Training-induced change of diastolic function in heart failure with preserved ejection fraction
Andreas B. Gevaert and Ephraim B. Winzer contributed equally to the study.
Summary tweet: #ExerciseEcho in #HFpEF reveals increased E/e′ and poor RV reserve. After 3 months training → small improvement in E/e′. But not enough to explain improved V̇O2peak @ESC_Journals #EchoFirst.
Registration: Optimizing Exercise Training in Prevention and Treatment of Diastolic Heart Failure (OptimEx-Clin); NCT02078947.
Abstract
Aims
Exercise training improves aerobic capacity (V̇O2peak) in patients with heart failure and preserved ejection fraction (HFpEF), but underlying mechanisms remain unclear. We aimed to evaluate whether exercise training could improve systolic and diastolic function during exercise.
Methods
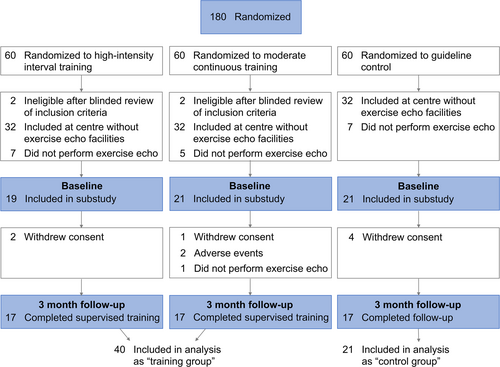
This was a substudy of the multicentre Optimizing Exercise Training in HFpEF (OptimEx-Clin) trial, in which 180 patients with HFpEF were randomized 1:1:1 to guideline control, moderate continuous training or high-intensity interval training. All patients included at two out of five participating sites underwent exercise echocardiography at baseline and 3 months. Patients of both training groups were pooled and compared with guideline control.
Results
A total of 61 patients (mean age 73 ± 7 years, 72% female) were included. At baseline, E/e′ increased from 17.0 ± 5.7 to 19.5 ± 6.1 and systolic pulmonary artery pressure from 31 ± 8 to 51 ± 11 mmHg (both P < 0.001). Right ventricular function did not change significantly (maximal tricuspid annular plane systolic excursion 24.7 ± 4.0 mm, P = 0.051 vs. baseline).
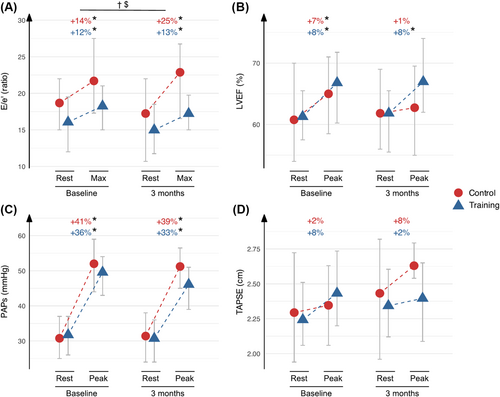
At 3 months, patients randomized to exercise training improved V̇O2peak (control +0.2, training +2.7 mL/kg/min, P = 0.006) and demonstrated small but significant improvements in exercise E/e′ (control 21.7 ± 7.5 to 22.8 ± 9.2, training 18.3 ± 5.0 to 17.2 ± 4.1, P = 0.044). No significant changes were observed in ejection fraction, mitral or tricuspid annular plane systolic excursion, S′, A′ or systolic pulmonary artery pressure (P > 0.05). Changes in E/e′ were not associated with the change in V̇O2peak.
Conclusions
In patients with HFpEF, exercise echocardiography revealed increases in filling pressures as well as a failure to augment right ventricular function during exercise. After 3 months of exercise training, HFpEF patients demonstrated a small improvement in diastolic function (exercise E/e′), but this did not explain the improved aerobic capacity.
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is one of few cardiovascular diseases with increasing prevalence.1 Despite recent advances in drug management, HFpEF treatment remains challenging.2 Exercise capacity and quality of life remain poor even after treatment with sodium-glucose transporter 2 inhibitors.3
In HFpEF patients, supervised exercise training (ET) is able to improve quality of life as well as aerobic capacity, measured objectively as peak oxygen uptake (V̇O2peak).4 The mechanisms responsible for this improvement in V̇O2peak are however still controversial. Small studies have suggested that ET improves resting systolic function by increasing left ventricular ejection fraction (LVEF) or diastolic function by reducing the E/e′ ratio and altering deceleration time at rest.5, 6 However, other studies indicated that the change in V̇O2peak is primarily associated with changes in peripheral oxygen extraction with a higher peak arterial–venous oxygen difference.7, 8
Improvements in cardiac function may be more evident during exercise testing, as systolic and diastolic function may be within normal ranges at rest.2 Exercise right heart catheterization is considered the gold standard in this setting, but is not in general use due to its invasive nature and limited availability. Exercise echocardiography serves as a more widely available and clinically applicable non-invasive examination, demonstrating technical feasibility and acceptable correlation with invasive measurements of LV filling pressure.9, 10 Only one study assessed the effects of ET on exercise echo parameters in HFpEF, and this did not include diastolic function parameters.7
Optimizing Exercise Training in HFpEF (OptimEx-Clin) was a randomized multicentre trial assessing the effect of different training modalities on V̇O2peak in patients with HFpEF. The primary endpoint of the main trial was a change in V̇O2peak at 3 months; while V̇O2peak improvement was significantly higher in moderate continuous training (MCT) and high-intensity interval training (HIIT) groups as compared with guideline control, the predefined margin of clinical relevance (2.5 mL/kg/min) was not met.11
In this prespecified subanalysis of OptimEx-Clin, we assessed whether ET could improve parameters of systolic and diastolic function assessed during exercise echocardiography.
