Frailty determinants in heart failure: Inflammatory markers, cognitive impairment and psychosocial interaction
Abstract
Aims
This study aimed to identify factors associated with frailty in heart failure (HF) patients, focusing on demographic, biochemical and health-related variables. It also explored the correlation between frailty and comorbidities such as malnutrition, cognitive impairment and depression, assessing how these factors interact to influence frailty risk.
Methods
A total of 250 HF patients (mean age 73.5 ± 7.2 years; 45.6% female) hospitalized for acute decompensated HF were included. Frailty was assessed using Fried phenotype criteria. Cognitive function, depression and nutritional status were evaluated using validated instruments [Mini-Mental State Examination (MMSE), Patient Health Questionnaire-9 (PHQ-9) and Mini Nutritional Assessment (MNA)]. Biochemical markers included C-reactive protein (CRP), N-terminal prohormone of brain natriuretic peptide (NT-proBNP), haemoglobin, estimated glomerular filtration rate (eGFR) and systolic blood pressure (SBP). Statistical analyses, including logistic regression, were performed to assess associations and odds ratios (ORs) for frailty, adjusted for inflammation and HF type.
Results
Frailty was present in 60.4% of patients. Frail individuals exhibited significantly higher CRP (median 4.60 vs. 2.54 mg/L, P < 0.001) and NT-proBNP (median 2558.8 vs. 1102.6 pg/mL, P = 0.001) and lower haemoglobin (13.7 vs. 14.3 g/dL, P = 0.012), eGFR (62 vs. 71 mL/min/1.73 m2, P = 0.025) and SBP (130 vs. 134 mmHg, P = 0.026). Each 10% increase in CRP was associated with a 5.5% increase in frailty odds (P < 0.001). Frailty was linked to cognitive impairment (OR 2.1, P = 0.018), malnutrition (OR 3.0, P < 0.001) and depression (OR 3.1, P < 0.001), while high adherence to treatment reduced frailty risk by 78.9% (P = 0.027). Interactions were observed between cognitive impairment and body mass index (BMI) (P = 0.020), where higher BMI mitigated the frailty odds difference between cognitively impaired and unimpaired patients. Depression's association with frailty odds varied by adherence levels (P = 0.034) and central obesity (P = 0.047), with the absence of depression offering protection against frailty in patients with central obesity. These interactions remained significant after adjustment for HF type and left ventricular ejection fraction (LVEF) and were consistent across stratifications by these factors.
Conclusions
Frailty in HF is influenced by inflammatory markers, cognitive impairment and psychosocial factors. Elevated CRP and NT-proBNP were strong predictors of frailty. Cognitive impairment and depression were key modifiable factors, interacting with BMI, adherence and obesity. Targeting these factors with early interventions could mitigate frailty risk, improving outcomes and quality of life in HF patients.
Introduction
Frailty is a multifaceted, intricate syndrome that manifests as a decrease in physiological reserves in the cognitive, physical and social domains, rendering individuals more vulnerable to acute stressors. In patients with heart failure (HF), frailty is associated with adverse outcomes, such as an elevated risk of disability, mortality and hospitalization.1 This vulnerability is ascribed to factors including sarcopenia, heightened inflammatory responses and neurohormonal dysregulation, prevalent in patients with HF, with frailty impacting 40%–80% based on the HF subtype.1 In HF with preserved ejection fraction (HFpEF), frailty prevalence is notably elevated, impacting up to 90% of patients, attributable to advanced age and increased comorbidity burden.2 Patients with HF with reduced ejection fraction (HFrEF) also demonstrate frailty, with prevalence rates ranging from 30% to 60%.3
Frailty is widely acknowledged as a unique biological illness, independently linked to considerable physical and cognitive deficits, regardless of age or other concomitant conditions.4 In HF, frailty has been found to elevate all-cause mortality and hospitalizations by 1.5–2 times.5 Indeed, frailty may be a more significant predictor of negative outcomes in HF than conventional cardiovascular risk factors,6 highlighting its critical role in disease management. The relationship between HF and frailty involves several interrelated pathophysiological mechanisms. Chronic inflammation in HF, marked by elevated cytokines, promotes muscle catabolism, contributing to sarcopenia and frailty.7 In addition to these considerations, HF induces a pro-inflammatory condition via neurohormonal and adrenergic overstimulation, which exacerbates sarcopenia—muscle atrophy that further deteriorates frailty by reducing muscular mass and strength.8 Neurohormonal activation, particularly of the renin–angiotensin–aldosterone system and sympathetic nervous system, further worsens frailty by increasing muscle wasting. HF also causes skeletal muscle changes, including fibre type shifts and mitochondrial dysfunction, which reduce strength and endurance.7 Chronic congestion and hypoxia resulting from HF, together with comorbidities such chronic kidney disease, contribute to multi-organ dysfunction and diminished physiological reserves, intensifying frailty.9
In patients with HF, frailty is correlated with higher biomarkers, such as natriuretic peptides and high-sensitivity troponin, signifying persistent myocardial damage and heightened risk of unfavourable clinical outcomes.10 Malnutrition, frequently caused by intestinal oedema, malabsorption and cognitive impairment, exacerbates frailty, especially when aggravated by dietary restrictions and depression.11 Nutritional inadequacies in patients with HF are particularly detrimental, resulting in early satiety and diminished meal consumption, hence exacerbating frailty.12 In addition to physical frailty, cognitive frailty, which is a combination of physical frailty and cognitive impairment without dementia, is present in one out of every four elderly patients with HF and is associated with an increase in mortality and hospitalizations.13 Social frailty, impacting two thirds of elderly patients with HF, is correlated with worse symptoms, increased mortality and elevated HF-related hospitalizations.14 Psychological frailty, encompassing sadness and anxiety, significantly contributes to deteriorating outcomes, resulting in heightened hospital admissions and mortality rates.15
Considering the complex nature of frailty and its significant effect on patients with HF, this study seeks to examine the factors that contribute to frailty in HF, encompassing demographic, biochemical and health-related variables. Additionally, it will examine the correlation between frailty and comorbidities, including malnutrition, cognitive impairment and depression. Furthermore, the study will evaluate the interaction of these factors on frailty risk, aiming to guide interventions that may alleviate the burden of frailty in patients with HF. By exploring these interactions, the study allows for addressing the existing gap in knowledge, ultimately contributing to a better understanding of frailty in HF and guiding future interventions.
