17 (47) | 10 (56) | 0.564 |
| Posterior-lateral | 9 (17) | 8 (22) | 1 (6) | 0.121 |
| Posterior | 0 (0) | 0 (0) | 0 (0) | — |
| Long axis | ||||
| Basal | 21 (39) | 16 (44) | 5 (28) | 0.236 |
| Mid | 31 (57) | 18 (50) | 13 (72) | 0.120 |
| Apex | 2 (4) | 2 (6) | 0 (0) | 0.308 |
| Echocardiographic data | ||||
| LVEDV (mL) | 223 ± 132 | 234 ± 155 | 201 ± 57 | 0.391 |
| LVESV (mL) | 161 ± 94 | 172 ± 109 | 141 ± 52 | 0.270 |
| LVEF (%) | 27 ± 8 | 27 ± 9 | 28 ± 5 | 0.331 |
| GLS (%) | −6.4 ± 2.8 | −5.9 ± 2.7 | −7.3 ± 2.8 | 0.067 |
| LV dyssynchrony | ||||
| SPWMD (ms) | 122 ± 64 | 125 ± 59 | 116 ± 74 | 0.647 |
| Ts-SD (ms) | 49 ± 27 | 49 ± 27 | 49 ± 28 | 0.922 |
| Ts sep-late (ms) | 61 ± 56 | 46 ± 31 | 68 ± 65 | 0.202 |
| RS sep-post (ms) | 164 ± 140 | 150 ± 118 | 195 ± 176 | 0.270 |
| RS-SD (ms) | 95 ± 69 | 89 ± 48 | 107 ± 88 | 0.392 |
| RSmean (%) | 15 ± 10 | 15 ± 10 | 15 ± 8 | 0.997 |
| i-Index (ms%) | 1254 ± 1152 | 1177 ± 958 | 1409 ± 1487 | 0.490 |
| Septal flash | 21 (39) | 12 (33) | 9 (50) | 0.236 |
| Apical rocking | 24 (44) | 13 (36) | 11 (61) | 0.081 |
| Cardiac work | ||||
| GWI (mmHg%) | 469 ± 248 | 409 ± 216 | 590 ± 271 | 0.010 |
| GCW (mmHg%) | 658 ± 316 | 592 ± 272 | 790 ± 362 | 0.028 |
| GWW (mmHg%) | 207 ± 97 | 208 ± 89 | 204 ± 114 | 0.896 |
| Septal MW (mmHg%) | 355 ± 277 | 308 ± 284 | 450 ± 244 | 0.077 |
| Lateral MW (mmHg%) | 570 ± 405 | 502 ± 371 | 704 ± 446 | 0.085 |
| Late-sep MW difference (mmHg%) | 341 ± 366 | 347 ± 395 | 329 ± 310 | 0.867 |
| Septal CW (mmHg%) | 515 ± 275 | 451 ± 261 | 641 ± 263 | 0.015 |
| Lateral CW (mmHg%) | 734 ± 439 | 656 ± 892 | 892 ± 545 | 0.061 |
| Late-sep CW difference (mmHg%) | 341 ± 295 | 344 ± 280 | 335 ± 332 | 0.910 |
| Septal WW (mmHg%) | 225 ± 162 | 219 ± 140 | 236 ± 202 | 0.711 |
| Lateral WW (mmHg%) | 167 ± 148 | 166 ± 163 | 170 ± 118 | 0.927 |
| Late-sep WW difference (mmHg%) | 150 ± 154 | 151 ± 167 | 149 ± 127 | 0.965 |
- Values are expressed as numbers (percentages) or mean ± standard deviation.
- ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BNP, brain natriuretic peptide; CW, constructive work; eGFR, estimated glomerular filtration rate; GCW, global constructive work; GLS, global longitudinal strain; GWI, global work index; GWW, global wasted work; i-Index, product of RS-SD and RSmean; late-sep work difference, the absolute difference of the work between the lateral and septal walls; LV, left ventricle; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MW, myocardial work index; MRA, mineralocorticoid receptor antagonist; NICD, nonspecific intraventricular conduction delay; RBBB, right bundle branch block; RS sep-post, absolute difference time between the septal and posterior segments at radial strain; RSmean, mean radial strain of six LV segments; RS-SD, standard deviation of the time-to-peak systolic radial strain of six LV segments; SGLT2, sodium-glucose cotransporter 2; SPWMD, septal-posterior wall-motion delay; Ts sep-late, absolute difference time between the septal and lateral wall at tissue Doppler imaging; Ts-SD, standard deviation of time-to-peak systolic velocity for 12 left ventricular segments at tissue Doppler imaging; WW, wasted work.
3.1 Response to cardiac resynchronization therapy
Six months after implantation, 18 patients (33%) responded to CRT. The absolute LVEF improvement for responders and non-responders were 5.5% ± 6.9% and 1.3% ± 7.5%, respectively (P = 0.021). Compared with the responders, the non-responders had a higher prevalence of male sex (10 [56%] vs. 29 [81%]; P = 0.053). There was no significant difference in baseline QRS duration or LV lead position between the two groups. Interestingly, no significant differences were observed in the echocardiographic parameters of LV dyssynchrony (e.g., SPWMD, Ts-SD, TS sep-late) between the two groups (Table 1). The responder group demonstrated greater GLS than the non-responder group, but the difference was not statistically significant (−7.3% ± 2.8% vs. −5.9% ± 2.7%; P = 0.067).
As for the myocardial work evaluation, GWI (590 ± 271 vs. 409 ± 216 mmHg%; P = 0.010) and GCW (790 ± 362 vs. 592 ± 272 mmHg%; P = 0.028) were significantly higher in the response group than in the non-response group (Table 1). In the detailed LV region analyses, the values of myocardial and constructive work in the septal region were lower than that in the lateral region in the responder and non-responder groups (Table 1 and Figure S1). The septal constructive work was significantly higher in the responder group than in the non-responder group (641 ± 263 vs. 451 ± 261 mmHg%; P = 0.015). The interclass correlation of the intra-observer and inter-observer variability for assessing GWI were 0.932 (95% CI, 0.767–0.983) and 0.933 (95% CI, 0.765–0.983), respectively.
3.2 Predictors for response to cardiac resynchronization therapy
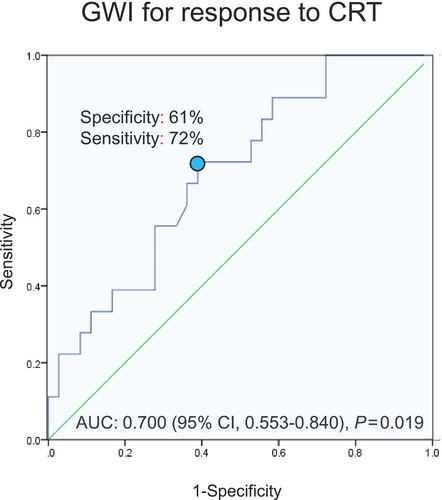
Univariable logistic regression analysis demonstrated that GWI and GCW were significantly associated with CRT response. In the subsequent multivariable analysis, only GWI was independently associated with response to CRT (odds ratio [OR], 1.109; 95% CI, 1.013–1.213; P = 0.024) (Table 2). AUC of GWI for responders was 0.700 (95% CI, 0.553–0.840; P = 0.019) based on ROC curve analysis (Figure 3). The cut-off value of the GWI was determined to be 456 mmHg% for responders, with a specificity of 61% and a sensitivity of 72%.