Methods
Main trial design
The OptimEx-Clin trial was conducted between September 2014 and July 2018 at the University Hospital ‘Klinikum rechts der Isar’ (Technical University of Munich), Heart Centre Leipzig, Charité Universitätsmedizin Berlin in Germany and Antwerp University Hospital in Belgium.11, 12 A total of 180 HFpEF patients were enrolled. HFpEF was defined as LVEF ≥ 50%, with signs and symptoms of HF and elevated LV filling pressure (E/e′ septal ≥ 15 or E/e′ septal ≥ 8 with concurrent elevated natriuretic peptides).11 Inclusion criteria are listed in Table S1.
Patients were randomized 1:1:1 to MCT (5 times per week, 40 min exercise at 35%–50% of heart rate reserve), HIIT (3 times per week, 38 min exercise: 4 × 4 min intervals at 80%–90% heart rate reserve preceded by 10 min warm up and interspaced by 3 min exercise, both at 35%–50% heart rate reserve) or guideline control (physician recommendation to perform regular exercise). For MCT and HIIT, exercise sessions were supervised in cardiac rehabilitation centres three times per week for 3 months. The remaining exercise sessions were performed at home using telemonitoring supervision: patients were asked to wear a heart rate monitor (Polar, Finland), and heart rate data were transferred digitally via a smartphone application (Polar, Finland) at the end of the session. Adherence was reviewed weekly by the study team and telephonic motivational feedback was provided in case of non-adherence to protocol.
The study complied with the Declaration of Helsinki and was approved by the local ethics committees at the participating centres. We obtained written informed consent from each participant.
Exercise echocardiography substudy design
All patients recruited at Heart Centre Leipzig and Antwerp University Hospital were invited to undergo symptom-limited exercise echocardiography at baseline and 3 months, and included in the current substudy (Figure 1). As the main analysis of change in V̇O2peak did not demonstrate meaningful differences between MCT and HIIT groups, we pooled both ET groups for this substudy, to increase statistical power.
A protocol of 20 W with 10 W increments per minute was used for exercise testing on a semi-supine bicycle until exhaustion (peak exercise). At each increment, patients indicated dyspnoea level on a 15-step Borg scale (6–20), and blood pressure was measured. Heart rate and electrocardiogram were recorded continuously. Echocardiographic images (Vivid E9, GE Healthcare, Norway) were acquired at each increment, including parameters of systolic and diastolic function, left atrial size and right ventricular (RV) function. Reported values of diastolic function include both resting measurements, the value recorded at 50% of peak workload (‘submaximal’) and the highest value obtained during exercise (‘maximal’). As for the remaining echocardiographic parameters, we reported values at both rest and peak exercise (‘peak’).
Echocardiographic data were analysed offline (EchoPAC, GE Medical Systems, Norway). All measurements were averaged over three cardiac cycles for patients in sinus rhythm and five cycles for patients with atrial fibrillation. LV ejection fraction was quantified by the modified Simpson's method in the apical four- and two-chamber view. Left atrial volume was measured using the area–length method in the apical four- and two-chamber view and indexed to body surface area. Mitral E and A wave velocities were measured with pulsed wave Doppler at the tips of the mitral leaflets in the apical four-chamber view. Early relaxation (e′) and systolic (S′) velocities were obtained by tissue Doppler imaging at the septal and lateral mitral annulus at rest. During exercise, only septal e′ and S′ were recorded due to time constraints.13 Systolic pulmonary artery pressure (PAP) was calculated as the sum of the maximal trans-tricuspid pressure gradient and an estimate of the resting right atrial pressure based on the inferior caval vein dimension and collapsibility. Mitral and tricuspid annular plane systolic excursion (TAPSE) was measured at the septal and lateral annulus, respectively. RV–vascular coupling was measured by TAPSE/PAPs ratio.14 The procedure and analysis were performed by cardiologists with extensive experience in exercise echocardiography (E. B. W, C. M. V. D. H. and A. B. G.).