Materials and methods
Participants
The study included 250 patients with HF who were admitted to the cardiology department due to acute decompensated HF. To be eligible for participation, patients had to meet the following criteria: age 60 or older, a confirmed diagnosis of HF based on European Society of Cardiology (ESC) guidelines,16 HF duration of at least 6 months, New York Heart Association (NYHA) Functional Classes II–IV, hospitalization for acute HF, absence of active infections such as pneumonia, no active cancer and preserved cognitive function as measured by a Mini-Mental State Examination (MMSE) score of 24 or higher. Exclusion criteria consisted of NYHA Class I, an MMSE score below 24, diagnosed and treated depressive disorders, or refusal to participate in the study.
Data collection
Patients were enrolled from the Institute of Heart Diseases at the University Hospital in Wrocław, Poland, between September 2022 and June 2023. Trained professionals gathered data from the patients using a standardized protocol. This information was collected during their hospitalization, following the successful treatment of acute decompensated HF, with clinical stability confirmed before discharge. Clinical data, including biomarker levels (Table 1), were obtained from the patients' electronic medical records to ensure comprehensive analysis. The study adhered to the STROBE guidelines for observational research.
|
Variable (quantitative) |
Risk of frailty (n = 99) | Frailty (n = 151) | P | ||||
|---|---|---|---|---|---|---|---|
| Median | 1st Q | 3rd Q | Median | 1st Q | 3rd Q | ||
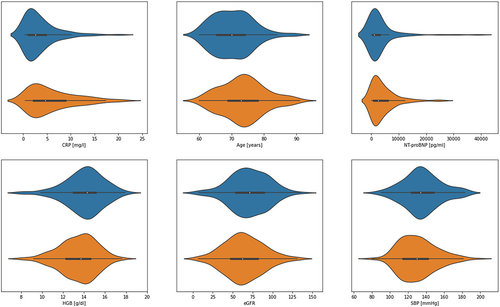
| CRP (mg/L) | 2.54 | 1.07 | 4.77 | 4.60 | 2.15 | 8.96 | <0.001 |
| Age (years) | 70.00 | 65.00 | 74.00 | 73.00 | 69.00 | 78.00 | <0.001 |
| NT-proBNP (pg/mL) | 1102.60 | 605.40 | 3001.50 | 2558.80 | 943.90 | 5937.20 | 0.001 |
| HGB (g/dL) | 14.30 | 13.00 | 15.10 | 13.70 | 12.30 | 14.60 | 0.012 |
| eGFR | 71.00 | 54.00 | 89.00 | 62.00 | 47.00 | 82.00 | 0.025 |
| SBP (mmHg) | 134.00 | 123.00 | 149.00 | 130.00 | 114.00 | 142.00 | 0.026 |
| DBP (mmHg) | 80.00 | 70.00 | 88.00 | 76.00 | 70.00 | 85.00 | 0.080 |
| CREA (mg/dL) | 1.05 | 0.84 | 1.24 | 1.11 | 0.88 | 1.44 | 0.098 |
| Mean education length (years) | 12.00 | 10.00 | 14.00 | 11.00 | 10.00 | 14.00 | 0.138 |
| BMI (kg/m2) | 28.07 | 25.35 | 32.74 | 27.73 | 24.62 | 32.61 | 0.355 |
| BUN (mg/dL) | 45.50 | 35.00 | 58.00 | 44.50 | 37.00 | 68.00 | 0.361 |
| WC (cm) | 103.00 | 93.00 | 111.00 | 104.00 | 95.00 | 116.00 | 0.414 |
| LVEF | 0.45 | 0.33 | 0.58 | 0.44 | 0.32 | 0.55 | 0.448 |
| HR (b.p.m.) | 76.00 | 66.00 | 84.00 | 75.00 | 67.00 | 85.00 | 0.735 |
|
Variable: Category (qualitative) |
Observed n | Frequency (%) | Observed n | Frequency (%) | P |
|---|---|---|---|---|---|
| Nutritional status: Risk of malnutrition/malnutrition | 21 | 21.21 | 73 | 48.34 | <0.001 |
| Depression: Yes | 37 | 37.37 | 98 | 64.90 | <0.001 |
| NYHA: II | 54 | 54.55 | 55 | 36.42 | 0.001 |
| NYHA: III | 38 | 38.38 | 61 | 40.40 | |
| NYHA: IV | 7 | 7.07 | 35 | 23.18 | |
| Cognitive impairment: Yes | 17 | 17.17 | 46 | 30.46 | 0.018 |
| Adherence: High | 62 | 62.63 | 73 | 48.34 | 0.027 |
| Diabetes: Yes | 46 | 46.46 | 91 | 60.26 | 0.032 |
| CAD: Yes | 70 | 70.71 | 89 | 58.94 | 0.059 |
| Tumour disease: Yes | 12 | 12.12 | 31 | 20.53 | 0.085 |
| Peptic ulcers: Yes | 8 | 8.08 | 23 | 15.23 | 0.093 |
| Connective tissue diseases: Yes | 17 | 17.17 | 39 | 25.83 | 0.108 |
| Comorbidity severity: Mild | 6 | 6.06 | 12 | 7.95 | 0.262 |
| Comorbidity severity: Moderate | 35 | 35.35 | 39 | 25.83 | 0.262 |
| Comorbidity severity: Severe | 58 | 58.59 | 100 | 66.23 | 0.262 |
| Professional activity: Active | 15 | 15.15 | 16 | 10.60 | 0.285 |
| CVDs in the family: Yes | 31 | 31.31 | 38 | 25.17 | 0.288 |
| Renal diseases: Yes | 36 | 36.36 | 65 | 43.05 | 0.292 |
| Civil status: Single | 30 | 30.30 | 55 | 36.42 | 0.318 |
| Sex: Male | 72 | 72.73 | 101 | 66.89 | 0.328 |
| COPD/asthma: Yes | 15 | 15.15 | 30 | 19.87 | 0.343 |
| CS: Yes | 15 | 15.15 | 18 | 11.92 | 0.460 |
| Central obesity: Yes | 80 | 80.81 | 126 | 83.44 | 0.592 |
| Location: Village | 23 | 23.23 | 39 | 25.83 | 0.642 |
| Alcohol consumption: Yes | 4 | 4.04 | 8 | 5.30 | 0.649 |
| HT: Yes | 89 | 89.90 | 133 | 88.08 | 0.655 |
| Liver diseases: Yes | 9 | 9.09 | 16 | 10.60 | 0.698 |
| Smoking: Past/present | 56 | 56.57 | 89 | 58.94 | 0.710 |
| MI: Yes | 38 | 38.38 | 61 | 40.40 | 0.750 |
| HFpEF | 39 | 39.39 | 60 | 39.74 | 0.823 |
| HFmrEF | 20 | 20.20 | 26 | 17.22 | |
| HFrEF | 40 | 40.40 | 65 | 43.05 |
