Familial restrictive cardiomyopathy with novel missense variant of uncertain significance in the FLNC gene
Introduction
Filamin C (FLNC) is a structural protein that has an actin-binding domain. Variants of the FLNC gene (FLNC) have been associated with myofibrillar myopathy and cardiomyopathies, such as dilated cardiomyopathy, hypertrophic cardiomyopathy and restrictive cardiomyopathy (RCM).1 Because FLNC variants are less prevalent in RCM than in other cardiomyopathies, the role of FLNC in RCM is difficult to determine. Herein, we present cases of familial RCM associated with a novel FLNC variant.
Case series
A woman in her 40s (II-3), who had been found while a teenager to have atrial fibrillation, was admitted to our hospital owing to worsening dyspnoea on exertion (Figure 1A). Upon admission, the findings of examinations were consistent with the clinical features of RCM accompanied by right-sided heart failure. Electrocardiogram showed atrial fibrillation, right bundle branch block, right axis deviation, and ST depression in the inferior and precordial leads (Figure S1E). Chest X-ray demonstrated cardiomegaly with pulmonary congestion at the right lower lobe (Figure 1B). Echocardiography showed preserved left ventricular systolic function with significant biatrial enlargement (Figures 1C and S1A). Mitral annular tissue Doppler showed that medial e′ velocity was lower than lateral e′ velocity, indicating no mitral annulus reversus, which is consistent with RCM. Right heart catheterization revealed restrictive physiology with a mean pulmonary artery pressure of 34 mmHg and a pulmonary artery wedge pressure of 24 mmHg. Furthermore, simultaneous right and left ventricular pressure via right heart catheterization did not show the finding of dip and plateau, but demonstrated inspiratory variability with a left ventricular end-diastolic pressure of 24 mmHg, right ventricular end-diastolic pressure of 14 mmHg, suggesting the difference with more than 5 mmHg. Magnetic resonance imaging of the heart showed no late gadolinium enhancement (Figures 1D and S1C). An endomyocardial biopsy had no specific findings. Immediately after the patient was discharged with improved symptoms, heart failure was exacerbated despite high doses of diuretic agents. She was placed on a waiting list for heart transplantation while being treated with an intravenous inotropic agent but died of septic shock.
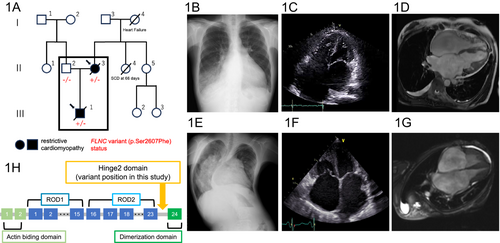
The patient had a significant family history, including her son (III-1) dying of RCM in his 20s, her mother (I-4) dying of heart failure in her 50s and her sibling (II-4) dying of an unknown cause within 3 months after birth.
When the woman's son (III-1) was a teenager, he was referred by a children's hospital to our affiliated institution for the follow-up management of RCM. Coarctation of the aorta, small ventricular and atrial septal defects, and a patent ductus arteriosus had been diagnosed 1 day after birth and were treated surgically with subclavian flap aortoplasty and patent ductus arteriosus ligation 6 days after birth. He had congenital constriction band syndrome. In addition, pulmonary hypertension was diagnosed at the age of 3 years via right heart catheterization, which revealed a mean pulmonary artery pressure of 60 mmHg and pulmonary vascular resistance of 7.3 Wood units.
Upon arrival, he was examined and found to have a height of 127 cm and a body weight of 23 kg. Echocardiography showed preserved left ventricular systolic function without ventricular hypertrophy or dilatation and significant biatrial enlargement (Figures 1F and S1B). Magnetic resonance imaging showed no late gadolinium enhancement (Figures 1G and S1D). Family tree analysis with whole exome sequencing identified a heterozygous missense variant of FLNC (chr7: 128858047 C>T; GRCh38/hg38: NM_001458.5: c.7820C>T: p.Ser2607Phe) in the woman (II-3) and her son (III-1) but not in her husband (II-2) (Supporting information method). This FLNC variant was absent in public databases, predicted as pathogenic in multiple in-silico pipelines (SIFT-4G: damaging; PolyPhen2: deleterious; CADD: 27.1, AlphaMissense: likely pathogenic) and resulted in its classification as a variant of uncertain significance according to the guidelines of the American College of Medical Genetics and Genomics (PM2 and PP3).2 No other likely pathogenic variants of genes related to cardiomyopathy were identified. Although this FLNC variant is classified as a variant of uncertain significance, considering its cosegregation with clinical features, it is potentially associated with the occurrence of RCM in this pedigree.