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.021 (0.970–1.074) | 0.426 | ||
| Male sex | 0.302 (0.087–1.046) | 0.059a | ||
| Ischaemic cardiomyopathy | 0.284 (0.056–1.453) | 0.131 | ||
| NYHA functional class | 1.204 (0.577–2.511) | 0.621 | ||
| Systolic blood pressure (mmHg) | 1.015 (0.970–1.061) | 0.527 | ||
| BNP levels (pg/dL) | 1.000 (1.000–1.001) | 0.565 | ||
| QRS width (ms) | 1.012 (0.986–1.040) | 0.362 | ||
| RBBB | 2.800 (0.829–9.458) | 0.097 | ||
| LVEF (%) | 1.040 (0.962–1.123) | 0.326 | ||
| SPWMD (ms) | 0.998 (0.989–1.007) | 0.641 | ||
| i-Index | 1.000 (1.000–1.001) | 0.486 | ||
| Apical rocking | 2.780 (0.866–8.926) | 0.086a | ||
| GLS (%) | 0.822 (0.662–1.019) | 0.073a | 1.936 (0.895–4.185) | 0.093 |
| GWI (per 10 mmHg%) | 1.032 (1.005–1.059) | 0.018a | 1.109 (1.013–1.213) | 0.024 |
| GCW (per 10 mmHg%) | 1.021 (1.001–1.042) | 0.040a | ||
| GWW (per 10 mmHg%) | 0.996 (0.938–1.057) | 0.893 | ||
| Enrolment period (years) | 1.017 (0.899–1.151) | 0.788 | ||
- The other abbreviations are listed in Table 1.
- CI, confidence interval; OR, odds ratio.
- a Parameters were included in the multivariate analysis using the forward stepwise method. Enrolment period was calculated as the time lapsed from the start of the enrolment period (February 1, 2007) to patient enrolment date.
When the population was limited to 19 patients who had advanced left ventricular remodelling (LVEF < 30%) and impaired renal function (estimated glomerular filtration rate < 60 mL/min/1.73 m2), the GWI value remained significantly associated with CRT response (OR, 1.075; 95% CI, 1.013–1.141; P = 0.016).
3.3 Comparison of characteristics and outcomes between patients with GWI > 456 mmHg% and those with GWI ≤ 456 mmHg%
The population was divided into patients with GWI > 456 mmHg% and those with GWI ≤ 456 mmHg% (27 patients in each group); then, the baseline characteristics and clinical outcomes were compared between the groups (Table 3). Patients with GWI ≤ 456 mmHg% had significantly lower systolic blood pressure, LVEF, and GLS and larger LV volume than those with GWI > 456 mmHg%. Both GWI and GCW, in addition to GWI, were significantly lower in patients with GWI ≤ 456 mmHg%. Septal and lateral myocardial work and constructive work were also lower in patients with GWI ≤ 456 mmHg%. At 6 months, the response rates for CRT were 48% and 19% in patients with GWI > 456 and GWI ≤ 456 mmHg%, respectively, accompanied with a significant difference in brain natriuretic peptide levels at 6 months (287 ± 278 vs. 726 ± 698 pg/mL; P = 0.004).