- Note: Differences were tested with the Mann–Whitney U test (quantitative variables) or the χ2 test (qualitative variables).
- Abbreviations: BMI, body mass index; BUN, blood urea nitrogen; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CREA, creatinine; CRP, C-reactive protein; CS, cardiac surgery; CVDs, cardiovascular diseases; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HGB, haemoglobin; HR, heart rate; HT, hypertension; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; Q, quartile; SBP, systolic blood pressure; WC, waist circumference.
Research instruments
The MMSE is a commonly employed instrument for evaluating cognitive function.17 The MMSE covers various cognitive domains, including orientation to time and place, memory, attention, language and executive functioning. It consists of tasks and questions designed to evaluate skills such as memory recall, attention span, following simple commands and language use. The maximum score on the MMSE is 30 points, with scores below 24 suggesting possible cognitive impairments, including dementia, warranting further assessment. A Polish adaptation of the MMSE was used in this study.18
The Polish version of the Patient Health Questionnaire-9 (PHQ-9) was used to measure depression. The PHQ-9 has proven to be both a reliable and valid instrument for evaluating depression severity across various settings, such as primary care and specialized medical fields.19, 20 It contains nine basic questions, making it a comprehensive tool for assessing depression. The patient is asked about the occurrence of various symptoms and their frequency in the past 2 weeks. The total score ranges from 0 to 27. The higher the score, the greater the severity of depression. A score below 5 indicates no depression. The interpretation of the scores is as follows: no depression 0–4 points; mild depression 5–9 points; moderate depression 10–14 points; moderately severe depression 15–19 points; and severe depression 20–27 points.
Adherence in Chronic Diseases Scale (ACDS)
The level of treatment adherence was evaluated using the ACDS, specifically created for patients with chronic illnesses. This questionnaire consists of seven questions. Questions 1–5 focus on behaviours that directly impact adherence, while Questions 6 and 7 explore patients' beliefs and circumstances that may indirectly affect their adherence. Scores can range from 0 to 28, with higher scores indicating better adherence to treatment.21
Mini Nutritional Assessment (MNA)
The MNA was created in 1994 by a team of experts in nutrition, geriatrics and medicine.22 The MNA is a validated and reliable tool used by healthcare professionals and researchers to assess nutritional status, particularly in older adults (aged 65 and over). The MNA comprises two key parts. The screening part consists of six simple questions covering weight loss, appetite, dietary intake, mobility, self-perception of health and neuropsychological stress. Each question receives a score, with a maximum achievable score of 14 points. For a more comprehensive assessment, the second part of the ‘detailed patient assessment’ should be completed, which delves into specific aspects such as eating habits, the presence of bedsores, the use of medication and anthropometric measurements. The maximum score for this part is 16 points. Combining both parts, the MNA yields a total score between 0 and 30 points. Here is the interpretation of the overall score: 24–30 points: good nutritional status; 17–23 points: risk of malnutrition; and 0–16 points: malnutrition.23
Frailty phenotype
The Fried Scale is a tool that identifies people with a reduced ability to cope with everyday challenges, which can lead to a higher risk of falls, hospitalization and a poorer quality of life (QoL). The Fried Scale consists of five criteria: weight loss (unintentional weight loss in the past 12 months), muscle weakness (assessment of handgrip strength using a dynamometer), decreased physical activity, fatigue (feeling tired or lacking energy) and slowing down (slower gait speed). People who meet three or more of these criteria are considered frail. People who meet one or two of the conditions are at intermediate risk or are considered precariously frail.24
Ethical consideration
This study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of the Wroclaw Medical University (No. KB-651/2022). Written informed consent was obtained from participants prior to their inclusion in the study. All patient data were anonymized to ensure confidentiality.
