Cardiac myosin binding protein C correlate with cardiac troponin I during an exercise training program in patients with HFrEF
Abstract
Background
Cardiac myosin binding protein C (cMyC) is an emerging new biomarker of myocardial injury rising earlier and cleared faster than cardiac troponins. It has discriminatory power similar to high-sensitive troponins in diagnosing myocardial infarction in patients presenting with chest pain. It is also associated with outcome in patients with acute heart failure. It is currently unclear how it relates to cardiac troponins in patients with chronic heart failure undergoing exercise training.
Methods and results
This is a post hoc analysis of symptomatic heart failure patients in the multicentre randomized SMARTEX trial. Patients were randomized to one of three arms: high-intensity interval training, moderate continuous training and recommendation of regular exercise serving as control group (CG) for 12 weeks. As the training load in the two intervention arms was similar, these patients were merged and constituted the intervention group (IG). Clinical data and measurements were obtained at baseline and at 12 weeks. In 205 patients, serum was available for cMyC testing and in 196 patients, serum was available for hs-cTni testing. Due to non-normal distribution, cMyC and hs-cTnI measurements were log-transformed. A Bland–Altman plot was employed to evaluate the agreement of cMyC with hs-cTnI measurements. Lastly, a linear regression model was applied. No significant differences were observed in the change of cMyC levels between the groups throughout the intervention period (∆ cMyC IG: −0.5 [IQR: −3.4; 2.1] vs. ∆ cMyC CG: −0.7 [IQR: −2.7; 2.6]). The change in log hs-cTnI was significantly correlated with the change in log cMyC during the 12-week intervention period, with a Pearson correlation coefficient of R = 0.52 (95% CI 0.37–0.66, P < 0.001). For every 10% increase in cMyC levels, hs-cTnI levels rose by approximately 5%.
Conclusions
Changes in levels of the novel biomarker cMyC were significantly associated with hs-cTnI serum levels in patients with symptomatic chronic HFrEF during a structured 12-week exercise training programme. This may indicate that cMyC has a role as a future marker of subclinical myocardial damage.
Introduction
Cardiac myosin binding protein C (cMyC), a part of the cardiac contractile apparatus, is an emerging novel biomarker of myocardial injury. It rises earlier and is cleared faster from plasma than cardiac troponins (cTn) and is as such a promising tool for clinical use.1-5 In triage of chest pain patients, cMyC has been demonstrated to have discriminatory power similar to high-sensitive cardiac Troponin (hs-cTn).5 Additionally, it is associated with outcome in heart failure (HF) but with slightly lower predictive power than the clinical gold standard of N-terminal B-type natriuretic peptide (NT-proBNP).6 Experimental studies in knockout mice indicate that cMyC may also have a role in the pathogenesis of HF.7 Other studies show that cMyC is degraded into an N-terminal 40-kDa fragment (C0–C1f) following MI and this fragment is released into the circulation.8 This degradation fragment is associated with significant contractile dysfunction in HF. Furthermore, in animal models, cMyC has been shown to trigger the production of autoantibodies that generate experimental autoimmune myocarditis and dilated cardiomyopathy.9, 10
In contrast to cTn, cMyC does not increase over upper reference limit after strenuous exercise like a marathon run.11
We have previously demonstrated that exercise training is associated with reduction of serum cTn levels in patients with symptomatic HF with reduced ejection fraction.12, 13 However, there are no data pertaining to cMyC in this context, and we do not know if the serum levels parallel those of cTn.
The aim of this investigation was to assess the association between cMyC and hs-cTnI in patients with HFrEF during a 12-week structured exercise training programme.
Methods
This was a post hoc analysis of 215 patients with symptomatic HF with left ventricular ejection fraction (LVEF) < 35% and NYHA II-III enrolled in the SMARTEX-HF trial.14 Methods have been presented in the main study, but in brief patients were randomly assigned to one of three training groups: high-intensity interval training (HIIT, n = 77), moderate continuous training (MCT, n = 65) or recommendation of regular exercise (RRE) (n = 73) control group (CG), for 12 weeks. As the training load in the two intervention arms was similar, the HIIT and MCT groups were merged and served as the intervention group (IG). Clinical data and required measurements were acquired before and after the 12-week period of intervention. In 205 patients, serum was available for cMyC testing, and in 196 patients, serum was available for hs-cTnI testing. hs-cTnI was measured in serum using a commercially available assay hs-cTnI from Abbott Diagnostics, Illinois, USA (STAT Troponin-I analysed on an Abbott Architect SR2000i). The analytical range was 2.0–500 000 ng/L. cMyC was measured on the Erenna® platform by EMD Millipore Corporation, Hayward California: lower limit of detection 0.4 ng/L and lower limit of quantification (LLoQ) of 1.2 ng/L (LLoQ at 20% coefficient of variation [CV]).15
For the analysis, all cMyC and hs-cTnI measurements were log-transformed. A Bland–Altman plot was used to evaluate the agreement between the cMyC and hs-cTnI measurement methods. Linear regression models were applied to assess the relationship between changes in hs-cTnI values (outcome) and either baseline cMyC levels or changes in cMyC from baseline to follow-up (predictors), adjusting for age, aetiology and training group.
Results
There was no statistically significant difference between groups in change of cMyC levels during the intervention period (∆ cMyC IG: −0.5 [IQR: −3.4; 2.1] vs. ∆ cMyC CG: −0.7 [IQR: −2.7; 2.6]).