Other measurements
Cardiopulmonary exercise testing was performed on a separate day (within 7 days of exercise echocardiography) and analysed in a blinded manner at the study core laboratory (Munich), as described previously.15 Briefly, patients exercised until exhaustion on a bicycle ergometer while gas exchange (ventilation, oxygen uptake and carbon dioxide production) was measured continuously. V̇O2peak was defined as the highest 30 s average oxygen uptake within the last minute of exercise. Predicted V̇O2peak was calculated by the Jones formulas.16 Ventilatory efficiency was calculated using the entire exercise data. Patients with a relative increase of 6% in V̇O2peak were considered ‘responders’, based on a recent consensus statement as well as the mortality benefit associated with this threshold in patients with HFrEF.17, 18
HFpEF clinical scores (H2FPEF, HFA–PEFF) were calculated based on clinical and echocardiographic variables at rest, as published.19, 20 N-terminal pro B-type natriuretic peptide (NT-proBNP) values were analysed by a central core laboratory (Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Austria).
Sex was defined as ‘sex assigned at birth’ for all analyses.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or as median (25th–75th percentile) when distribution was skewed. Group comparisons (Table S2) were performed using one-way analysis of variance (continuous variables), Kruskal–Wallis test (skewed continuous variables) or Pearson's χ2 test (categorical variables). Correlations were analysed using Spearman's correlation coefficient.
Linear mixed models were used to analyse repeated measures data. An uncorrected model was constructed using randomization number as random factor and time, training group and their interaction as fixed factors. Logistic regression models were used to assess independent determinants of training response (change in V̇O2peak ≥ 6% or <6%).17 If anomalies were noted on quantile–quantile plots, residual histograms or standardized residuals versus fitted values plots, the analysis was repeated after log-transformation of the outcome variable.
Inter-observer reproducibility was assessed by the intraclass correlation coefficient on data measured independently by two experienced observers (A. B. G. and C. M. V. D. H.).
A two-sided P value < 0.05 was considered significant. All data were analysed using R v4.4.0 (R Foundation for Statistical Computing) with packages irr, multcomp, nlme and tidyverse.
Results
Patient characteristics
A total of 61 patients were included in this substudy: 21 randomized to guideline control and 40 randomized to ET, of which 19 were allocated to HIIT and 21 to MCT (Figure 1). Follow-up exercise echocardiography was performed in 34 patients (85%) allocated to training and 17 patients (81%) allocated to guideline control (Figure 1).
Patient characteristics are summarized in Table 1. Patients were older adults (mean age 73 ± 7 years), predominantly female (72% women) and overweight (mean body mass index 30.7 ± 5.9 kg/m2). Typical comorbidities included hypertension (93%), hyperlipidaemia (77%), chronic kidney disease (67%) and atrial fibrillation (38%). Natriuretic peptide levels and HFpEF clinical scores were elevated [median NT-proBNP 363 (243–820) pg/mL, median H2FPEF score 5 (4–6), median HFA–PEFF score 5 (4–6)].
| Control (n = 21) | Training (n = 40) | |
|---|---|---|
| Clinical examination | ||
| Age (years) | 73 ± 8 | 73 ± 6 |
| Sex (n, % female) | 15 (71) | 29 (73) |
| Body mass index (kg/m2) | 30.6 ± 3.7 | 30.7 ± 6.9 |
| New York Heart Association class | ||
| II (n, %) | 11 (52) | 22 (55) |
| III (n, %) | 10 (48) | 18 (45) |
| Systolic blood pressure (mmHg) | 129 ± 16 | 128 ± 15 |
| Diastolic blood pressure (mmHg) | 71 ± 12 | 69 ± 10 |
| Rest heart rate (min−1) | 62 ± 8 | 65 ± 10 |
| Past medical history | ||
| Atrial fibrillation (n, %) | 6 (29) | 17 (43) |
| Chronic kidney disease (n, %) | 12 (57) | 29 (73) |
| Coronary heart disease (n, %) | 5 (26) | 5 (13) |
| Diabetes (n, %) | 5 (24) | 11 (28) |
| Hypertension (n, %) | 20 (95) | 37 (93) |
| Hyperlipidaemia (n, %) | 17 (81) | 29 (74) |
| Sleep apnoea (n, %) | 5 (24) | 9 (23) |
| Medication use | ||
| ACE inhibitor or ARB (n, %) | 16 (76) | 31 (78) |
| Anti-coagulant (n, %) | 6 (28) | 20 (50) |
| Anti-platelet (n, %) | 2 (10) | 2 (5) |
| Beta-blocker (n, %) | 19 (91) | 29 (73) |
| Calcium antagonist (n, %) | 13 (62) | 18 (45) |
| Diuretic (n, %) | 17 (81) | 28 (70) |
| Glucose lowering (n, %) | 3 (14) | 4 (10) |
| Statin (n, %) | 12 (57) | 22 (55) |
| Laboratory analysis | ||
| eGFR (mL/min/1.73 m2) | 62 ± 19 | 59 ± 21 |
| Haemoglobin (g/L) | 128 ± 12 | 131 ± 16 |
| Leukocytes (×106/L) | 6.8 ± 2.1 | 6.4 ± 1.6 |
| NT-proBNP (pg/mL) | 354 (294–1,038) | 392 (230–755) |
| H2FPEF logistic (%) | 90 (67–97) | 94 (70–97) |
| H2FPEF score | 5 (3–6) | 5 (4–6) |
| HFA–PEFF score | 5 (4–6) | 5 (4–6) |
- Note: Continuous variables are represented as mean ± standard deviation and were compared using one-way analysis of variance. Skewed continuous variables are represented as median (25th–75th percentile) and were compared with Kruskal–Wallis test. Categorical variables are represented as n (%) and were compared with Pearson's χ2 test.
- Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker, EGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro B-type natriuretic peptide.
Of note, patients included in this substudy had a more severe HFpEF phenotype compared to the other patients in OptimEx-Clin. They were older, more symptomatic, had more comorbidities and higher NT-proBNP (Table S2).
Baseline exercise echocardiography
Exercise echocardiography was performed until exhaustion in all patients (mean Borg score ≥ 16). Most patients had echocardiographic signs of elevated filling pressures already at rest, including low e′ (mean 5.8 ± 1.6 cm/s), high E/e′ ratio (mean 17.0 ± 5.7), high left atrial volume index (mean 40 ± 14 mL/m2) and high PAPs (mean 31 ± 8 mmHg). In total, 34 patients (60%) had E/e′ ≥ 15 at rest, and 23 patients (51%) had PAPs ≥ 30 mmHg at rest. In contrast, parameters of LV and RV systolic function were within normal ranges at rest (mean LV ejection fraction 61 ± 8%, mean TAPSE 22.6 ± 3.8 mm).
At peak exercise, significant increases were seen in all parameters, except TAPSE (nonsignificant increase, P = 0.051) and RV–vascular coupling (significant decrease, P < 0.001, Table 2). Changes were similar in control and ET group at baseline, although LVEF and MAPSE increase did not reach statistical significance in controls (Table 2).
| Control | Training | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | Time | Group | Interaction | ||
| Exercise | ||||||||
| Workload (W) | Peak | 70 (50–80) | 70 (50–80) | 60 (50–80) | 70 (60–70) | 0.663 | 0.251 | 0.629 |
| Borg dyspnoea scale | Rest | 8 ± 2 | 8 ± 2 | 9 ± 2 | 9 ± 3 | 0.682 | 0.346 | 0.814 |
| Peak | 17 ± 1* | 17 ± 1* | 17 ± 1* | 17 ± 1* | 0.222 | 0.334 | 0.210 | |
| Heart rate (min−1) | Rest | 62 ± 10 | 65 ± 13 | 67 ± 11 | 66 ± 10 | 0.287 | 0.145 | 0.337 |
| Peak | 98 ± 19* | 101 ± 17* | 99 ± 22* | 100 ± 16* | 0.348 | 0.791 | 0.572 | |
| Change rest–peak | 36 ± 16 | 36 ± 16 | 33 ± 19 | 33 ± 13 | 0.807 | 0.572 | 0.954 | |
| Left ventricular systolic | ||||||||
| Left ventricular ejection fraction (%) | Rest | 61 ± 10 | 62 ± 8 | 61 ± 7 | 62 ± 7 | 0.802 | 0.806 | 0.930 |
| Peak | 65 ± 8 | 63 ± 10 | 67 ± 7* | 67 ± 7* | 0.232 | 0.391 | 0.280 | |
| Change rest–peak | 5 ± 9 | 1 ± 5 | 6 ± 10 | 5 ± 7 | 0.170 | 0.599 | 0.375 | |
| Mitral annular plane systolic excursion (cm) | Rest | 1.29 ± 0.15 | 1.26 ± 0.24 | 1.22 ± 0.18 | 1.30 ± 0.19 | 0.569 | 0.316 | 0.177 |
| Peak | 1.45 ± 0.33 | 1.34 ± 0.35 | 1.44 ± 0.32* | 1.48 ± 0.22* | 0.254 | 0.974 | 0.219 | |
| Change rest–peak | 0.16 ± 0.29 | 0.09 ± 0.21 | 0.22 ± 0.25 | 0.17 ± 0.18 | 0.517 | 0.458 | 0.895 | |
| S′ septal (cm/s) | Rest | 5.18 ± 1.20 | 5.28 ± 1.24 | 5.53 ± 1.15 | 5.42 ± 1.21 | 0.852 | 0.278 | 0.521 |
| Peak | 7.11 ± 1.78* | 6.72 ± 2.08* | 7.00 ± 1.65* | 7.25 ± 2.04* | 0.180 | 0.831 | 0.130 | |
| Change rest–peak | 1.93 ± 1.03 | 1.44 ± 1.25 | 1.54 ± 1.42 | 1.81 ± 1.84 | 0.131 | 0.300 | 0.072 | |
| Left ventricular diastolic | ||||||||
| E wave (cm/s) | Rest | 106 ± 25 | 110 ± 45 | 101 ± 26 | 94 ± 43 | 0.762 | 0.700 | 0.535 |