Statistical methods
Data pre-processing and statistical analysis were performed with the STATISTICA 13.3 package on the licence of Wrocław Medical University. Python 3.10.7 packages were employed for data visualization. An alpha value of 0.05 was assumed for statistical inference. The Mann–Whitney U test was used in assessing the difference in distribution of continuous variables, while the χ2 test was utilized in the case of the assessment of categorical variables. The association between the selected variables and the odds of frailty was analysed with the use of logistic regression. Continuous variables were checked for linearity with the log-transformed odds through analysis of plotted functions and the results of the Box–Tidwell test. Variables that did not meet this assumption [age, C-reactive protein (CRP) and N-terminal prohormone of brain natriuretic peptide (NT-proBNP)] were log-transformed. All the continuous variables were centred based on the integers closest to median values from the population sample. Collinearity between the continuous variables was checked based on the Spearman ρ. Subsequently to univariate analysis, interactions between all selected variables and cognitive impairment or depression were analysed based on the Type 1 likelihood ratio (LR) test (comparing the informativeness of each interaction vs. the naïve model). Selected interactions that were significant according to this test were further explored in full factorial models adjusted for inflammation severity (expressed as log1.1(CRP)). The P values for the β coefficients from the models were based on the Wald statistic.
Results
Patient characteristics in context of frailty
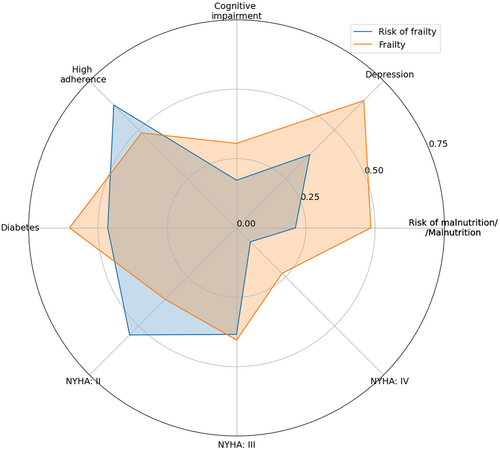
The population sample was divided into two groups: with risk of frailty and with frailty (Figure 1, Table 1). Individuals with frailty were older (P < 0.001) and showed higher inflammation severity (CRP, P < 0.001), as well as higher NT-proBNP (P < 0.001). Moreover, this group was characterized by lower haemoglobin (HGB) (P = 0.012), estimated glomerular filtration rate (eGFR) (P = 0.025) and systolic blood pressure (SBP) (P = 0.026). These differences are featured in Figure 2. There were no other statistically significant differences in values of quantitative parameters between the two groups. Individuals suffering from frailty more often showed risk of malnutrition or malnutrition based on the MNA scale (P < 0.001). Frailty was associated with a higher frequency of NYHA IV class at the cost of a lower frequency of NYHA II class (P = 0.001). Additionally, depression (assessed with the PHQ-9 scale), cognitive impairment (assessed with the MMSE scale) and diabetes were more frequent in this group (P < 0.001, P = 0.018 and P = 0.032, respectively). Interestingly, patients with frailty showed lower adherence (assessed with the ACDS) compared with those with risk of frailty (P = 0.027).
Exploring the odds of frailty—A univariate approach
All of the formerly analysed (Table 1) parameters were checked in the context of their potential univariate modulation of the odds of frailty, revealing several statistically significant findings (Figure 3).
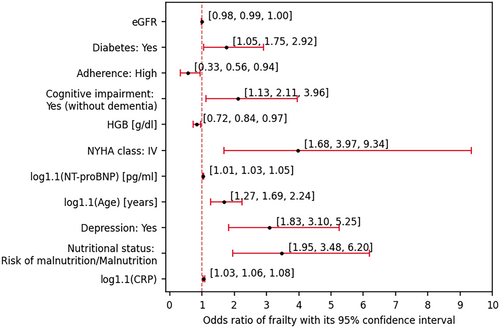
With each 10% increase in CRP, age and NT-proBNP, the odds increased by 5.60% (P < 0.001), 68.60% (P < 0.001) and 3.20% (P = 0.002), respectively. Moreover, each one-unit increase in HGB and eGFR deflated the odds by 19.33% (P = 0.018) and 1.01% (P = 0.049), respectively.
Individuals posing malnutrition or risk of malnutrition showed 3.476-fold higher odds compared with those of normal nutritional status (P < 0.001), while individuals with diabetes were of 74.7% higher odds compared with their non-diabetic peers (P = 0.033). Individuals of NYHA IV class showed 3.97-fold (P = 0.002) higher odds compared with those of Class II or III.
