Immune cell dynamics in heart failure: implicated mechanisms and therapeutic targets
Abstract
The relationship between heart failure (HF) and immune activation has garnered significant interest. Studies highlight the critical role of inflammation in HF, affecting cardiac structure and function. Despite promising anti-inflammatory therapies, clinical trials have faced challenges, indicating an incomplete understanding of immune mechanisms in HF. Immune cells, which are key cytokine sources, are pivotal in HF progression. In this review, the authors provide a comprehensive overview of the complex role of different types of immune cells and their cell subtypes in HF. In addition, the authors summarize the available targets and animal experimental evidence for targeting immune cells for the treatment of HF. Future research directions will focus on the roles of immune cells and their interrelationships at different stages of HF, aiming to develop more targeted therapeutic strategies that can achieve more precise interventions in the pathological process of HF.
Introduction
Heart failure (HF) is a condition of impaired heart function, defined by the inability of the heart to pump an adequate volume of blood to meet the body's metabolic demands. HF is not a single pathological diagnosis but a clinical syndrome consisting of cardinal symptoms (e.g. breathlessness, ankle swelling, and fatigue) that may be accompanied by signs (e.g. elevated jugular venous pressure, pulmonary crackles, and peripheral oedema).1 The causes of HF are numerous and diverse. According to the Global Burden of Disease Study 2010, there are an estimated 17 primary causes of HF. Furthermore, more than two-thirds of HF is attributed to four diseases: ischaemic heart disease, chronic obstructive pulmonary disease (COPD), hypertensive heart disease, and rheumatic heart disease.2 In developed regions, ischaemic heart disease and COPD are the main causes of HF. In contrast, in some developing regions, the most common causes are hypertensive heart disease, rheumatic heart disease, cardiomyopathy, and myocarditis.2 HF is a serious condition affecting millions worldwide, serving as a principal cause of mortality and significantly impacting patients' quality of life and survival. The prevalence of HF in adults is approximately 1–2%, with an increase with age, exceeding 10% in those aged 70 and older.1 Despite significant advancements in pharmacological treatments and management strategies over the past decades, the prevalence of HF remains high. This highlights the urgent need to enhance the understanding of HF's complex pathophysiological mechanisms and develop novel therapeutic strategies.
The advancement of medical research and technological innovations has led to a shift in the understanding of HF from a simplistic view of cardiac pumping dysfunction to a more nuanced perspective that recognizes it as a complex disease involving multiple systems and factors. The development and progression of HF is a complex biological process involving a multitude of mechanisms, which are currently recognized to include, but are not limited to, alterations in cardiac structure, activation of the neuronal humoral system, activation of the immune system, involvement of biochemical alterations, abnormalities of metabolism, and oxidative stress.3 Elevated plasma cytokine levels have been identified in patients with HF as early as two decades ago,4, 5 indicating that immune system activation represents an important pathway for disease progression in HF. The initial cytokines identified as being associated with HF were predominantly TNF-α, IL-1, IL-6, and IL-18. Subsequently, a number of other biologically active substances, including chemokines, were identified as being associated with HF.6, 7 Initially, anti-inflammatory therapies targeting cytokines were regarded as a promising avenue of treatment. However, subsequent clinical trials, including studies of infliximab and etanercept targeting TNF-α, not only failed to demonstrate benefit for patients with chronic HF but also increased the risk of all-cause mortality with high doses of infliximab and the risk of hospitalization for HF.8, 9 A review of the literature on HF and inflammation indicates that the majority of clinical trials investigating the efficacy of anti-inflammatory therapies for HF have yielded disappointing results.10 This may be indicative of our incomplete comprehension of the immune system and the intricate inflammatory network that exists in HF.
In recent years, with the advent of new technologies such as genomics, proteomics, single-cell sequencing, and others, the role of immune cells in the pathogenesis of HF has received considerable attention and has gradually become a focal point for scientific research. These cells are involved in the process of cardiac inflammation and fibrosis by releasing cytokines, chemokines, or other mediators, thereby playing a role in cardiac injury and repair and ultimately affecting cardiac function and structure. In the case of inflammatory cardiomyopathy, which is a form of HF, immune cells are of great importance and can be considered central to the progression of the disease.11 Recent studies have demonstrated that the modulation of immune cells and immune responses may hold significant clinical implications for the treatment of HF. Targeting specific immune cell subsets or inflammatory pathways may help limit cardiac damage and improve remodelling. For instance, recent studies have demonstrated that the blockade of distinct phases of macrophage inflammatory activation is efficacious in the improvement of cardiac function and the attenuation of fibrosis in mice with HF.12-14 These findings emphasize the potential of immunomodulatory therapies in the clinical management of HF. The translation of these findings into clinical practice holds the potential for the development of more effective treatments, enhanced patient outcomes, and a reduction in rehospitalisation rates. In light of the aforementioned considerations, the aim of this review is to examine the interactions between HF and immune cells. In particular, this review will investigate how different immune cell types contribute to the development of HF through their respective mechanisms, with a view to elucidating the immunoregulatory mechanisms involved in HF. Furthermore, potential future therapeutic strategies will be explored with the objective of providing novel insights and directions for the management of HF.