| Maximal | 167 ± 62 | 189 ± 43 | 150 ± 41 | 137 ± 34 | 0.289 | 0.312 | 0.148 | |
| Change rest-maximal | 62 ± 61 | 79 ± 43 | 47 ± 42 | 42 ± 53 | 0.843 | 0.035 | 0.247 | |
| e′ septal (cm/s) | Rest | 5.43 ± 1.39 | 5.72 ± 1.24 | 5.98 ± 1.65 | 5.89 ± 1.53 | 0.624 | 0.312 | 0.665 |
| Maximal | 7.63 ± 3.13* | 8.04 ± 2.30* | 7.93 ± 1.93* | 8.08 ± 2.49* | 0.937 | 0.732 | 0.916 | |
| Change rest-maximal | 2.20 ± 2.98 | 2.32 ± 2.30 | 2.08 ± 1.75 | 2.03 ± 2.97 | 0.731 | 0.899 | 0.900 | |
| E/e′ ratio septal | Rest | 18.68 ± 5.89 | 17.24 ± 7.29 | 16.06 ± 5.52 | 14.99 ± 4.75 | 0.351 | 0.091 | 0.941 |
| Submaximal | 20.30 ± 5.97 | 19.17 ± 9.13 | 17.16 ± 3.35 | 16.67 ± 3.81 | 0.524 | 0.010 | 0.776 | |
| Maximal | 21.70 ± 7.47* | 22.88 ± 9.23* | 18.26 ± 5.01* | 17.23 ± 4.14* | 0.080 | 0.041 | 0.044 | |
| Change rest–maximal | 3.11 ± 4.4 | 5.43 ± 3.7 | 1.75 ± 4.68 | 2.74 ± 3.36 | 0.050 | 0.261 | 0.379 | |
| Left atrial | ||||||||
| A′ septal (cm/s) | Rest | 6.30 ± 1.52 | 6.59 ± 1.89 | 7.01 ± 2.13 | 7.22 ± 1.38 | 0.761 | 0.268 | 0.536 |
| Peak | 8.67 ± 3.80* | 10.92 ± 4.14* | 8.37 ± 2.88* | 10.03 ± 3.69* | 0.603 | 0.814 | 0.577 | |
| Change rest–peak | 2.27 ± 2.97 | 4.39 ± 2.94 | 1.36 ± 2.07 | 2.65 ± 3.21 | 0.156 | 0.392 | 0.672 | |
| Right ventricular | ||||||||
| Systolic pulmonary artery pressure (mmHg) | Rest | 31 ± 8 | 31 ± 12 | 32 ± 8 | 31 ± 11 | 0.705 | 0.671 | 0.517 |
| Peak | 52 ± 13* | 51 ± 10* | 50 ± 10* | 46 ± 11* | 0.785 | 0.395 | 0.304 | |
| Change rest–peak | 21 ± 11 | 19 ± 10 | 18 ± 9 | 14 ± 10 | 0.617 | 0.168 | 0.574 | |
| Tricuspid annular plane systolic excursion (cm) | Rest | 2.29 ± 0.46 | 2.43 ± 0.59 | 2.24 ± 0.33 | 2.34 ± 0.32 | 0.453 | 0.746 | 0.880 |
| Peak | 2.35 ± 0.32 | 2.63 ± 0.61 | 2.43 ± 0.39 | 2.40 ± 0.48 | 0.129 | 0.767 | 0.186 | |
| Change rest–peak | −0.03 ± 0.54 | 0.14 ± 0.76 | 0.18 ± 0.48 | 0.04 ± 0.36 | 0.729 | 0.553 | 0.429 | |
| Right ventricular–vascular coupling (mm/mmHg) | Rest | 0.71 ± 0.18 | 0.69 ± 0.24 | 0.73 ± 0.21 | 0.78 ± 0.19 | 0.841 | 0.953 | 0.314 |
| Peak | 0.45 ± 0.10* | 0.52 ± 0.16* | 0.47 ± 0.16* | 0.55 ± 0.12* | 0.439 | 0.225 | 0.702 | |
| Change rest–peak | −0.28 ± 0.16 | −0.33 ± 0.16 | −0.20 ± 0.20 | −0.22 ± 0.19 | 0.531 | 0.538 | 0.784 | |
- Note: All values displayed as mean ± standard deviation except workload as median (first quartile–third quartile) because of skewed distribution. P values from linear mixed models using patient ID as random effect and time, training group and their interaction as fixed effects. ‘Peak’ denotes the value obtained at peak exercise, ‘maximal’ denotes the highest value obtained during exercise and ‘submaximal’ denotes measurement taken at 50% of the peak load at baseline CPET. Bold and italic: P < 0.05.
- * P < 0.05 when comparing peak/max with rest values (linear mixed models).
Inter-observer reproducibility was assessed for E/e′ and TAPSE: for E/e′ at rest, intraclass correlation was 0.95 (95% confidence interval 0.91–0.98), for maximal E/e′ 0.92 (0.83–0.97), for TAPSE at rest 0.77 (0.53–0.89) and for TAPSE at peak 0.66 (0.42–0.82).