Depression and cognitive impairment (without dementia) were associated with, respectively, 3.098-fold (P < 0.001) and 2.113-fold (P = 0.019) higher odds of frailty. Interestingly, patients of high adherence showed 78.89% (P = 0.027) lower odds compared with those of low or moderate willingness to cooperate with medical staff.
More thorough report from this analysis, which spanned across all variables, is shown in Table S2.
Significant interactions associated with cognitive impairment and depression—Insights from inflammation severity-adjusted full factorial models
Upon analysing the interactions in regard to their informativeness compared with the naïve model (Type 1 LR test), the following interactions were statistically significant: cognitive impairment × waist circumference (P = 0.0064), cognitive impairment × body mass index (BMI) (P = 0.010), depression × adherence (P = 0.016) and depression × central obesity (P = 0.029). Because both waist circumference and BMI are used to detect obesity and waist circumference is associated with age (the addition of which would further expand the regression model), only the interaction between cognitive impairment and BMI was further analysed out of the two mentioned interactions associated with cognitive impairment. CRP was incorporated in all these models so as to adjust the results for variable inflammation severity. The simplified information from the analysed models is given in Table 2, whereas the more thorough report is featured in Table S3.
| A. Exploring the interaction between cognitive impairment status and BMI, adjusting for variable inflammation severity (measured with CRP) | |||||
|---|---|---|---|---|---|
| Effect/interaction | Description | Estimate | Estimate | Estimate | P |
| −95% CI | 95% CI | ||||
| (A) Intercept | The odds of frailty of an individual with cognitive impairment, BMI = 28 and CRP = 3 mg/L | 2.687 | 1.452 | 4.970 | 0.002 |
| (B) Cognitive impairment | Fold difference in (A) if the individual did not suffer from cognitive impairment | 0.466 | 0.234 | 0.928 | 0.030 |
| (C) BMI | Fold difference in (A) upon one-unit increase in BMI | 0.870 | 0.768 | 0.985 | 0.028 |
| log1.1(CRP) | Fold difference in (A) upon 10% increase in CRP | 1.055 | 1.028 | 1.083 | <0.001 |
| (D) Cognitive impairment × BMI | Fold difference in (B) if both compared individuals were of one-unit higher BMI | 1.173 | 1.025 | 1.343 | 0.020 |
| or | |||||
| Fold difference in (C) if an individual did not suffer from cognitive impairment | |||||
| B. Exploring the interaction between depression and adherence, adjusting for variable inflammation severity (measured with CRP) | |||||
|---|---|---|---|---|---|
| Effect/interaction | Description | Estimate | Estimate | Estimate | P |
| −95% CI | 95% CI | ||||
| (A) Intercept | The odds of frailty of an individual with depression, low or moderate adherence and CRP = 3 mg/L | 4.326 | 2.298 | 8.145 | <0.001 |
| (B) Depression | Fold difference in (A) if the individual did not suffer from depression | 0.182 | 0.075 | 0.440 | <0.001 |
| (C) Adherence | Fold difference in (A) if the individual was of high adherence | 0.366 | 0.161 | 0.830 | 0.016 |
| log1.1(CRP) | Fold difference in (A) upon 10% increase in CRP | 1.047 | 1.020 | 1.075 | 0.001 |
| (D) Depression × adherence | Fold difference in (B) if both compared individuals were of high adherence | 3.383 | 1.094 | 10.463 | 0.034 |
| or | |||||
| Fold difference in (C) if both compared individuals did not suffer from depression | |||||
| C. Exploring the interaction between depression and central obesity, adjusting for variable inflammation severity (measured with CRP) | |||||
|---|---|---|---|---|---|
| Effect/interaction | Description | Estimate | Estimate | Estimate | P |
| −95% CI | 95% CI | ||||
| (A) Intercept | The odds of frailty of an individual with central obesity, depression and CRP = 3 mg/L | 3.041 | 1.938 | 4.771 | <0.001 |
| Central obesity | Fold difference in (A) if the individual did not suffer from central obesity | 0.392 | 0.153 | 1.004 | 0.051 |
| (B) Depression | Fold difference in (A) if the individual did not suffer from depression | 0.274 | 0.149 | 0.507 | <0.001 |
| log1.1(CRP) | Fold difference in (A) upon 10% increase in CRP | 1.050 | 1.023 | 1.078 | <0.001 |
| (C) Central obesity × depression | Fold difference in (B) if both compared individuals did not suffer from central obesity | 4.092 | 1.016 | 16.472 | 0.047 |
- Note: The ‘Estimate’ referred to in the columns represents baseline odds (in case of the intercept terms), odds ratios (in case of the effects) or fold change in odds ratios (in case of interactions).
- Abbreviations: BMI, body mass index; CI, confidence interval; CRP, C-reactive protein concentration.
Exploring the interaction between cognitive impairment and BMI
The model (Tables 2A and S3A) estimated the odds of death in reference to an individual with cognitive impairment, BMI 28 and CRP 3 mg/L (typical values, close to the median in the population sample). The odds of frailty of the said individual was 2.687 (P = 0.002). For a person of the same BMI and CRP, without cognitive impairment, the odds would be ~2.146-fold lower (P = 0.030), yielding odds equal to 1.252. This fold difference would remain constant between individuals of variable CRP concentration. Moreover, regardless of either cognitive impairment status or BMI, each 10% increase in CRP would be associated with a 5.5% increase in the odds of frailty.