Immune cells in the heart
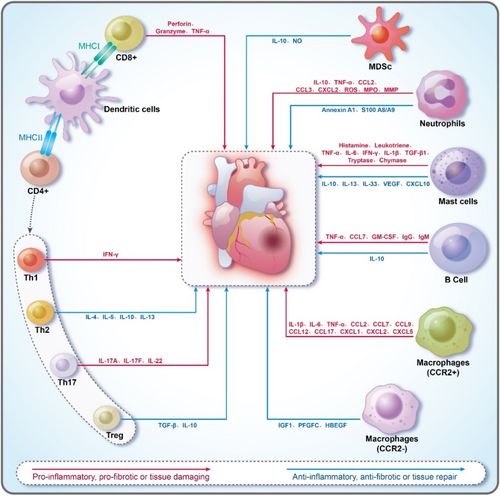
The healthy adult mouse heart contains a diverse array of immune cells, including various leucocyte types such as macrophages, B cells, T cells, neutrophils, mast cells, dendritic cells, and myeloid-derived suppressor cells (MDSCs)15 (Figure 1). The heart of healthy mice contains a considerable number of leucocytes, with the number of leucocytes in the heart of mice measured by flow cytometry at approximately 1000 per milligram of cardiac tissue.16 This is 12 times higher than in skeletal muscle. These immune cells play a pivotal and complex role in maintaining cardiac homeostasis, responding to injury, and modulating the inflammatory response, which in turn influences cardiac tissue repair and remodelling during HF development.17, 18 However, this role is twofold: On the one hand, a moderate immune response undoubtedly contributes to the repair of cardiac tissue after injury. On the other hand, an excessive, dysregulated immune response may lead to further cardiac damage and accelerate the progression of HF.15 A single-cell sequencing study in transverse aortic constriction (TAC) mice demonstrated that the immune activation in the hearts of TAC mice induced by pressure overload includes a significantly broader array of cell types than previously recognized, covering the entire spectrum of immune cells.19 This suggests that the mechanism of immune cell action in HF is more complex than previously assumed. In addition to the TAC-induced animal model of HF, immune cell activation was observed in a variety of other animal models of HF, thereby highlighting the general importance of immune cells in HF.20
Macrophage
Although macrophages constitute a smaller fraction than cardiomyocytes, endothelial cells, and fibroblasts in a healthy heart, they are the most abundant immune cell type, with their numbers increasing in disease states.21, 22 Macrophages in the heart are sourced from two major pathways: those that originate from tissue-resident macrophages during embryonic development and those that derive from blood monocytes in adulthood.23, 24 Tissue-resident macrophages in the heart are primarily originating from early embryonic development. Studies have shown that these macrophages are initially originating from the primitive yolk sac and later from precursor cells in the embryonic liver. During embryonic development, these precursor cells migrate to the heart, differentiating into mature macrophages.25, 26 These macrophages are sufficient to self-renew and sustain their numbers without bone marrow replenishment.27 They play a critical role in normal cardiac development, maintaining tissue homeostasis, and early immune surveillance. In addition to tissue-resident macrophages, macrophages in the heart can also be derived from blood monocytes during adulthood. These monocytes circulate in the bloodstream and enter cardiac tissues during cardiac damage or inflammation via a CCR2-dependent mechanism, differentiating into mature macrophages. This source is particularly important for the cardiac inflammatory response and subsequent tissue repair processes.28, 29 Furthermore, it has been demonstrated that following the transient depletion of tissue-resident macrophages, blood monocyte-derived macrophages are capable of transforming into a persistent macrophage population, complementing tissue-resident macrophages.30, 31
Macrophages play a pivotal role in the pathogenesis of HF. A study of 176 patients with dilated cardiomyopathy revealed that 80% of cardiac tissue samples contained activated macrophages.32 A study of 41 patients who died from acute myocardial infarction found a significant increase in macrophage numbers in both infarcted and non-infarcted areas, peaking 4–10 days post-infarction, with a positive correlation between macrophage numbers and infarction duration.33 Studies in patients with myocarditis also demonstrated a significant increase in macrophages in cardiac tissue.34 Histological examination of cardiac, splenic, and bone marrow tissues from 28 patients who died from acute myocardial infarction showed a significant increase in CD14+ monocytes in the heart and a significant decrease in the spleen and bone marrow.35 Macrophage and monocyte numbers were increased in both cardiac tissues and peripheral blood in patients with heart failure with preserved ejection fraction (HFpEF).36 High peak peripheral blood monocyte counts were associated with poor prognosis in patients with acute myocardial infarction, serving as an independent predictor of adverse cardiac events.37, 38 In patients with chronic HF, elevated peripheral blood monocyte counts were robust prognostic indicators for adverse cardiopulmonary events.39 Furthermore, elevated monocyte/macrophage levels have been documented in cardiac tissue and peripheral blood in a number of mouse models of HF.40-45 The aforementioned evidence collectively indicates that monocytes/macrophages play a pivotal role in the pathogenesis of HF.
In the healthy adult mouse heart, macrophages can be distinguished into three major subpopulations based on their expression of CCR2 and MHC-II: CCR2−MHCIIlow, CCR2−MHCIIhigh, and CCR2+MHCIIhigh.30, 46, 47 The CCR2− macrophages, the cardiac resident macrophages mentioned earlier, and the CCR2+ macrophages, the monocyte-derived macrophages, are relatively distinct in their specific functions. Transcriptomic data of macrophages from the hearts of HF patients demonstrated that CCR2+ macrophages expressed many chemokines, chemokine receptors, and IL-1, IL-6, and NF-κB signalling genes. In contrast, CCR2− macrophages exhibited high expression of growth factors, extracellular matrix components, and transducer genes, suggesting that CCR2+ macrophages primarily engage in pro-inflammatory processes, while CCR2− macrophages are more involved in anti-inflammatory and tissue repair functions.31 Flow cytometry quantification of CCR2+ and CCR2− macrophages in the hearts of TAC mice showed an increase in both populations, with a more pronounced increase in CCR2+ macrophages.40
CCR2− macrophages play a pivotal role in cardiac development and the maintenance of normal cardiac homeostasis and function. For instance, CCR2− macrophages are implicated in the formation of coronary arteries through the secretion of insulin-like growth factor (IGF),26 in the maintenance of metabolic stability and homeostatic function of cardiomyocytes through the removal of dysfunctional mitochondria excreted by cardiomyocytes,48 and in the promotion of electrical conduction by coupling to cardiomyocytes through connexin 43,49 among other functions. In disease states, CCR2− macrophages can secrete growth factors such as IGF1, platelet-derived growth factor C (PDGFC), and heparin-binding epidermal-like growth factor (HBEGF), thereby exerting tissue repair.31, 50 Furthermore, CCR2− macrophages, which abundantly express the calcium channel protein transient receptor potential vanilloid 4 (TRPV4), promote the expression of pro-vascular growth factors in a TRPV4-dependent manner upon mechanical stretch, thereby attenuating myocardial remodelling in HF and protecting the heart.51
In contrast to CCR2− macrophages, CCR2+ macrophages have undergone more extensive investigation in the context of HF. In the initial phase of pressure loading, elevated chemokine expression in the heart recruits CCR2+ macrophages in a CCR2-dependent manner. The activated CCR2+ macrophages secrete a variety of chemokines and cytokines, including CCL2, CCL7, CCL12, CCL17, TNF-α, IL-1β, and IL-6, which promote the recruitment of macrophages and other immune cells, activate local inflammation, and thereby advance HF. For instance, CCL17, secreted by CCR2+ macrophages, exacerbates myocardial injury by inhibiting Treg cell recruitment. The knockdown of CCL17 exhibits a protective effect on cardiac inflammation and ventricular remodelling.52 Additionally, CCR2+ macrophages secrete CXCL5 via the TLR9/MyD88/CXCL5 signalling pathway, which facilitates neutrophil recruitment to the injured heart.53 Despite promising results in animal models, therapeutic approaches targeting CCR2 have yet to be applied clinically. The use of CCR2 antagonists has demonstrated a marked reduction in macrophage infiltration within the hearts of mice afflicted with HF, thereby improving cardiac function.54 Additionally, CCR2 antagonists have been shown to reduce infarct size in infarcted mice and attenuate cardiac hypertrophy in the late stages of HF.40, 55 Furthermore, silencing or knocking down of CCR2 in experimental autoimmune myocarditis (EAM) mice has been observed to have a therapeutic effect and to reduce macrophage infiltration in the heart.44, 56 Knockdown of CCL2, CCR2, or antibody neutralization of CCL2 and CCR2 reduces macrophage infiltration in the heart after HF and myocardial infarction, thereby attenuating ventricular remodelling.21, 57-62 The use of nanoparticles to deliver siRNA targeting CCR2 has demonstrated reductions in monocyte recruitment, attenuated inflammatory cell infiltration, and inhibited left ventricular remodelling.63 These findings collectively highlight the therapeutic potential of targeting CCR2.