Effect of exercise training
Patients randomized to ET demonstrated excellent adherence: participation in exercise sessions was 88% (77%–95%). Aerobic capacity significantly increased in patients randomized to ET at 3 months compared with controls: V̇O2peak (+2.7 vs. +0.2 mL/kg/min, P-interaction = 0.006), percentage of predicted V̇O2peak (+7% vs. +1%, P-interaction = 0.021) and peak workload (+11 vs. −2 Watt, P-interaction < 0.001) (Table 3). NT-proBNP did not change significantly in patients randomized to control or training (P-interaction = 0.516). Several resting echo parameters (LV end-diastolic diameter, septum thickness and mass index) changed significantly in the control group at 3 months (Table S3).
| Control | Training | P value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | Time | Group | Interaction | |
| V̇O2peak (mL/kg/min) | 17.3 ± 4.7 | 17.5 ± 5.5 | 16.8 ± 5.7 | 19.5 ± 5.6 | 0.318 | 0.797 | 0.006 |
| Percent predicted V̇O2peak (%) | 87 ± 20 | 88 ± 25 | 85 ± 26 | 92 ± 22 | 0.354 | 0.976 | 0.021 |
| Peak workload (W) | 86 ± 26 | 84 ± 28 | 81 ± 29 | 92 ± 23 | 0.111 | 0.449 | <0.001 |
| Peak heart rate (bpm) | 109 ± 21 | 110 ± 22 | 111 ± 22 | 118 ± 19 | 0.980 | 0.508 | 0.269 |
| Peak systolic blood pressure (mmHg) | 169 ±28 | 170 ±23 | 169 ±26 | 169 ±24 | 0.576 | 0.917 | 0.559 |
| V̇E/V̇CO2 slope | 30.8 ± 5.7 | 30.3 ±3.5 | 30.6 ± 3.6 | 31.3 ±6.0 | 0.859 | 0.587 | 0.987 |
- Note: P values from linear mixed models using patient ID as random effect and time, training group and their interaction as fixed effects. Bold and italic: P < 0.05.
- Abbreviations: V̇CO2, carbon dioxide production; V̇E, ventilation; V̇O2, oxygen uptake.
A significant difference in exercise E/e′ ratio was seen between ET and control groups at 3 months (Table 2, Figure 2A). This was due to an exaggerated E/e′ increase during exercise in controls. Also, E wave was significantly higher in controls overall (P-time = 0.035, P-interaction however did not reach statistical significance). No other between-group differences were found for resting or exercise echocardiography measurements (Table 2, Figure 2B–D).
Four patients had AF at baseline and follow-up. Additionally, two patients had sinus rhythm at baseline but AF at follow-up; sensitivity analysis excluding these two patients did not change our findings.
Associations with training response
In total, 60% of patients meaningfully increased V̇O2peak after training (>6% increase).17 Compared with those with <6% increase, at baseline, these ‘responders’ had lower body mass index (28.8 ± 4.5 vs. 34.1 ± 9.0 kg/m2, P = 0.023), lower TAPSE at rest (20.9 ± 2.9 vs. 24.6 ± 2.6 mm, P = 0.022) and lower TAPSE/PAPs ratio at rest (0.55 ± 0.15 vs. 0.87 ± 0.05 mm/mmHg, P = 0.010). Of note, 93% of non-responders were on a beta-blocker, compared with 59% of responders (P = 0.054). Other patient characteristics and echo parameters were similar.
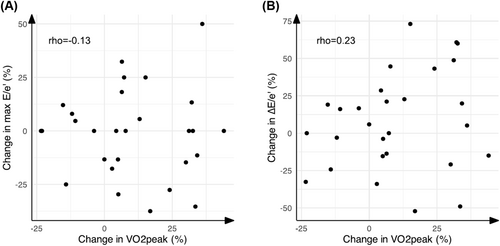
In univariate logistic regression, only few parameters were associated with the training-induced V̇O2peak increase body mass index, beta blocker use and TAPSE (Table S4). None of these variables was significantly associated with the V̇O2peak increase in multivariate logistic regression analysis (Table S4). Changes in exercise echocardiographic parameters between baseline and 3 months did not correlate with the change in V̇O2peak (all P > 0.05, all rho < 0.5). More specifically, changes in E/e′ did not relate to the V̇O2peak training response (Figure 3).
Discussion
This substudy of HFpEF patients included in the OptimEx-Clin trial is the largest to report on the effect of training on exercise echocardiography parameters in HFpEF and the first to include diastolic function parameters. We report the following main findings: (1) while parameters of LV and RV systolic function were both within normal ranges at rest, at peak exercise, LV systolic function increased whereas RV systolic function did not, leading to poor RV–vascular coupling. (2) Despite good adherence to training, patients randomized to ET had only a small difference in exercise E/e′ compared with control at 3 months. (3) The V̇O2peak response to ET was not associated with exercise echocardiography parameters.