The influence of the interaction between cognitive impairment and BMI (P = 0.020) could be understood in two possible ways. Firstly, it could be said that the odds ratio (OR) between the individuals of no cognitive impairment and those suffering from it increases by 17.3% with every one-unit increase in BMI. This would mean that, the higher the BMI, the odds in the two groups of patients would become closer to each other, eventually becoming higher among those of no cognitive impairment at BMI 33 (OR = 1.035 at this BMI value in both individuals) or higher. The other way of interpreting the interaction is that the fold difference in the odds associated with every one-unit increase in BMI would be different between the two groups (decreasing by 14.94% among those of cognitive impairment, while increasing by 2.05% among those of no such impairment). The overall odds of frailty according to the model are plotted in Figure 4. Additional adjustment for HF type or left ventricular ejection fraction (LVEF) yielded similar estimates, and the way in which cognitive impairment interacted with BMI would be similar upon subsequent stratification by HF type or LVEF, as shown in Table S4A (HF type) and Table S5A (LVEF).
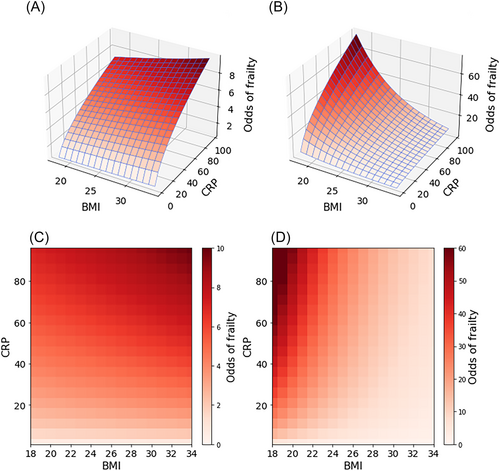
Interaction between depression status and adherence and its modulation of the odds of frailty
An individual with CRP 3 mg/L and low or medium adherence suffering from depression was referred to in the model (Tables 2B and S3B). The odds for such a person would be equal to 4.326, accounting for markedly higher likelihood of frailty compared with the risk of frailty. Regardless of depression and adherence status, an increase of CRP by 10% would inflate the odds by 4.70% (P = 0.001). The effect of depression and adherence on the odds varied because the interaction between these variables was statistically significant (P = 0.034). In a person of low or medium adherence, the lack of depression would result in 5.495-fold (P < 0.001) lower odds. Interestingly, this lack-of-depression-associated fold decrease would be 62.41% in an individual of high adherence. On the other hand, based on the model, it could also be stated that in an individual with depression, 2.732-fold (P = 0.016) lower odds would be associated with high adherence, while such adherence would be associated with 1.238-fold higher odds in individuals not suffering from depression. Additional adjustment for HF type or LVEF yielded similar estimates, and the way in which depression status interacted with adherence would be similar upon subsequent stratification by HF type or LVEF, as shown in Table S4B (HF type) and Table S5B (LVEF).
How central obesity and depression interact to influence frailty risk
The model (Tables 2C and S3C) referred to an individual suffering from either depression or central obesity (odds = 3.041, P < 0.001). Caution is advised in trusting this interaction because the P value is close to the α (P = 0.047). Regardless of both these diseases, a 10% increase in CRP would increase the odds by 5.0% (P < 0.001).
In an individual with central obesity, the lack of depression would decrease the odds by 3.649-fold (P < 0.001). However, the effect of the lack of depression would be antagonistic (12.12% increase) in a person with no central obesity.
Based on the fact that the confidence interval (CI) of the OR associated with the lack of central obesity only slightly crosses 1.0 (P = 0.051, 95% CI = 1.004), it could be hypothesized that the lack of obesity would decrease the odds of frailty in a person with depression, although its estimated effect remains unknown. Moreover, the magnitude of this influence would be different (not necessarily antagonistic as −95% CI = 0.149) in depression-free individuals. Additional adjustment for HF type or LVEF yielded similar estimates, and the way in which central obesity interacted with depression status would be similar upon subsequent stratification by HF type or LVEF, as shown in Table S4C (HF type) and Table S5C (LVEF).
Discussion
This study examined determinants of frailty in HF patients, focusing on various factors and their interactions. The results confirmed known relationships, emphasizing the roles of inflammatory markers, cognitive impairment, central obesity and psychosocial factors. These findings underline the complexity of frailty and the need for a comprehensive approach in treatment. Our analysis highlighted significant differences between frail and pre-frail HF patients, allowing the identification of modifiable factors to prevent frailty progression, which is linked to worse clinical outcomes in HF patients.3, 5 Frail patients were older, with higher CRP and NT-proBNP and lower HGB, eGFR and SBP. They were also more likely to suffer from malnutrition or its risk, NYHA Class IV symptoms, depression, cognitive dysfunction, diabetes and lower adherence. These determinants and their interactions are discussed below.