Additionally, other macrophage surface receptors have been demonstrated to play a role in the initiation and progression of HF. In the heart of the HFpEF mouse model, the number of macrophages significantly increased, with high expression of CXCR4 in these macrophages. Conversely, the specific knockdown of CXCR4 significantly reduces inflammatory cell infiltration, improves diastolic function, and inhibits cardiac hypertrophy and fibrosis.64 The use of CXCR4 antagonists and blockers has also demonstrated protective effects against myocardial infarction.65-68 Nevertheless, some studies have reported contradictory results. For instance, CXCR4 antagonists have been shown to exacerbate cardiac insufficiency and cardiac remodelling after myocardial infarction.69 The reason for this paradox remains unclear.
B-lymphocyte
The number of B cells in the heart of healthy mice is not insignificant, comprising approximately 10% of CD45+ cells.19 B cells perform three main functions: antibody production to mediate immune response, antigen presentation, and immunomodulation.70 The changes in B-cell counts in patients with HF remain contentious. One case report demonstrated substantial B-cell infiltration in endomyocardial biopsies obtained from patients diagnosed with inflammatory dilated cardiomyopathy.71 Another study of 56 patients with dilated cardiomyopathy revealed a significant increase in B cells in the peripheral blood.72 A significant increase in B-cell proportion was also observed in the peripheral blood of patients with acute myocardial infarction.73 However, another study found no significant increase in B cells in the heart tissue of patients with inflammatory cardiomyopathy.74 Furthermore, a study of 92 patients with HF and reduced ejection fraction showed significant reductions in both relative and absolute B-lymphocyte counts in the patients' peripheral blood.75 In contrast to human data, increased B-cell infiltration in the heart has been observed in various animal models of HF.19, 76-78
The mechanisms of B-cell involvement in HF are broadly categorized into antibody-mediated and inflammatory factor-mediated.
Antibody-mediated: B cells differentiate into plasma cells, which produce antibodies. Numerous studies have reported an increase in a variety of antibodies in the hearts and blood of patients with HF. In a study involving 100 patients with end-stage HF, histologic examination of cardiac specimens revealed immunoglobulin (IgG) deposits in most patients' hearts and complement activation in a few patients.79 In another study involving 20 patients with end-stage HF, testing of cardiac specimens and peripheral blood peripheral blood mononuclear cell (PBMC) showed increased IgG deposition in the myocardium and peripheral circulation.80 An infarction model using antibody-deficient mice showed that, relative to the control group, although antibody deficiency did not alter the survival rate of mice after myocardial infarction, it significantly reduced the infarct size and improved cardiac function, suggesting the important role of antibodies in infarction modelling.81 Autoantibodies target a variety of antigens, including proteins on the cardiomyocyte membrane and intracellular proteins, such as heat shock protein-60 (HSP-60),82 β1-adrenergic receptors,83 muscarinic acetylcholine receptor-2 (M-2),84 myosin heavy chain (MyHC),85 Na-K-ATPase,86 cardiac troponin I (c-TnI),87 and others. Although many cardiac injury-related autoantibodies against various antigens have been identified, the specific mechanisms underlying their involvement in myocardial injury are still not well understood.
Inflammatory factor-mediated: B cells exhibit immunomodulatory functions and secrete various cytokines and chemokines, including IL-2, IL-6, IL-10, TNF-α, IFN-γ, GM-CSF, and CCL7.88 Some of these cytokines are involved in the pathogenesis of HF. Studies of peripheral blood and cardiac magnetic resonance imaging (MRI) in patients with dilated cardiomyopathy have demonstrated that B cells contribute to myocardial fibrosis via TNF-α secretion.72 In a mouse model of acute myocardial infarction, mature B cells induce monocyte recruitment to the heart through the production of C-C motif chemokine ligand 7 (CCL7). B-cell depletion, impeding CCL7 production and monocyte mobilization, attenuates myocardial injury and improves cardiac function.77 The B cells involved in this process originate from the splenic marginal zone, where they are recruited to the heart and produce CCL7, inducing monocyte recruitment.89 Subsequent clinical data from 1000 patients with heart attacks demonstrated that patients with detectable CCL7 levels at admission had a significantly higher risk of death and recurrent myocardial infarction after 2 years of follow-up compared to those without detectable CCL7.77 Another animal experiment demonstrated that B-cell depletion and neutralization of granulocyte–macrophage colony-stimulating factor (GM-CSF) in an acute myocardial infarction model reduced neutrophil infiltration and cardiac fibrosis and improved cardiac function.90 Additionally, B cells are recruited to the heart through the CXCL13–CXCR5 axis and secrete TGF-β1.78
Although the prevailing opinion is that B cells facilitate the development of HF, some argue that B cells exert beneficial effects on the heart. For instance, during acute myocardial infarction, mouse pericardial adipose tissue is enriched with CD5+ B cells, which secrete IL-10 and exert anti-inflammatory effects.91 Furthermore, the transfer of B cells isolated from bone marrow into the myocardium following myocardial infarction improves cardiac function and reduces apoptosis.92, 93 Additionally, although B-cell depletion in mice with acute viral myocarditis reduces myocardial inflammation, it also disrupts Treg cell homeostasis in the spleen and heart. This suggests that B cells might play a beneficial role in viral myocarditis.94
Therapies targeting B cells have shown promise in animal experiments, but few clinical studies exist. A clinical trial demonstrated the safety of a single rituximab injection in patients with acute ST-segment elevation myocardial infarction (STEMI). This treatment reduced B- and T-cell counts in peripheral blood.95 Furthermore, rituximab administration has been shown to enhance cardiac function in patients with dilated cardiomyopathy.71 This suggests that B cells could be a potential target for future HF treatments.