| GWI > 456 mmHg% (n = 27; 50%) | GWI ≤ 456 mmHg% (n = 27; 50%) | P value | |
|---|---|---|---|
| Clinical data | |||
| Age (years) | 63 ± 12 | 61 ± 12 | 0.592 |
| Male sex | 18 (67) | 21 (78) | 0.544 |
| Body weight (kg) | 58 ± 10 | 58 ± 18 | 0.981 |
| Ischemic cardiomyopathy | 5 (19) | 8 (30) | 0.526 |
| Systolic blood pressure (mmHg) | 108 ± 10 | 93 ± 11 | <0.001 |
| Diastolic blood pressure (mmHg) | 61 ± 9 | 59 ± 11 | 0.228 |
| NYHA functional class | |||
| II | 10 (37) | 4 (15) | 0.062 |
| III | 8 (30) | 13 (48) | 0.163 |
| IV | 9 (33) | 10 (37) | 0.776 |
| Medications | |||
| Beta-blockers | 22 (81) | 22 (81) | 1.000 |
| ACE-I/ARB | 20 (74) | 15 (63) | 0.208 |
| ARNI | 0 (0) | 1 (4) | 1.000 |
| MRA | 20 (74) | 18 (67) | 0.551 |
| SGLT2 inhibitors | 1 (4) | 6 (22) | 0.100 |
| Laboratory data | |||
| BNP levels (pg/mL) | 487 ± 871 | 938 ± 1,082 | 0.100 |
| Creatinine levels (μmol/L) | 1.2 ± 0.7 | 1.5 ± 1.0 | 0.172 |
| eGFR (mL/min/1.73 m2) | 57 ± 24 | 49 ± 20 | 0.178 |
| Electrocardiographic data | |||
| QRS duration (ms) | 144 ± 22 | 144 ± 26 | 0.959 |
| RBBB | 10 (37) | 6 (22) | 0.372 |
| NICD | 17 (63) | 21 (78) | 0.372 |
| Atrial fibrillation | 6 (22) | 6 (22) | 1.000 |
| LV lead position | |||
| Short-axis | |||
| Anterior | 1 (4) | 1 (4) | - |
| Anterior-lateral | 8 (30) | 8 (30) | - |
| Lateral | 13 (48) | 14 (52) | 1.000 |
| Posterior-lateral | 5 (19) | 4 (15) | 1.000 |
| Posterior | 0 (0) | 0 (0) | - |
| Long-axis | |||
| Basal | 9 (33) | 12 (44) | 0.577 |
| Mid | 17 (63) | 14 (52) | 0.583 |
| Apex | 1 (4) | 1 (4) | - |
| Echocardiographic data | |||
| LVEDV (mL) | 181 ± 50 | 265 ± 171 | 0.018 |
| LVESV (mL) | 126 ± 39 | 197 ± 118 | 0.005 |
| LVEF (%) | 31 ± 6 | 23 ± 7 | <0.001 |
| GLS (%) | −8.5 ± 2.1 | −4.3 ± 1.7 | <0.001 |
| LV dyssynchrony | |||
| SPWMD (ms) | 122 ± 76 | 122 ± 50 | 0.998 |
| Ts-SD (ms) | 49 ± 29 | 49 ± 26 | 0.963 |
| Ts sep-late (ms) | 52 ± 26 | 70 ± 77 | 0.280 |
| RS sep-post (ms) | 161 ± 132 | 169 ± 150 | 0.826 |
| RS-SD (ms) | 89 ± 69 | 101 ± 71 | 0.524 |
| RSmean (%) | 18 ± 9 | 13 ± 10 | 0.047 |
| i-Index (ms%) | 1,452 ± 1,350 | 1,056 ± 895 | 0.210 |
| Septal flash | 12 (44) | 9 (33) | 0.402 |
| Apical rocking | 12 (44) | 12 (44) | 1.000 |
| Cardiac work | |||
| GWI (mmHg%) | 663 ± 190 | 276 ± 107 | <0.001 |
| GCW (mmHg%) | 893 ± 267 | 422 ± 129 | <0.001 |
| GWW (mmHg%) | 235 ± 111 | 178 ± 72 | 0.030 |
| Septal MW (mmHg%) | 532 ± 235 | 179 ± 72 | <0.001 |
| Lateral MW (mmHg%) | 762 ± 464 | 377 ± 206 | <0.001 |
| Septal CW (mmHg%) | 682 ± 263 | 347 ± 163 | <0.001 |
| Lateral CW (mmHg%) | 990 ± 470 | 479 ± 189 | <0.001 |
| Septal WW (mmHg%) | 246 ± 195 | 203 ± 119 | 0.333 |
| Lateral WW (mmHg%) | 194 ± 198 | 140 ± 64 | 0.181 |
| Responder after 6 months | 13 (48) | 5 (19) | 0.04 |
| LVEF after 6 months (%) | 34 ± 8 | 25 ± 7 | <0.001 |
| BNP after 6 months (pg/mL) | 287 ± 278 | 726 ± 698 | 0.004 |
- Values are expressed as numbers (percentages) or mean ± standard deviation; the other abbreviations are listed in Table 1.