High filling pressures at rest
More than half of the patients included in this study already had evidence of elevated filling pressures at rest, including high E/e′ ratio (60%) and high PAPs (51%), indicating a relatively ‘advanced’ stage of HFpEF in our population. This is certainly influenced by the inclusion criteria for OptimEx-Clin, which mandated either resting E/e′ ≥ 15 or E/e′ 8–15 with elevated natriuretic peptides.12 Additionally, the patients included in this substudy demonstrated more advanced disease than those not included in the substudy (Table S2) reflected by older age, higher prevalence of chronic kidney disease, higher NT-proBNP levels, more advanced NYHA class, lower V̇O2peak and higher HFA–PEFF scores. This could arise from differences in referral patterns or regional differences in healthcare practice (lack of NT-proBNP reimbursement in Belgium could favour inclusion by echo criteria and thus more advanced disease).
However, other HFpEF randomized clinical trials found a similar number of patients with elevated LV filling pressures. For example in the Reduce Elevated Left Atrial Pressure in Patients With Heart Failure II (REDUCE-LAP-HF-II) trial, 71% of patients had elevated LV filling pressures at rest.21 Patients with ‘early’ HFpEF may present with normal LV filling pressures at rest, as such our findings cannot be extrapolated to this subset of patients. Indeed exercise echocardiography has been mostly advocated as a means to enhance diagnosis of HFpEF at an early stage, as it is known that exercise may unmask discrepant elevation of filling pressures during exercise non-invasively.22-24
Importance of RV reserve during exercise in HFpEF
While LV and RV systolic function parameters were within normal ranges in most patients at rest, during exercise, HFpEF patients demonstrated impaired RV reserve (failure to increase TAPSE) despite increasing PAPs. Thus, RV–vascular coupling measured by TAPSE/PAPs was severely impaired in this population. Borlaug et al. previously demonstrated impaired RV–vascular coupling in HFpEF patients despite preserved RV function at rest using exercise right heart catheterization.25 Of note, the authors found that abnormal RV–vascular coupling was strongly correlated with biventricular filling pressures. The TAPSE/PAPs ratio, while being a broad indicator of RV–vascular coupling, is of prognostic value in HF and may discern different HFpEF phenotypes.26
It is known that RV structure and function deteriorates over time after a diagnosis of HFpEF.27 Unfortunately, ET did not improve RV resting or exercise parameters in our ‘advanced’ HFpEF population. However, it cannot be excluded that at earlier stages of HFpEF, modulation of RV function and RV–vascular coupling is still possible.
Improvements by training in HFpEF
Despite the relatively ‘advanced’ HFpEF population included in the present substudy, patients had good adherence to the ET protocol and significantly increased V̇O2peak (+2.7 mL/kg/min). Nevertheless, only small changes in exercise echocardiography parameters were seen after 3 months in patients randomized to ET, and the changes in E/e′ ratio did not correlate to V̇O2peak increase. Thus, it is unlikely that these are responsible for the increase in aerobic capacity. Haykowsky et al. previously performed exercise echocardiography in HFpEF patients before and after training and found that increases in arteriovenous oxygen difference could explain most of the changes induced by training.7 The current study adds to the knowledge that changes in LV diastolic function are likely not responsible for the increase in exercise capacity after training.
We have previously demonstrated that training had no effect on vascular function and HIIT induced important changes in skeletal muscle: reduced markers of muscle atrophy, increased mitochondrial enzyme activity and expression and increased amount of satellite cells.28, 29 Of note, the patients included in the skeletal muscle study and the exercise echocardiography study overlap. These changes occurred despite a focus on aerobic ET in OptimEx-Clin while resistance training or combined training may provide incremental benefit in patients with cardiovascular disease.30 The Personalized Remotely Guided Preventive Exercise Therapy in HFpEF (PRIORITY) study will further explore the effects of combined aerobic and resistance training in HFpEF patients.31 In the current study, we demonstrate that cardiac parameters remained mostly unchanged; thus, it is most likely that the V̇O2peak increase is mediated by changes in peripheral oxygen extraction at the level of the skeletal muscle.32
As not all HFpEF patients increase V̇O2peak meaningfully by training, we explored whether we could predict which patients would respond favourably using baseline parameters. None of the baseline clinical, resting echocardiography or exercise echocardiography parameters could predict the V̇O2peak response to training. Previously, we demonstrated that peak O2 pulse (by CPET) and microRNA-181c (by polymerase chain reaction) could predict the V̇O2peak response in the same population.15, 33
The overall high prevalence of comorbidities underlines the multifactorial nature of HFpEF. Even subtle impact of ET on these comorbidities might contribute to improved exercise tolerance. We have previously demonstrated that the OptimEx-CLIN population is phenotypically heterogeneous, reflecting a general variation in HFpEF patient characteristics which is seen in all HFpEF studies.34, 35 Training response could certainly vary according to patient phenotype; however, the sample size of the current substudy precludes further subdivision for such an analysis.