T-lymphocyte
T cells are derived from pluripotent stem cells in the bone marrow, undergoing positive and negative selection in the thymus to become CD4+ T cells and CD8+ T cells. These naïve T cells migrate to peripheral lymphoid organs, where they encounter antigenic stimuli and differentiate into various subtypes.96 CD8+ T cells differentiate into effector T cells and memory T cells, whereas CD4+ T cells have at least four subtypes: Th1, Th2, Th17, and Treg.97, 98 T cells are crucial in the onset and progression of HF. Patients with HF show significantly elevated T-cell numbers in both the heart and peripheral blood. For instance, endomyocardial biopsies of patients with dilated cardiomyopathy demonstrated a significant increase in T cells.32 Myocardial tissue analysis of 16 patients with acute myocardial infarction revealed increased activated T cells in the infarcted area, with 11 cases also showing an increase in the non-infarcted area.99 Increased T-cell numbers were also observed in the cardiac tissue of patients with myocarditis.34 Enhanced expression of T-cell inflammatory cytokines and activation markers in the peripheral blood of patients with chronic HF indicates increased T-cell activation and numbers.100 A significant elevation in the proportion of Th1 cells was observed in the peripheral blood of patients with ischaemic cardiomyopathy and idiopathic dilated cardiomyopathy.101 Additionally, the percentage of Th1 cells was significantly higher in the peripheral blood of patients with acute myocardial infarction and unstable angina.102 Significant T-cell infiltrates were observed in the hearts of various mouse models of HF.19, 103-106 Different T-cell types exhibit distinct roles and mechanisms in the context of HF.
CD8+ T cells: The number of CD8+ T cells significantly increased in the heart and peripheral blood of TAC mice.107 Following an acute myocardial infarction in mice, CD8+ T cells are recruited and activated in ischaemic cardiac tissues, releasing granzyme B that causes apoptosis, poor ventricular remodelling, and deteriorated myocardial function. Depleting CD8+ T cells has been shown to reduce apoptosis within the ischaemic myocardium, impede the inflammatory response, limit myocardial injury, and improve cardiac function.105 Single-cell sequencing data from HF mice indicate that depleting CD8+ T cells protects TAC mice from pressure overload-induced HF, possibly through the regulation of macrophage function.108
CD4+ T cells: In animal models of HF, increased infiltration of Th1, Th2, and Th17 cells has been observed in the myocardium.107, 109 Th1 cells produce IFN-γ, exerting a predominantly pro-inflammatory effect. Th2 cells produce IL-4, IL-5, IL-10, and IL-13, exerting a predominantly anti-inflammatory effect. The ratio of Th1/Th2 cells somewhat reflects the pro-inflammatory and anti-inflammatory balance. A positive correlation has been observed between the number of peripheral blood Th1 cells and the severity of HF in patients with chronic HF. Peripheral blood Th1 cell numbers were higher in HF patients with ischaemic cardiomyopathy than in those with dilated cardiomyopathy.101 Th1 cell numbers were significantly elevated in the peripheral blood of patients with acute myocardial infarction, persisting for at least 1 week or even 1 month following disease onset.102 This indicates that Th1 cells may be involved in the immune-mediated ventricular remodelling process following acute myocardial infarction. Additionally, Th1 cells interact with cardiac fibroblasts and mediate cardiac fibrosis.110 T-bet, a Th1 cell-specific transcription factor, and its knockdown attenuated cardiac remodelling and myocardial inflammation in rats with HF, demonstrating the crucial role of Th1 cell subsets in cardiac remodelling and myocardial inflammation.111 Another clinical study found that a higher proportion of CD4+ Th1 cells in peripheral blood was significantly linked to a reduced risk of heart failure with reduced ejection fraction (HFrEF).112 However, the reason for this contradiction is unclear. Th17 cells produce IL-17A, IL-17F, and IL-22, primarily exerting pro-inflammatory effects. A case–control study found that both IL-17 levels and the number of Th17 cells were significantly elevated in the peripheral blood of patients with chronic HF.113 IL-17A supplementation to IL-17A-specific knockout mice exacerbated myocardial infarction and cardiac remodelling.114 Blocking IL-17 with a monoclonal antibody has been shown to ameliorate myocardial fibrosis in HF mice115 and improve cardiac function and reduce infarct size in infarcted mice.116 Treg cells produce TGF-β and IL-10, exerting a predominantly anti-inflammatory effect. The ratio of Th17/Treg can indicate the balance between pro-inflammatory and anti-inflammatory effects. The proportion of Treg cells in the peripheral blood is reduced in patients with chronic HF.107, 117 Clinical studies have shown that patients with HFrEF with a Treg/CD4+ T-cell ratio of less than 6% in peripheral blood have a significantly higher risk of rehospitalization due to HF deterioration within 12 months,118 suggesting that the ratio of Treg cells may serve as a prognostic indicator in HF.