Frailty syndrome (FS) often resembles physiological ageing, but advanced age alone does not define frailty in HF patients.25 In our study, frail patients were significantly older than pre-frail individuals. Age is recognized as a key non-modifiable factor influencing frailty in HF, with ~25% of older patients exhibiting frailty, increasing sharply with age. The FRAIL-HF study reported that over 70% of HF patients aged 80 and older are frail,1, 25 highlighting the high prevalence of frailty and functional impairments in this group. It emphasized the significant role of clinical and social factors in post-discharge outcomes, underscoring the need for comprehensive frailty assessment to better understand its impact on prognosis.26
Our findings also demonstrated significantly lower SBP in frail patients. Reduced SBP is associated with diminished functional capacity and increased fall risk in older HF patients, with SBP levels below 110 mmHg linked to higher mortality, particularly in frail individuals.27 In patients with advanced stages of HF, more aggressive pharmacological interventions are often required, which can lead to further reductions in SBP. Managing hypotension in these patients is particularly challenging, as they may experience symptomatic hypotension during drug titration or as a side effect of their medications.28
Frail patients were more frequently classified in NYHA Class III/IV, reflecting greater HF severity and poorer functional status.29 In a cohort of 3429 HF patients, 26% exhibited NYHA III/IV symptoms, with frail individuals more frequently presenting advanced HF stages compared with non-frail counterparts. Our study similarly reported NYHA IV in 23% and NYHA III in 40% of frail patients.30
NT-proBNP, a marker released in response to cardiac stress, holds significant prognostic value in older adults. Elevated NT-proBNP levels not only indicate HF severity but also signal a higher risk of frailty, reinforcing the role of cardiovascular dysfunction in frailty pathogenesis.31 A meta-analysis of 53 studies revealed that frail HF patients exhibit significantly higher NT-proBNP levels, with a standardized mean difference of 0.33 (95% CI = 0.25–0.40). In our study, NT-proBNP levels were significantly elevated in frail patients compared with those at risk, correlating with worse clinical outcomes, including higher NYHA classification and increased mortality risk.10
In our study, frail patients had lower HGB and eGFR levels than pre-frail patients. Frailty in HF exacerbates anaemia and renal dysfunction, creating a vicious cycle that worsens both conditions and elevates the risk of adverse outcomes. Patients with HF, frailty, low HGB and impaired kidney function face a 1.5- to 2-fold higher risk of all-cause mortality compared with non-frail individuals.32
Walston et al. reported diabetes prevalence at 24.5% in pre-frail and 32.4% in frail individuals.33 In our study, diabetes affected 60% of frail patients versus 45% of pre-frail patients, largely due to older age inclusion. A bidirectional relationship exists between diabetes and frailty, where diabetes heightens frailty risk, while frailty worsens diabetic complications. Sarcopenia, chronic low-grade inflammation and insulin resistance link diabetes and frailty, contributing to functional decline and accelerated ageing, with elevated cytokines further connecting the two conditions.34
Nutritional status is also a significant determinant of FS. In our study, malnutrition or risk of malnutrition was significantly more common in frail patients than in pre-frail ones. Univariate analysis showed that patients at risk of malnutrition (MNA scale) had a three-fold higher risk of frailty compared with those with normal nutritional status. Malnutrition contributes to muscle mass loss, impaired physical performance and immune dysfunction, reducing resistance to infections. Age-related weight loss also plays a crucial role in frailty pathophysiology.35
Our analysis found that higher BMI reduced frailty risk differences between individuals with and without cognitive impairment, suggesting it may protect against cognitive impairment's negative effects on frailty. This aligns with the ‘obesity paradox’, which posits that overweight or mildly obese individuals may have better outcomes in cardiovascular diseases, including HF. However, obesity does not negate the increased frailty risk in higher BMI patients. Despite potential protective benefits, the overall interaction between high BMI and frailty is concerning due to its impact on mortality and QoL.36 We also observed that in patients with central obesity, the absence of depression reduced frailty risk by 3.649 times, whereas the absence of depression in non-obese individuals paradoxically increased frailty risk.
Research shows that depression accelerates frailty progression in HF patients. Depressive symptoms are often linked to declining physical function, contributing to the frailty phenotype.37 Reduced physical activity, social isolation and chronic inflammation further explain this association.38 A study of 622 HF patients found significant links between frailty, depression and 1 year mortality, with frail patients showing a much higher risk of death compared with non-frail individuals (16.9% vs. 4.8%, P < 0.001).37 Addressing psychosocial factors such as depression may improve self-care in HF patients, potentially reducing hospitalization rates.32
Our research shows that both depression and adherence with health recommendations significantly influence frailty risk. Individuals without depression who adhere to guidelines have a markedly lower risk of frailty. Conversely, those with depression face an elevated risk, even with high adherence. Adherence to medication and lifestyle changes is essential for managing HF, as poor adherence worsens symptoms, increases hospitalizations and raises mortality rates.1 Factors affecting adherence in HF patients include cognitive decline, depression, anxiety and treatment complexity.37 The relationship between adherence and frailty is bidirectional: Frailty can reduce adherence due to physical limitations, while poor adherence worsens HF symptoms and exacerbates frailty.32 Depression influences adherence through both biological mechanisms, including increased inflammation, neurohormonal dysregulation and cognitive impairment, and psychosocial factors, such as decreased motivation, reduced social support and negative psychological factors. In our findings, the absence of depression was associated with significantly better adherence, especially in patients with high adherence levels, while depression led to a marked decrease in adherence.39, 40 This highlights the need for treatment strategies addressing both physical and psychological factors. Enhancing adherence in frail HF patients can improve outcomes, reduce hospitalizations and enhance QoL.