Limitations
Our findings need to be interpreted in the light of the following limitations. Sample size calculation for OptimEx-Clin was based on the primary endpoint (change in V̇O2peak at 3 months). Given the sample size and the precision of the measured variables, it is not possible to completely rule out a minor effect of ET on cardiac function, particularly within specific patient subgroups. However, to the best of our knowledge, this study represents the largest investigation to date comparing exercise echocardiography before and after ET in patients with HFpEF. Even in populations with HFrEF or coronary artery disease, available datasets on exercise echocardiography pre- and post-training interventions are notably smaller. We cannot rule out that a longer ET intervention could lead to more favourable effects on cardiac function.
We have pooled the MCT and HIIT training groups to increase statistical power. While neither the main study nor most substudies have demonstrated relevant differences between MCT and HIIT groups,11, 15, 29, 33, 34 in theory, a significant effect in either training modality could be influenced by a neutral effect in the other training modality.
Body habitus of the HFpEF population precluded accurate measurement of PAPs in a subset of patients. RV fractional area change was not measured: image acquisition during exercise was constrained in HFpEF patients with limited exercise tolerance. Anticipating poor RV-focused views in elderly and overweight patients, we prioritized to obtain high-quality TAPSE measurements during the exercise echocardiography assessment. Also, image quality was insufficient for measuring speckle-tracking strain in the majority of patients. We did report the more easily obtained MAPSE and septal S′. These measurements are not as dependent on image quality as the speckle-tracking strain, and they equally reflect longitudinal LV function.36
Diagnosis of HFpEF has evolved significantly since the start of the OptimEx-CLIN study in 2014. Diagnostic HFpEF scores did not exist yet, and thus, our inclusion criteria were based on the European Society of Cardiology 2012 guidelines.37 Nevertheless, we demonstrate in Table 1 that the study population has a diagnosis of HFpEF also according to more recent diagnostic scores.
Finally, our study protocol did neither include the measurement of stroke volume, which would have allowed the calculation of cardiac output, nor simultaneous measurement of V̇O2 and echocardiographic parameters during exercise, which could provide further insight in the discrimination of cardiac versus peripheral limitations of patients with HFpEF, but was not the focus of the current study.22
Conclusions
We performed exercise echocardiography in HFpEF patients who were recruited for the OptimEx-Clin study and randomized to ET or guideline control. While LVEF and RV parameters were within normal range at rest, patients were unable to increase RV systolic function during exercise, resulting in impaired RV–vascular coupling. ET increased V̇O2peak after 3 months, whereas only minor changes in diastolic reserve were observed (smaller increase in E/e′ during exercise). Furthermore, the E/e′ change was not related to the V̇O2peak response. These minor cardiac changes contrast with the important molecular and structural effects of ET in skeletal muscle, which we have previously demonstrated in the same population. As such, the V̇O2peak response to training in HFpEF patients cannot be explained by improved diastolic function or other cardiac changes determined by exercise echocardiography.
Acknowledgements
The authors thank the staff of the University Hospital ‘Klinikum rechts der Isar’ of the Technical University of Munich, Antwerp University Hospital, Heart Centre Leipzig and Charité Universitätsmedizin Berlin who were involved in recruitment, evaluation, administration and support during the conduct of the OptimEx-Clin study.
Conflict of interest statement
A. B. G. reported receiving research grants to his institution by Abbott and Boehringer Ingelheim outside the submitted work and lecture/advisory fees paid to his institution by Abbott, AstraZeneca, Boehringer Ingelheim, Johnson and Johnson, Novartis, Novo Nordisk and Menarini outside the submitted work. E. B. W. reported grants from Boehringer Ingelheim and personal fees from Amarin, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, CVRx, Daiichi Sankyo, Novartis and Pfizer outside the submitted work. S. M. reported receiving personal fees from Bristol-Myers Squibb outside the submitted work. B. P. reported receiving personal fees from AstraZeneca (lectures), Bayer (steering committee, lectures), Bristol-Myers Squibb (lectures), Medscape (lectures), Merck (steering committee, lectures), Novartis (steering committee, lectures) and Servier outside the submitted work. M. H. reported receiving grants from Novartis and personal fees from Abbott (advisory board), AstraZeneca, Bayer, Berlin Chemie-Menarini, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Novartis, Roche, Sanofi and Pfizer and serves as an advisor for Medical Park SE, Germany, outside the submitted work. E. M. V. C. reported receiving grants from Vifor Pharma outside the submitted work. No other disclosures were reported. C. M. V. D. H. reported receiving personal fees from Abbott, Daiichi-Sankyo, Bayer and Edwards Lifesciences (lectures) outside the submitted work.
Funding
This work was supported by the European Commission, Framework Program 7 (EU-FP7; Brussels, Belgium) (grant number: EU 602405-2); the Deutsche Forschungsgemeinschaft (DFG) through the TUM International Graduate School of Science and Engineering (IGSSE; Garching, Germany) (S. M., M. H.); University of Antwerp (BOF DOCPRO4 51265 to S. D. S.); and the Flanders Research Foundation (FWO; Brussels, Belgium) (senior clinical investigator grant to E. M. V. C.). The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Open Research
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.