Neutrophil
Neutrophils are the most abundant leucocytes in human blood, making up 50–70% of total leucocytes and 10–25% of total leucocytes in mouse peripheral blood.119 Neutrophils play a pivotal role in both acute and chronic inflammatory responses.120 Following tissue injury, neutrophils are recruited to the site by cytokines and chemokines. Through phagocytosis, they engulf pathogens and damaged cells and produce cytokines and chemokines to recruit other immune cells.121, 122 Following ischaemic injury or pressure overload in the heart, neutrophils are the first innate immune cells recruited in significant numbers.18
Neutrophils are strongly associated with several cardiovascular diseases. A study revealed that patients with myocardial infarction showed significant neutrophil infiltration in their cardiac tissue.123-125 Peripheral blood neutrophil counts were significantly elevated and positively correlated with infarct size.126, 127 Neutrophil counts were highly correlated with HF and directly correlated with infarct size and decreased left ventricular ejection fraction (LVEF) in infarction patients.128, 129 Other studies have demonstrated that neutrophil count is an independent predictor of mortality or myocardial infarction in patients with coronary artery disease (CAD) and that the predictive efficacy of the neutrophil/lymphocyte ratio (NLR) is higher.130 Elevated NLR has been associated with an increased risk of mortality or cardiac transplantation in patients with advanced HF, increased long-term mortality in patients with acute decompensated HF, and increased long-term mortality in patients with STEMI.131-133 In a mouse model of myocardial infarction, significant neutrophil infiltration was observed at the border of the infarcted area within 1–2 days after infarction, peaking on the third day, then gradually decreasing.43, 76 The reduction of neutrophils in myocardial tissue is considered a principal indicator of inflammatory regression.134 In a mouse model of viral myocarditis, neutrophil inhibition ameliorated the acute inflammatory response of the heart.135
The prevailing view is that neutrophils play a pro-inflammatory role in the heart, promoting the development of HF. Nevertheless, experiments have demonstrated that the depletion of neutrophils in infarcted mice does not confer a protective effect but rather leads to the deterioration of cardiac function, the exacerbation of fibrosis, and the progression to HF.136 Consequently, the prevailing opinion is that neutrophils play a dual role in cardiac injury and repair, exerting both pro-inflammatory effects that cause tissue damage and anti-inflammatory, pro-angiogenic, and pro-repair effects.137, 138 It is known that neutrophils cause cardiac injury through three main mechanisms. Neutrophils release reactive oxygen species (ROS), which directly cause tissue damage. Additionally, neutrophils release a variety of granule proteins, including myeloperoxidase (MPO), matrix metalloproteinase (MMP), serine protease, lysozyme, and lactoferrin.139, 140 Of these proteins, the relationship between MPO and infarction is more pronounced, and elevated plasma levels of MPO in infarcted patients have diagnostic value for myocardial infarction.141 Additionally, elevated levels of MPO serve as a risk factor for long-term mortality in myocardial infarcted patients.142 Knocking down MPO resulted in a reduction of leucocyte infiltration in the hearts of mice with myocardial infarction, an inhibition of left ventricular dilatation, and a significant improvement in cardiac function.143 Similar results were observed with an MPO inhibitor.144 Furthermore, neutrophils secrete a diverse array of cytokines and chemokines, which exert pro-inflammatory effects. These include IL-1β, TNF-α, CCL2, CCL3, and CXCL2.145, 146 Neutrophils participate in the repair of the injured heart by influencing macrophages. Neutrophils secrete annexin A1, which converts macrophages into a pro-angiogenic phenotype.147 Additionally, neutrophils induce nuclear receptor subfamily 4 group A member 1 (Nr4a1) expression by secreting S100A8/A9, promoting the conversion of inflammatory monocytes into reparative macrophages. Prolonged (21-day) inhibition of S100A8/A9 led to deterioration of cardiac function and left ventricular dilatation in myocardial infarction mice.148 In a model of autoimmune myocarditis, administration of S100A8/A9 to rats demonstrated anti-inflammatory effects by inhibiting multiple pro-inflammatory cytokines.149 Another study demonstrated that a short-term (3-day) administration of S100A8/A9 blockers improved cardiac function in mice with myocardial infarction.150 Given the dual role of neutrophils, therapeutic approaches targeting neutrophils should consider the timing of treatment to balance preventing excessive damage and preserving their repair function.
Mast cell
Mast cells represent a crucial component of the innate immune system and are extensively distributed in various tissues throughout the body, particularly on surfaces that are frequently exposed to the external environment, including the skin, intestines, respiratory tract, and genitourinary tract. Mast cells originate in the bone marrow and subsequently migrate to peripheral tissues, where they differentiate and mature to carry out a variety of functions. Mast cells are essential for the maintenance of organismal health, as they play roles in inflammatory responses, allergic reactions, antiparasitic defences, tissue repair, and modulation of immune responses.151-154 New research has identified a role for mast cells in both physiological and pathological processes in the heart.155-157 The activation of mast cells releases a variety of vasoactive substances and inflammatory mediators, including chemokines, cytokines, growth factors, heparin, histamine, leukotrienes, prostaglandin D2, and several proteases. These mediators contribute to the pathogenesis of various cardiac diseases.
Mast cells play a significant role in various cardiac conditions, including HF and cardiomyopathy. Research has shown that patients with severe congestive HF and left ventricular dysfunction have significantly elevated numbers of mast cells in left ventricular biopsy tissue.158 Similarly, studies involving patients with end-stage HF have reported a notable increase in mast cell numbers within cardiac tissue. For instance, a study of 20 patients with end-stage HF found a significant elevation in mast cells.159 In another study involving 10 patients with end-stage cardiomyopathy and 20 patients with end-stage HF due to idiopathic dilated cardiomyopathy, a significant increase in mast cell density in cardiac tissue was observed.160, 161 Additionally, patients with dilated cardiomyopathy and ischaemic cardiomyopathy exhibited a significant rise in mast cell density, along with elevated levels of histamine and tryptase in heart tissue.162 In a mouse model of myocardial infarction, a significant increase in cardiac tissue infiltrated with mast cells was observed, and mast cell defects were protective against myocardial infarction.163 In an animal model of autoimmune myocarditis, a significant correlation was observed between myocardial fibrosis and mast cell density in the heart. Furthermore, the mast cell stabilizer sodium cromoglycate has been shown to attenuate myocardial injury and myocardial fibrosis.164, 165 Chymase, a protease secreted by mast cells, has been demonstrated in several studies to be cardioprotective in various models of HF by means of chymase knockdown or inhibition.166-172
Although numerous studies have demonstrated a detrimental impact of mast cells on the progression of HF, there exists some controversy and evidence indicating that mast cells may exhibit resistance to cardiac fibrosis.173 Mast cells not only secrete pro-inflammatory factors but also produce anti-inflammatory and anti-fibrotic agents, including IL-10. IL-3, IL-33, and CXCL10.174-176 In a rat model of hyperhomocysteinaemia-induced cardiac remodelling, mast cell defects were observed to significantly exacerbate cardiac remodelling and myocardial fibrosis compared with controls.177 The administration of mast cell granules (MCG) to mice demonstrated a protective effect on the hearts in a myocardial infarction model.178 Mouse mast cell defects have been demonstrated to significantly impair myocardial fibroblast repair response and cardiac function following myocardial infarction, resulting in pronounced cardiac expansion.179 The role of mast cells in different experiments has produced contradictory results, indicating that the function and underlying mechanisms of mast cells remain poorly understood. Future studies should concentrate on elucidating the mechanisms of mast cells and exploring ways to inhibit their adverse effects and promote their beneficial effects.