Elevated CRP, a systemic inflammatory marker, is a key determinant of frailty in HF patients, highlighting the bidirectional link between frailty and cardiovascular health. High levels of CRP are commonly observed in patients with HF, which suggests that inflammation exacerbates both heart dysfunction and overall health deterioration. In our study, a 10% increase in CRP raised the likelihood of frailty by 5%, independent of cognitive function, BMI, depression or adherence. Similarly, the CHARLS study found that individuals with elevated high-sensitivity CRP had a 1.18 times greater risk of developing frailty over 3 years. Elevated CRP not only predicts frailty onset but also hinders recovery from a pre-frail state.41 A meta-analysis by Soysal et al.,42 including 32 cross-sectional studies, found that frailty and pre-frail status are associated with significant increases in inflammatory markers, particularly CRP and interleukin-6 (IL-6). Among frail and pre-frail patients, higher rates of disability and obesity further contributed to elevated inflammatory parameters.43 Monitoring CRP levels in older adults, particularly in pre-frail individuals, offers valuable insights into their risk of progressing to frailty. Early identification and targeted interventions to reduce inflammation could mitigate this risk and improve health outcomes.41 The systemic nature of HF means that inflammation not only affects the heart but also impacts other organs, including the brain, with inflammatory markers from peripheral tissues influencing cognitive function.44
Chronic inflammation can lead to neuroinflammation, further worsening cognitive impairment. Cognitive decline in HF patients is multifaceted, influenced by interconnected factors. Atherosclerosis contributes to coronary artery disease and HF, particularly HFrEF, where reduced cerebral perfusion due to decreased cardiac output is significant; hypoperfusion may cause ischaemic brain changes leading to cognitive deficits.45 Other factors associated with cognitive impairment in HF include calcium dysregulation, neurohormonal activation, structural brain changes, thromboembolic events and depression.44, 45 Cognitive impairments are predictors of FS and can indicate future cognitive disorders.45 In our univariate analysis, patients with cognitive impairment but no dementia had a 2.113-fold higher risk of frailty compared with those without dysfunction. Similarly, the FRAGILE-HF study found that 23% of elderly HF patients had cognitive frailty, which increased the risk of adverse events by 1.55-fold, including mortality and rehospitalization within 1 year, emphasizing the need to address cognitive health in HF management.13
The findings from this study provide essential insights for clinical practice concerning patients with HF. Regular monitoring of inflammatory markers, particularly CRP levels, could be considered as part of routine assessment for frailty risk in this population. However, while CRP serves as a valuable biomarker, its monitoring should be complemented by other clinical indicators of frailty and cardiovascular health. Effectively managing inflammation may significantly reduce this risk. Effectively managing inflammation may significantly reduce the risk of frailty in HF patients. This involves a multifaceted approach that includes pharmacological interventions targeting specific inflammatory pathways, lifestyle modifications, personalized treatment strategies based on individual patient profiles and regular monitoring of inflammatory markers. Continued research into the role of inflammation in these conditions is essential for developing effective therapies that can improve patient outcomes and QoL. Additionally, early identification and treatment of comorbid conditions, such as depression, diabetes and cognitive disorders, are crucial in patients with HF. Encouraging adherence to medical recommendations is vital, as higher adherence is associated with a lower risk of frailty. Health education and psychological support play important roles, especially for those dealing with depression. Health education and psychological support are crucial components, particularly for patients with depression. Patients presenting with depressive symptoms should undergo regular screening for this condition, and if diagnosed, they should be referred to a psychologist or psychiatrist for appropriate therapeutic intervention. Psychological approaches, such as cognitive–behavioural therapy, have been shown to enhance treatment adherence, alleviate depressive symptoms and improve patients' overall QoL. Finally, patients with cognitive impairments require focused attention, and integrated cognitive interventions can help prevent the onset of frailty in individuals with HF.
Despite the many valuable findings, our study has several limitations. Firstly, the cross-sectional design restricts our ability to make causal inferences. Consequently, we cannot clearly determine whether the observed factors are direct causes of frailty or merely outcomes of it. Secondly, the study did not account for several potentially significant variables and focused on specific inflammatory markers like CRP, while other markers [such as IL-6 and tumour necrosis factor-α (TNF-α)] might offer further insights into the inflammatory processes involved in the pathogenesis of frailty. Lastly, some factors, including physical activity levels, social support and economic status, which could influence the development of frailty, were not incorporated into the analysis.
In conclusion, our study highlights the complex interactions among factors determining frailty in HF patients. Frailty is associated with increased age, higher inflammation (CRP), elevated NT-proBNP levels, and lower HGB, eGFR and SBP. Cognitive disorders, depression, high CRP levels and central obesity all contribute to frailty risk, demonstrating their interplay. Notably, higher BMI may mitigate the effects of cognitive impairment on frailty, while the absence of depression offers protection even with central obesity. Elevated CRP levels consistently correlate with increased frailty risk, independent of cognitive status, BMI, depression or high treatment adherence, emphasizing the importance of managing inflammation. Strong adherence with health recommendations may be significantly associated with a lower risk of frailty, particularly in individuals without depression. Observed relationships between cognitive impairment and BMI, as well as between depression, adherence and central obesity, underscore the complex interplay between physical and mental health factors that may influence frailty in patients with HF. Moreover, these interactions were uniform among different HF types or LVEF values. Therefore, their association with the odds of frailty could be generalized for the entire HF cohort. Overall, addressing modifiable factors like inflammation, depression and adherence is essential to reduce frailty risk. An integrated approach considering both physical and psychological factors is crucial in preventing frailty in HF patients, ultimately aiming to improve patient outcomes and QoL.
Conflict of interest statement
The authors declare no conflicts of interest.
Funding
This research was funded by the National Science Centre, Poland [Narodowe Centrum Nauki (NCN), Grant OPUS 22 OPUS.E250.22.003].
Open Research
Data availability statement
Data are available from the authors upon reasonable request.