- HF, heart failure.
3.4 Changes in myocardial work after cardiac resynchronization therapy implantation
Myocardial work indices from baseline to 6 months after implantation, based on the CRT response, are presented in Figure S2. Although all myocardial work indices tended to improve after 6 months in both the responder and non-responder groups, significant improvements in GLS and GCW were predominantly observed in the responder group, whereas significant improvements in GWI and GWW were observed in the non-responders.
3.5 Follow-up outcomes
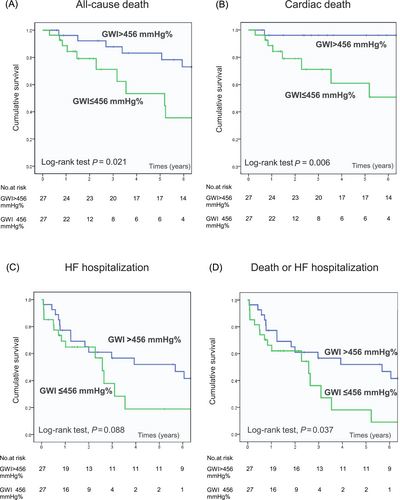
During the median follow-up period of 37 months, all-cause death, cardiac death, and HF hospitalization occurred in 21 (39%), 12 (22%), and 28 (52%) patients, respectively. Patients with GWI ≤ 456 mmHg% had a higher risk of all-cause death than those with GWI > 456 mmHg% (hazard ratio [HR], 2.721; 95% CI, 1.126–6.574; P = 0.026). In the multivariable Cox regression analysis including GWI ≤ 456 mmHg%, creatinine levels, and GLS, GWI ≤ 456 mmHg% were independently associated with increased risks of all-cause death (HR, 2.882; 95% CI, 1.157–7.176; P = 0.023) (Table 4). Kaplan–Meier survival curve analysis showed that patients with GWI ≤ 456 mmHg% had significantly poorer outcome of all-cause mortality compared with patients with GWI > 456 mmHg% (log-rank test, P = 0.021) (Figure 4). Additionally, GWI ≤ 456 mmHg% was associated with cardiac mortality (log-rank test, P = 0.006), HF hospitalization (log-rank test, P = 0.088), and composite events (death or HF hospitalization) (log-rank test, P = 0.037) (Figure 4).
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.032 (0.985–1.080) | 0.184 | ||
| Male sex | 1.631 (0.585–4.550) | 0.350 | ||
| Ischemic cardiomyopathy | 1.653 (0.629–4.342) | 0.308 | ||
| BNP (pg/mL) | 1.000 (1.000–1.000) | 0.588 | ||
| Creatinine (μmol/L) | 1.411 (0.940–2.118) | 0.096a | ||
| QRS width (ms) | 0.999 (0.980–1.019) | 0.940 | ||
| RBBB | 0.535 (0.195–1.469) | 0.225 | ||
| LVEF (%) | 0.961 (0.906–1.019) | 0.180 | ||
| GLS (%) | 1.212 (1.025–1.433) | 0.024a | ||
| GWI (per 10 mmHg%) | 0.978 (0.958–0.999) | 0.036 | ||
| GWI ≤ 456 mmHg% | 2.721 (1.126–6.574) | 0.026a | 2.882 (1.157–7.176) | 0.023 |
| Enrolment period (years) | 0.977 (0.865–1.149) | 0.968 | ||
- The other abbreviations are listed in Table 1.
- CI, confidence interval; HR, hazard ratio.
- a Variables included in the multivariate model using the forward stepwise method.