Dendritic cell
Dendritic cells play a vital role in the immune system, acting as crucial antigen-presenting cells that bridge innate and adaptive immunity. Dendritic cells are divided into two main subpopulations: conventional dendritic cells (cDC) and plasmacytoid dendritic cells (pDC). cDC are further divided into two subtypes, cDC1 and cDC2.180 Dendritic cells present antigens to T cells via MHC molecules, thereby promoting T-cell activation, proliferation, and differentiation. This represents a key step in the initiation of an adaptive immune response. In addition, dendritic cells regulate the immune response by producing various cytokines.181, 182 It has been demonstrated that dendritic cells significantly increase in the heart tissue of patients in the acute phase of myocarditis.34 Another study observed a significant decrease in dendritic cell counts in the peripheral blood, yet a significant increase in dendritic cells in the heart tissue of patients with acute myocarditis.183 Additionally, dendritic cell infiltration was observed in the heart tissue of patients with myocardial infarction.184 The proportion and count of dendritic cells significantly increased in the peripheral blood of patients with chronic HF.185 Nevertheless, a study involving 72 patients with dilated cardiomyopathy revealed a significant reduction in dendritic cells in the hearts of these patients.186 In an animal model of myocardial infarction, there was observed a significant increase in infiltrating dendritic cells in the heart,187-189 as well as a significant increase in dendritic cells in the peripheral blood.190 The selective depletion of dendritic cells using diphtheria toxin exacerbated left ventricular remodelling after myocardial infarction, suggesting that dendritic cells play a protective role via the regulation of monocyte/macrophage homeostasis.191 Tolerogenic dendritic cells (tDCs) were generated by treating bone marrow dendritic cells with cardiac lysate from mice with myocardial infarction. Administration of tDCs to mice with myocardial infarction resulted in a reduction in infarct size, attenuation of left ventricular dilation, and improvement in left ventricular contractile function.192 The administration of tDCs to mice with EAM resulted in a significant reduction in the infiltration of inflammatory cells in the heart. This reduction may be attributed to the induction of Treg cell production and the secretion of anti-inflammatory cytokines.193 These findings indicate that DCs might play a protective role in HF. Nevertheless, it has been proposed that distinct subtypes of DCs might not exhibit identical functional profiles in the context of HF. For instance, one study showed that the selective depletion of cDCs improved cardiac function in mice with myocardial infarction, whereas the selective depletion of pDCs had no effect on cardiac function.194 In a mouse model of coronary artery occlusion and subsequent reperfusion injury, pDC depletion reduced infarct size.195 Therefore, the role of DCs in HF remains to be fully elucidated. Future studies should focus on elucidating the role of different DC subgroups in greater depth.
Myeloid-derived suppressor cells
MDSCs are a heterogeneous population of immune cells with immunosuppressive functions, derived from myeloid precursor cells in the bone marrow.196 Based on cell surface markers and morphological features, MDSCs can be classified into two main subtypes: polymorphonuclear-MDSC (PMN-MDSC) and monocytic-MDSC (M-MDSC). In mice, PMN-MDSC are defined as CD11b+ Ly6G+ Ly6Clow, and M-MDSC are defined as CD11b+ Ly6G− Ly6Chigh.197 MDSCs were initially associated with tumours, but subsequent studies have shown that, in addition to tumours, MDSCs are involved in chronic inflammation, infections, and autoimmune diseases, obesity, graft-versus-host disease, and numerous other diseases.198, 199
Recent studies have indicated that MDSCs are involved in the onset and progression of HF. Studies have demonstrated that patients with acute myocardial infarction exhibit an elevated percentage of MDSCs in their peripheral blood.200, 201 In a mouse model of acute myocardial infarction, the proportion of MDSCs increased in the peripheral blood and heart while decreasing in the spleen. The intravenous injection of spleen-derived MDSCs into mice with myocardial infarction led to an increased proportion of MDSCs in the infarcted heart, cardiomyocyte apoptosis, exacerbated myocardial wounding, and a reduction in cardiac systolic function. These findings suggest that spleen-derived MDSCs play a deleterious role in infarction.202 In a mouse model of CVB3-induced viral myocarditis, the number of MDSCs in the heart and spleen increased, and depletion of MDSCs reduced viral load in the heart, lowered macrophage and T-cell infiltration, and reduced the expression of multiple pro-inflammatory factors.203 However, another study contradicted this, indicating that tail vein injection of CD11b+ Ly-6C+ MDSCs significantly attenuated the severity of CVB3-induced viral myocarditis. This was associated with a decrease in the activation and proliferation of cardiac CD4+ T cells, a reduction in the number of Th1 and Th17 cells in the heart, and an increase in the number of Th2 and Treg cells in the heart. These findings suggest that MDSCs might have a protective role.204 In peripheral blood samples from patients with HF, the number of M-MDSC significantly increased. In mouse models of HF induced by isoproterenol (ISO) or TAC, depletion of MDSCs exacerbated pathological cardiac remodelling and inflammation. In contrast, the transfer of MDSCs significantly attenuated cardiac hypertrophy, cardiac dysfunction, and inflammation levels. This suggests that MDSCs might have a protective role in HF, possibly through anti-inflammatory effects mediated by the release of IL-10 and NO.201 It has been demonstrated that exercise training (ET) may exert a protective effect on the heart in HF by stimulating the secretion of IL-10 from macrophages and increasing MDSCs through the IL-10/STAT3/S100A9 signalling pathway.205
Studies investigating the role of MDSCs in HF are limited. The current state of research indicates that the role of MDSCs in HF is complex and multifaceted. In some instances, MDSCs might exacerbate myocardial injury and inflammation, whereas in other instances, they might attenuate cardiac pathological remodelling and dysfunction. Future studies should investigate the functional differences between different MDSC subpopulations and the relationship between MDSCs and other immune cells more thoroughly.
Therapeutic strategies targeting immune cells in heart failure
- Antibodies, blockers, and antagonists targeting molecules or receptors on the surface of immune cells. This represents one of the most commonly employed therapeutic strategies. The activity of immune cells is regulated by targeting specific surface molecules or receptors. However, due to the wide range of gene expression, this approach lacks specificity and may lead to side effects. For example, CCR2 is expressed by a variety of cells in the human heart, including macrophages, plasma cells, neutrophils, T cells, cardiomyocytes, endothelial cells, and others. Antibodies and antagonists targeting CCR2 may affect all of these cells.
- Neutralizing antibodies, blockers, or antagonists of chemokines. This strategy is similar to the previous approach and is a common treatment modality. By interfering with the activity of chemokines to affect the migration of immune cells and the inflammatory response, this approach suffers from low specificity and a greater incidence of side effects.