There was also no significant change of cMyC during the intervention period neither in the entire population nor within groups.
For further analysis, cMyC and hs-cTnI values were log-transformed to stabilize variance and approximate a normal distribution. Bland–Altman plots were used to assess the agreement between Log (cMyC) and Log (hs-cTnI) at baseline and follow-up. These plots are presented in Figure 1.
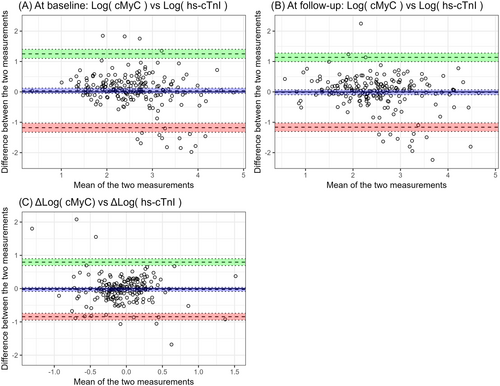
The (A) and (B) subfigures demonstrate poor agreement between hs-TnI and cMyC both at baseline and at follow-up, with a high prevalence of outliers.
In an adjusted model including aetiology, age, training group and change in log cMyC, only the latter is statistically predicting changes in hs-cTnI (Table 1) as illustrated in the scatter plot in Figure 2.
| Predictors | Model 1: Change in log hs-cTnI | Model 2: Change in log hs-cTnI | ||||
|---|---|---|---|---|---|---|
| Estimates | CI | P | Estimates | CI | P | |
| Baseline cMyC [log] | −0.05 | −0.14–0.04 | 0.232 | |||
| Change in log cMyC | 0.52 | 0.37–0.66 | <0.001 | |||
| Training group | −0.09 | −0.21–0.04 | 0.174 | −0.08 | −0.19–0.04 | 0.186 |
| Ischaemic aetiology | −0.05 | −0.18 – 0.07 | 0.413 | −0.04 | −0.16–0.07 | 0.448 |
| Age | 0.01 | −0.00–0.01 | 0.051 | 0.00 | −0.00–0.01 | 0.340 |
| Observations | 191 | 189 | ||||
| R2/R2 adjusted | 0.033/0.013 | 0.236/0.220 | ||||
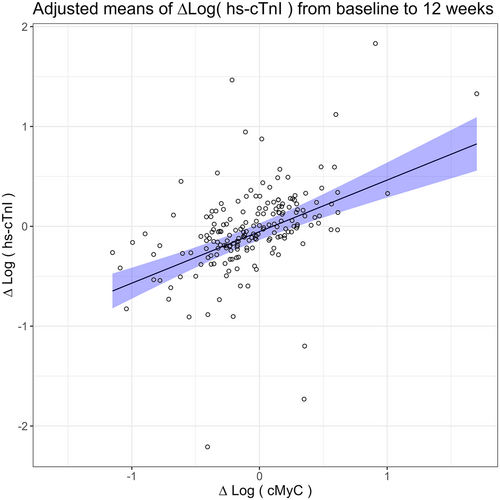
The change in log hs-cTnI and change in log cMyC correlated significantly (Pearson correlation R = 0.52 [95% CI 0.37–0.66], P < 0.001), during the intervention period of 12 weeks. For any 10% increase in cMyC level, the hs-cTnI level increased with approximately 5%.
Brief discussion
Few, if any, studies have investigated cMyC kinetics in HFrEF patients during an exercise training period. Our findings of a significant correlation between hs-cTnI and cMyC further suggest the potential clinical value of this novel biomarker also in HFrEF. In the early stage of myocardial infarction, cMyC has been shown to be detected earlier and has a steeper increase compared to hs-cTnI. This could indicate that cMyC might be more sensitive than cardiac troponins in early diagnosis of myocardial infarction.2, 4, 5 For HF patients, even slight elevations of biomarkers of cardiac injury are of prognostic significance.16 Whether cMyC is a more sensitive biomarker of subclinical myocardial injury in HFrEF is currently not known.
Altered levels of this novel biomarker might also affect parts of the pathways in the progression of HF.17 In experimental studies, during myocardial injury caused by ischaemia and reperfusion, cMyC is cleaved, releasing a C0–C1f fragment, which has been linked to detrimental effects on the myocardium.18, 19 Mutations of the gene, MYBPC3, encoding this protein is one of the preeminent causes of genetic cardiomyopathies in humans. In a study by Lynch and Sadayappan20 transgenic mice, completely lacking this region of the cMyC protein displayed dilated cardiomyopathy and increased fibrosis as well as altered myoarchitectural features and altered sarcomere contractility. When reintroduced in the form of C0–C2 domains, the myofilament function was restored. In vitro, the C0–C1f fragment induces depressed myofilament action and Ca2+ handling contributing to development of heart failure.21, 22 Whether this fragment is also present in sufficient levels to have a detrimental effect on myocardium in our study is not known, and future studies are warranted.
Conclusions
Changes in levels of the novel biomarker cMyC were significantly associated with hs-cTnI serum levels in patients with symptomatic chronic HFrEF during a structured 12-week exercise training programme. This may indicate that cMyC has a role as a future marker of subclinical myocardial damage.