3.6 Predictive value of the myocardial work index in right bundle branch block and non-specific intraventricular conduction delay
Baseline characteristics of the two QRS morphology groups (NICD group, n = 38; RBBB group, n = 16) are presented in Table S2. Response rate was lower in the NICD group than in the RBBB group (21% vs. 50%). In the NICD group, responders showed significantly higher GWI (615 ± 313 vs. 386 ± 219 mmHg%; P = 0.016) and GCW (834 ± 443 vs. 566 ± 267 mmHg%; P = 0.027) than non-responders at baseline (Figure S3). In contrast, in patients with RBBB, the GWI (559 ± 225 vs. 489 ± 195 mmHg%; P = 0.519) and GCW (730 ± 240 vs. 680 ± 288 mmHg%; P = 0.711) were not significantly different between the responders and non-responders. Meanwhile, no significant differences were observed in GWW between responders and non-responders in both groups (NICD: 205 ± 86 vs. 204 ± 86 mmHg%; P = 0.952; RBBB: 206 ± 54 vs. 219 ± 102 mmHg%; P = 0.763). A detailed analysis of the myocardial index among the LV regions revealed that higher values of myocardial and constructive work were observed in the posterior and lateral regions in the RBBB and NICD groups, respectively (Figure S4). However, wasted work in the septal region was remarkable in the NCID group.
3.7 Validations of global work index for predicting response to cardiac resynchronization therapy in K-fold cross-validation method
The performance of the fivefold cross-validation model in this dataset for predicting response to CRT was an accuracy of 0.70, precision of 0.76, recall of 0.84, and F-score of 0.79, which were not remarkably different from the accuracy of 0.80, precision of 0.79, recall of 0.94, and F-score of 0.86 observed in the original model.
4 Discussion
The present study investigated the usefulness of a non-invasive method, speckle-tracking echocardiography, for estimating myocardial work in response to CRT in patients with non-LBBB. Baseline GWI was independently associated with response to CRT after 6 months in the multivariable analysis, and its predictive value was superior to the classical parameters of QRS duration and GLS. A low GWI value (≤456 mmHg) was independently associated with a modest increased risk of all-cause death and the composite endpoint. The predictive value of the myocardial work index for response to CRT was predominantly observed in the NICD group compared with the RBBB group.
4.1 Poor response rate of cardiac resynchronization therapy in heart failure with non-left bundle branch block
The response rate after CRT in patients with non-LBBB decreases in comparison with those with LBBB, and its response rates are reported to be 30–50%,26, 27 which is consistent with the results of our study showing that only 33% of patients became responders. A meta-analysis of five randomized controlled trials reported that CRT did not reduce the risk of death or HF hospitalization in patients with non-LBBB.28 Another study reported a harmful effect of CRT on non-LBBB patients, with an increased risk of all-cause death.29 Left bundle branch area pacing is a promising alternative to biventricular pacing for improving outcomes in HF patients with non-LBBB; however, its long-term benefits need to be investigated further.30 Thus, it is essential to explore the appropriate pathology and indication for CRT in patients with non-LBBB using a high predictive modality beforehand.