- Drugs or DNA and RNA binding vectors targeting immune cells. This is a relatively recent therapeutic strategy, involving the use of carriers to deliver drugs, genes, or RNA to specific immune cells with the aim of regulating their functions. Currently known vectors include viral vectors, liposomes, lipid nanoparticles (LNPs), and exosomes. Novel carriers exhibit high targeting and specificity, as well as good plasticity, allowing modifications as needed.206-208 The development of novel vectors is crucial for the research of new drugs and therapeutic approaches, as well as for future clinical translation.
- The transfer of cells. Cell transfer involves transplanting immune cells with specific functions into the body for therapeutic purposes. For instance, immune cells with anti-inflammatory functions, such as Treg cells and MDSCs, are isolated from animals and then infused.201, 209-211 Furthermore, isolated monocytes/macrophages can be transfected with IL-10 in vitro and then injected into mice.212 Notably, chimeric antigen receptor-T (CAR-T) cell therapy has demonstrated efficacy in the treatment of HF. In 2019, Aghajanian et al. first employed CAR-T therapy in a mouse model of AngII and phenylephrine-induced HF. They designed a cell therapy targeting fibroblast activated protein (FAP) to specifically eliminate cardiac myofibroblasts. CAR-T therapy resulted in a significant reduction in cardiac fibrosis and an improvement in cardiac function.213 In conventional CAR-T therapy, T cells are isolated from patients or animals, modified and expanded in vitro, and then infused back into them. In 2022, Rurik et al. developed an in vivo method for generating CAR T cells by delivering mRNA designed against FAP to lymphocytes in vivo using LNP targeting CD5 to form transient anti-fibrotic CAR-T cells. This approach led to significant improvements in cardiac function and the degree of fibrosis in mice with AngII and phenylephrine-induced HF.214 Compared to conventional CAR-T, the approach of Rurik et al. represents a significant advancement. Firstly, CAR-T cells are generated in vivo, eliminating the process of extracting T cells and infusing them back. Secondly, the conventional CAR-T method involves transfection with a viral vector, which integrates the target gene into the genome. In contrast, Rurik et al.'s method employs LNP to deliver mRNA, which degrades post-translationally and does not integrate into the genome, reducing the risk of genetic alteration. CAR-T therapies provide a novel approach to the treatment of HF.
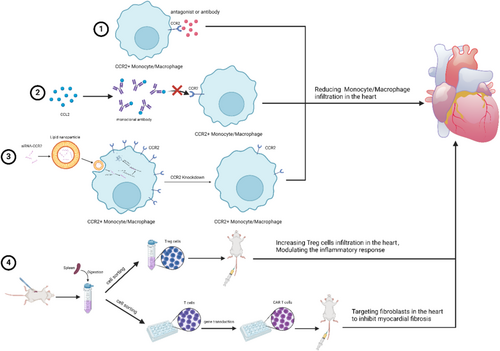
Although the aforementioned treatments have demonstrated therapeutic potential in animal models (Table 1), numerous challenges remain before their successful application to clinical treatment. For instance, the specificity, side effects, and long-term impacts of the treatments must be further studied and validated. Moreover, due to the complexity of the aetiology and pathogenesis of HF, personalized therapy is particularly crucial, as the underlying causes and disease states vary among patients. When formulating a treatment plan, it is essential to comprehensively consider the patient's disease status, identify the aetiology and specific subtype of HF, and determine the pathological characteristics and immune status of the patient. Multi-omics approaches, including genomics, transcriptomics, and proteomics, can be employed to assess the distribution and functional states of immune cells, the expression patterns of surface molecules on immune cells, and other key immune parameters. These techniques enable the construction of a cytokine-chemokine network for each patient, facilitating the identification of the most relevant immune cell subpopulations and the key active inflammatory pathways. Based on these insights, the most appropriate therapeutic strategies can be selected. During the treatment process, dynamic monitoring is essential to adjust drug dosages and administration schedules according to the evolving state of the patient's immune system. This approach aims to maximize therapeutic efficacy while minimizing adverse effects.
| Strategy | Model | Intervention | Result | Mechanism | References |
|---|---|---|---|---|---|
| 1 | TAC-induced HF | CCR2 antagonist and CCR2 antibody | Decreased fibrosis and improved cardiac function | Reduced macrophage and T-cell infiltration in the heart | 40 |
| 1 | TAC-induced HF | CCR2 antagonist | Improved cardiac function but no effect on cardiac hypertrophy and fibrosis | Reduced CCR2+ macrophage infiltration in the heart | 54 |
| 1 | TAC-induced HF | Rituximab | Inhibited cardiac dilatation, cardiomyocyte hypertrophy, fibrosis, and improved cardiac function | Inhibited B-cell activation and antibody production and suppressed pro-inflammatory cytokine expression | 215 |
| 1 | AngII-induced HF | CD22 antibody | Inhibited cardiomyocyte hypertrophy, decreased collagen deposition, and improved cardiac function | Inhibited B-cell activation and collagen production by cardiac fibroblasts | 216 |
| 1 | TAC-induced HF | CD3 antibody | Inhibited fibrosis and improved cardiac function | Reduced T-cell recruitment and inhibited pro-inflammatory cytokine production | 103 |
| 1 | TAC-induced HF | Abatacept | Inhibited cardiomyocyte hypertrophy, fibrosis, and improved cardiac function | Suppressed T-cell and macrophage activation and inhibited B-cell function | 217 |
| 1 | TAC-induced HF | CX3CR1 antagonist | Inhibited cardiomyocyte hypertrophy | Reduced expression of pro-fibrotic genes | 218 |
| 1 | Myocardial infarction | CXCR4 antagonist | Decreased size of the infarct scar, attenuated left ventricular remodelling, and improved cardiac function | Increased recruitment of Treg cells in the infarcted area | 66 |
| 1 | Myocardial infarction | CXCR4 antagonist | Decreased infarct size and improved cardiac function | Upregulated bone marrow endothelial nitric oxide synthase (eNOS) levels | 67 |
| 1 | Myocardial infarction | CXCR4 antagonist | Decreased incidence of left ventricular rupture and improved cardiac function | Inhibited monocyte and neutrophil infiltration in the heart | 219 |
| 1 | Myocardial infarction | CCR2 antagonist and CCR2 antibody | Decreased infarct size | Decreased monocyte recruitment in the heart and spleen | 55 |
| 1 | Myocardial infarction | CD20 antibody | Decreased infarct size, attenuated left ventricular remodelling, and improved cardiac function | Decreased macrophage recruitment and reduced pro-inflammatory cytokine production through depletion of B cells | 77 |