Two years later, this young man's heart failure had exacerbated with severe pulmonary hypertension. At a tertiary institution specializing in transplantation, he was assessed for possible simultaneous heart–lung transplantation. However, because of severe scoliosis (Figure 1E) and a thoracic deformity, which were considered high risk factors for surgery, he was considered a poor candidate for transplantation and not placed on a waiting list. He died of heart failure in his early 20s.
Discussion
These two patients with familial RCM had a newly identified missense variant in the FLNC gene. Both patients required advanced heart failure therapy including heart transplantation. Although the genetic basis of RCM is not well understood, most cases of idiopathic RCM evidently have a genetic background.3 The majority of mutations known to be associated with familial RCM are the following: mutations in sarcomeric proteins cardiac troponin-I (TNNI3),4 cardiac troponin-T (TNNT2),5 and β myosin heavy chain (MYH7),6; mutation in intermediate filament protein desmin (DES)7; and mutation in Z-disc protein filamin-C (FLNC).8 We described a comprehensive discussion comparing the novel FLNC variant with other known variants associated with RCM and other cardiomyopathies (Table S1). Further, accumulating evidence suggests that FLNC variants may lead to RCM through a complex interplay of disrupted cytoskeletal integrity, impaired signal transduction, defective sarcomere function and the development of fibrosis.9-11 The resultant stiffening of the ventricular walls and impaired diastolic filling are the clinical manifestations of this pathophysiology. Although RCM is an uncommon subtype of inheritable cardiomyopathy and is of uncertain pathophysiology, a possible cause is a missense variant in the ROD2 domain of FLNC.1, 9, 12 The variant in both patients was in the Hinge2 domain, which is near the ROD2 domain. To our knowledge, this variant is the first RCM-associated missense variant in the Hinge2 domain of FLNC (Figure 1H). From preclinical studies, the FLNC Hinge 2 domain regulates Z-disc maintenance by interacting with calpain 1.13 PKCα phosphorylation prevents cleavage, ensuring muscle fibre stability.14 The functional significance of this variant is challenging to discuss but will likely be elucidated with future case studies and functional analyses. Despite having the identical FLNC variant, the woman (II-3) and her son (III-1) had clinical characteristics, other than RCM, which differed significantly. A possible ‘second hit’ gene variant via paternal inheritance should be considered; however, whole exome sequencing did not identify any variants that could account for this difference. Considering FLNC is also causative for myofibrillar myopathy, with which scoliosis is known to occasionally occur,15 the FLNC variant by itself could partially account for the extracardiac conditions observed in the woman's son (III-1), but the mechanisms underlying the difference in expressivity between these two patients with the identical FLNC variant remain uncertain. Computational three-dimensional modelling algorithms predicted that this variant would cause no significant structural and electric changes compared with the wild-type gene. This lack of change might be due to technical limitations or to unassessed factors, such as hydrophilicity, playing a role in determining the pathogenicity of this variant (Figure S2). Lastly, our report has a potential limitation regarding the diagnosis of RCM in the woman's son (III-1) as he has congenital heart disease, including coarctation of the aorta, small ventricular and atrial septal defects and a patent ductus arteriosus.
Our findings highlight the importance of FLNC variants as the cause of familial RCM. Further RCM cases with FLNC variants should be reported to clarify the causal relationships and genotype–phenotype correlations.
Acknowledgements
The authors are grateful to Prof. Masao Okazaki at The Jikei University School of Medicine for his editing contribution.
Conflict of interest statement
The authors declare no competing interest.