4.2 Myocardial work and cardiac resynchronization therapy in patients with non-left bundle branch block
Speckle-tracking echocardiography analysis of the pressure–strain loop curve enables the non-invasive estimation of myocardial work. The reliability of this method, in comparison to invasive myocardial work estimation, has been validated through experimental studies and mathematical models.5, 24 Regional disparities in myocardial work, as measured by pressure–strain loops, exhibit a strong correlation with the extent of myocardial glucose metabolism assessed by an imaging study of 18F-fluorodeoxyglucose positron emission tomography.5, 24 These results suggest that variations in myocardial work derived from pressure–strain loops at baseline represent cardiac residual metabolism and contractile reserve capacity. Consequently, differences in baseline myocardial function may elucidate the role of predicting the CRT response and long-term survival after CRT. Several reports have evaluated myocardial work using strain analysis on echocardiography in patients undergoing CRT and have demonstrated its efficacy and feasibility in stratifying the response to CRT and clinical outcomes. Galli et al. reported the usefulness of GCW > 1057 mmHg% in addition to septal flash at baseline for predicting a response to CRT.6 A similar study from the same laboratory team added the essential value of myocardial work on speckle-tracking echocardiography including GWW > 384 mmHg% and GCW > 1057 mmHg% in predicting CRT responders.8 Decreased GCW (≤888 mmHg%) at baseline was also reported to predict cardiac mortality after CRT independently.7 However, most studies involved patients with LBBB and non-LBBB together in the analyses. Currently, only one study evaluated myocardial work on the echocardiography focusing on patients with non-LBBB, and they reported that GWW ≥ 200 mmHg% and longitudinal strain septal contraction pattern were associated with CRT response in those patients.9 However, they assessed GWW only as a parameter for estimating myocardial work in the analysis. Our study is unique in that it evaluated myocardial work by including all possible parameters of GWI, GCW, and GWW in patients with non-LBBB and confirmed that GWI, a total myocardial work based on the pressure–strain loop, independently predicted the response and long-term survival after CRT. GWI is a balanced parameter of myocardial work consisting of both the effects of GCW and GWW and can represent the amount of residual contractility and myocardial energy loss during the cardiac cycle. Thus, this total index estimating eligible myocardial work for reverse remodelling may be better for accurately predicting a response after CRT. In our sub-study, GWI was also useful in predicting CRT response in patients with more advanced left ventricular contractility loss and renal dysfunction that were unlikely to have a residual contractile reserve, suggesting that even in patients with deteriorated clinical parameters, the underlying possibility of recovery of myocardial remodelling for CRT response might be stratified by the GWI value. However, in contrast to the previous study, the GWW was not significantly different between the responders and non-responders in our study. Although the exact reason for this is not specified, the small sample size with different characteristics in the study population (lower GWW [207 vs. 267 mmHg%] and shorter QRS duration [144 vs. 150 ms] in the total population of our study compared with those in the former study) might be responsible for this result, which requires further investigation in a large-scale study. Moreover, there was a significant reduction in the GWW in the non-responders, but this small absolute reduction and relatively low baseline value of the GWW might not have any positive impact on the prognosis.
4.3 Other traditional factors for cardiac resynchronization therapy response
No parameters of LV dyssynchrony, including QRS duration or echocardiographic parameters, were able to predict the CRT response or long-term survival after CRT in our study. In addition, difference in myocardial work between lateral and septal walls, which can help identify CRT responders in a population mostly consisting of LBBB patients, failed to predict outcomes.25 Previous studies failed to demonstrate a significant association between baseline QRS duration, CRT response, and long-term survival after CRT in non-LBBB patients.4, 31 In patients with NICD, electrical activation sequences of the human heart showed that QRS duration did not differ between patients with and without delayed LV lateral wall activation, which is a fundamental component of the electrical substrate for the response to CRT.32 As for RBBB patients, the earliest ventricular activation usually occurs in the LV myocardium, whereas electrical activation of the right ventricle occurs slowly,33 suggesting that a longer QRS duration does not completely correlate with LV lateral wall activation delay. Probably, the mean QRS duration at baseline in our cohort was not wide (144 ms); thus, estimating the response using QRS duration might have been limited in the present study. In such cases, strain-based speckle-tracking echocardiography may be helpful in identifying the underlying mechanical dyssynchrony behind the long QRS duration in the non-LBBB group, but previous studies did not prove an improvement in long-term outcome for non-LBBB patients,17, 34 which may alternatively underscore the importance of global myocardial performance beyond the assessment of LV dyssynchrony. In this regard, the evaluation of myocardial work has the merit of estimating residual myocardial contractility and variability in addition to mechanical dyssynchrony. Specifically, the myocardial work index was significantly associated with response in the NICD group but not in the RBBB group in our study. Although the number of patients in the RBBB group was relatively small, the relatively high myocardial work value in the lateral LV region where LV pacing is typically applied may represent a preserved residual myocardial variability in the NICD group associated with a better response to CRT in that population.35
However, the AUC of the GWI in the present study was not definitive, with a moderate predictive value for CRT response, requiring a combined assessment with the well-known parameters of LV dyssynchrony to increase the predictive response to CRT, as demonstrated in previous studies.6, 8, 9 The assessment of global myocardial viability might be helpful in selecting CRT candidates, and a combination with another potential parameter of electromechanical dyssynchrony or region of the myocardial scar is essential.36 This novel, non-invasive approach for estimating global myocardial work may be feasible in predicting outcomes and prognosis after CRT based on cardiac haemodynamics with a balance of residual contractility and wasted energy and may play an important role in stratifying the response to CRT in patients with non-LBBB.