| 1 | Myocardial infarction | CD3 antibody | Decreased size of the infarct scar | Inhibited the function of T cells | 220 |
| 1 | Myocardial infarction | CXCR4 antagonist | Improved cardiac function | Inhibited pro-inflammatory cytokine production and mobilized mesenchymal stem cells (MSCs) | 68 |
| 1 | EAM | CCR5 antibody | Reducing the severity of myocarditis | Inhibited infiltration of multiple immune cells in the heart | 221 |
| 1 | EAM | CCR1 antagonist | Inhibited the development of myocarditis and improved cardiac function | Inhibited T-cell proliferation and pro-inflammatory cytokine expression | 222 |
| 1 | Spontaneous hypertension | CXCR2 inhibitor | Lowered blood pressure, inhibited cardiomyocyte hypertrophy and fibrosis, and improved cardiac function | Reduced macrophage recruitment, pro-inflammatory cytokines, and ROS production | 223 |
| 1 | Diabetic cardiomyopathy | CCR2 inhibitor | Improved cardiac function | Inhibited cardiomyocyte apoptosis and inhibited macrophage polarization to M1 subtype | 224 |
| 2 | Myocardial infarction | CCL2 competitive inhibitor | Decreased infarct size and inhibited fibrosis | Decreased monocyte recruitment in the heart | 225 |
| 2 | Myocardial infarction | CXC chemokine inhibitor and CC chemokine inhibitor | Decreased infarct size | Reduced inflammatory cell infiltration and ROS production in the heart by depleting T cells | 226 |
| 2 | Myocardial infarction | CCL5 antibody | Decreased infarct size | Reduced immune cell infiltration in the heart, inhibited chemokine expression, and suppressed oxidative stress and apoptosis | 227 |
| 2 | Myocardial infarction | CCL5 antibody | Decreased infarct size and improved cardiac function | Reduced macrophage and neutrophil infiltration in the heart | 228 |
| 2 | Myocardial infarction | MPO inhibitor | Inhibited ventricular remodelling and dilatation and improved cardiac function | Inhibited the recruitment of Ly-6Chigh monocytes | 144 |
| 2 | Myocardial infarction | CCL2 antibody | Decreased infarct size | Inhibited macrophage infiltration in the heart | 229 |
| 2 | Brief repetitive myocardial ischaemia and reperfusion | CCL2 antibody | Inhibited fibrosis and improved cardiac function | Inhibited macrophage infiltration in the heart | 61 |
| 2 | EAM | CCL2, CCL3 antibody | Reduced prevalence of myocarditis in mice | Reduced production of pro-inflammatory cytokines | 44 |
| 3 | Myocardial infarction | CCR2 siRNA | Inhibited ventricular remodelling and improved cardiac function | Decreased monocyte recruitment in the heart and spleen and reduced expression of inflammatory factors in the infarcted area | 63 |
| 3 | EAM | CCR2 siRNA | Improved cardiac function | Decreased cardiac monocyte recruitment and migration of bone marrow granulocyte macrophage progenitor cells to the peripheral blood | 56 |
| 3 | Myocardial infarction | IRF5 siRNA | Improved healing of the infarct site | Inhibited macrophage polarization to M1 subtype and suppressed pro-inflammatory cytokine expression | 230 |
| 3 | Myocardial infarction | CXCR7 shRNA | Decreased infarct size and improved cardiac function | Reduced the number of M1 macrophages and inhibited the production of pro-inflammatory cytokines | 231 |
| 4 | Myocardial infarction | Treg cells' adoptive transfer | Inhibited ventricular remodelling and improved cardiac function | Inhibited macrophage, neutrophil, and lymphocyte infiltration and reduced pro-inflammatory cytokine production in the heart | 209 |
| 4 | Viral myocarditis | Treg cells' adoptive transfer | Improved cardiac function | Decreased macrophage recruitment and reduced production of pro-inflammatory cytokines in the heart | 210 |
| 4 | Viral myocarditis | Treg cells' adoptive transfer | Inhibited fibrosis | Treg cells exert antifibrotic effects by producing IL-10 | 211 |
| 4 | EAM | Adoptive transfer of CD11b+ monocytes/macrophages transfected with IL-10 | Inhibited fibrosis | CD11b+ monocytes/macrophages overexpressing IL-10 secrete IL-10 to exert anti-inflammatory and antifibrotic effects | 212 |
| 4 | ISO or TAC-induced HF | MDSCs' adoptive transfer | Reduced cardiac hypertrophy and inflammation levels and improved cardiac function | MDSCs released IL-10 and NO to exert anti-inflammatory effects | 201 |
Conclusion
The aetiology of HF is complex and diverse, and its pathogenesis remains incompletely understood. In recent years, there has been a notable increase in research activity concerning the role of immune cells in the pathogenesis of HF. Nevertheless, it is not feasible to categorize the role of immune cells as either ‘beneficial’ or ‘harmful’ due to the inherent dynamism of their functions and the influence of multiple factors. The functions of immune cells are subject to regulation by a number of factors, including the specific cell type, subpopulation, level of activation, interactions between different cells, the underlying aetiology of HF, the progression of the disease, as well as the age, gender and health status of patients with HF. Therefore, therapies that merely remove or transplant a specific type of immune cell are insufficient for ensuring therapeutic efficacy and may disrupt immune homeostasis, potentially resulting in adverse effects. Furthermore, the results of disparate animal experiments may be inconsistent, thereby highlighting the limitations of this therapeutic approach. It is therefore recommended that future studies focus on more precise targets and cell subpopulations in order to gain a deeper understanding of how to maintain immune balance at specific disease processes and critical time points. This is of paramount importance for the effective treatment of HF. This may require the integration of personalized medicine to develop more precise treatment plans, tailored to the specific circumstances and pathophysiological state of each patient. Such studies will facilitate a deeper comprehension of the immunological mechanisms underlying HF and provide crucial guidance and support for the development of more efficacious therapeutic strategies.
Funding
This work was supported by the National Natural Science Foundation of China (82241034, 82330010 and 824B2007) and National Key Research and Development Program of China (2024YFC3044500).
Author contributions
G.L. and D.W.W. conceived the paper. G.L. wrote the article. W.H. participated in the literature search and provided helpful suggestions. D.W.W. revised the figures and reviewed the article. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no competing interests.