4.4 Study limitations
This retrospective study was conducted at a single institution with the sample size. While the baseline parameters explored were comprehensive, the limited sample size raises the possibility of false positive due to multiple comparisons. The multivariable analyses may be limited due to the small sample size or the number of events, along with the common rule of thumb.37 Due to the limited sample size in this study, we were unable to establish a separate validation group. This limitation should be considered when interpreting the cut-off of GWI. Moreover, this study only had 16 patients with RBBB; thus, we could not validate the prognostic value of myocardial work according to the response in the subanalysis. The study period spanned over 15 years from the year 2007, and treatments for HF may have varied depending on the enrolment period. Specifically, sodium-glucose cotransporter 2 inhibitors and angiotensin receptor-neprilysin inhibitors were unavailable in the early years, although the enrolment study period was not significantly associated with CRT response or all-cause death, as shown in Tables 2 and 4. The present study included considerable patients with a QRS width of 120–149 ms, who were less likely to respond to CRT according to the recommendations of the guidelines.12 In addition, the indications for CRT were determined according to Japanese guidelines, which have been revised over the duration of the study period, as shown in Table S1. Specifically, in the recent revised guidelines, the cut-off line of QRS duration in class IIa recommendation has increased from 120–130 ms to 150 ms in non-LBBB.10-12 Moreover, specific patients with a QRS width of 120–129 ms who were not eligible for CRT implantation (according to the 2016 and 2021 ESC guidelines)1, 38 could have been included during the early phases of our study, and the EchoCRT trial could have significantly affected patient selection and outcomes before and after the study period.39 The calculation of myocardial work proceeded automatically after the acquisition of the strain values and systolic arterial pressure; however, the exact parameters should be obtained through the extraction of acceptable, high-quality images in all the assessed LV segments. One-time non-invasive measurement of blood pressure could have affected the estimation of the LV strain–pressure loop, and this non-invasive estimation of myocardial work might be less accurate than the invasive measurement, but this concern has been validated in previous studies.5, 24 As myocardial work was correlated with myocardial variability, we did not examine imaging modalities such as cardiac magnetic resonance imaging and nuclear modalities to assess the agreement of the myocardial scar in each corresponding region.
5 Conclusions
The GWI assessed by speckle-tracking echocardiography was useful in predicting a favourable response to CRT in patients with non-LBBB. Patients with GWI ≤ 456 mmHg% had a nearly threefold increased risk of all-cause death after the implantation. This non-invasive approach for quantifying myocardial variability and residual contractility has the potential for growth in assessing CRT candidates and more accurate stratification for patient selection, requiring a further large multicentre study.
Conflict of interest
Satoshi Yanagisawa is affiliated with a department-sponsored by Medtronic Japan. Shun Kondo, Yasuya Inden, Kiichi Miyamae, Hiroyuki Miyazawa, Takayuki Goto, Masaya Tachi, Tomoya Iwawaki, Ryota Yamauchi, Kei Hiramatsu, Masafumi Shimojo, Yukiomi Tsuji, and Toyoaki Murohara declare that they have no conflict of interest.
Funding
The authors did not receive support from any organization for the submitted work.




