Exercise-induced dynamic mitral regurgitation is associated with outcomes in patients with ischaemic cardiomyopathy
Abstract
Aims
Ischaemic mitral regurgitation (MR) is a dynamic condition influenced by global and regional left ventricular remodelling as well as mitral valvular deformation. Exercise testing plays a substantial role in assessing the haemodynamic relevance of MR and is recommended by current guidelines. We aimed to assess the prevalence, haemodynamic consequences, and prognostic impact of dynamic MR using isometric handgrip exercise.
Methods and results
Heart failure patients with ischaemic cardiomyopathy and at least mild MR who underwent handgrip echocardiography at the University Hospital Duesseldorf between January 2018 and September 2021 were enrolled. Patients were followed-up for 1 year to assess a combined endpoint including all-cause mortality, heart failure hospitalization, mitral valve surgery/interventions, ventricular assist device implantation and heart transplantation. One hundred thirty-three patients with ischaemic cardiomyopathy were included (age 75 ± 10 years; 21% female; LVEF 35 ± 9%). At rest, 70 patients (53%) presented with mild MR, 54 patients had moderate MR (41%), and 9 patients (7%) showed severe MR. Twenty-five patients (20%) with non-severe MR at rest, developed severe MR during handgrip exercise. Patients with dynamic MR had larger left atrial dimensions, increased wall motion score index and larger tenting area at rest. Multivariate analysis identified MR severity during exercise [HR 1.998 (1.367–2.938)] and exercise TAPSE [HR 0.913 (0.853–0.973)] as predictors of the combined endpoint.
Conclusions
The haemodynamic changes provoked by isometric exercise unmasked dynamic severe MR in a significant number of patients with non-severe MR at rest. These data may have implications for therapeutic decision-making in symptomatic patients with non-severe MR at rest.
1 Introduction
Ischaemic mitral regurgitation (MR) occurs in patients with coronary heart disease and carries a dismal prognosis.1, 2 It has been associated with a two-fold increase in mortality rates and reduced survival following surgical or percutaneous revascularization.3-5 Furthermore, there is a graded relationship between secondary MR severity, as assessed using echocardiography, and reduced survival.6 Chronic ischaemic MR is known to be of dynamic nature and its severity various according to haemodynamic conditions.7, 8 Dynamic MR results in worsening of dyspnoea and contributes to progressive left ventricular dilatation and dysfunction.9, 10
Previous studies have demonstrated the prognostic benefit of bicycle exercise testing in patients with ischaemic MR.8, 11 Thus, current guidelines recommend exercise testing in various clinical scenarios in patients with valvular heart disease.12, 13
Isometric handgrip exercise resembles an alternative exercise intervention, which can also be performed in frail patients with multiple co-morbidities.14 Until now, there are no data on the prognostic impact of handgrip echocardiography in heart failure (HF) patients with ischaemic MR. We hypothesized that handgrip exercise during echocardiography may unmask dynamic MR in a significant proportion of patients, which could potentially alter therapeutic decision-making. In this regard, we assess the prevalence, haemodynamic consequences and prognostic impact of dynamic MR during isometric handgrip exercise in HF patients with ischaemic MR.
2 Methods
2.1 Study population
Consecutive patients with ischaemic cardiomyopathy (LVEF <50%), stable coronary artery disease and at least mild MR who underwent exercise echocardiography at the University Hospital Duesseldorf, Germany, were enrolled between 2018 and 2021. In all patients, echocardiography at rest and during specified handgrip exercise following a standardized protocol was performed. The majority of patients presented with HF symptoms and at least mild MR at rest. The study was approved by the ethics committee of the Heinrich-Heine University Duesseldorf and executed in accordance with the Declaration of Helsinki.
2.2 Echocardiographic examinations
Echocardiographic examinations were conducted using a GE Vivid E90 (Chicago, Illinois, United States) and a Philips iE33 (Amsterdam, Netherlands). Assessment of MR severity was performed according to current ESC guidelines.12 An integrative approach using semi-quantitative and at least one quantitative parameter was used to assess the severity of MR, which was graded mild, moderate or severe. A detailed description of MR assessment is given in supplemental material. Ischaemic MR was defined in patients with known ischaemic heart disease and reduced LV function (LVEF <50%) with global and/or regional wall motion abnormalities, and with the mitral valve itself remaining intact (Carpentier IIIb).
Mitral annulus was measured at end-diastole and mid-systole in the parasternal long-axis view and in apical four chamber view and then averaged. Mitral valve (MV) tenting was measured using established MV tenting parameters (tenting area; tenting height), and assessed in the parasternal long axis view during mid-systole according current recommendations.15 Interpapillary muscle distance was assessed from parasternal short axis view in systole and diastole.
Right and left ventricular function and volumes were assessed according to current recommendations. Systolic pulmonary artery pressure (SPAP) was estimated from the regurgitant jet of tricuspid regurgitation (TR) with peak systolic trans-tricuspid pressure gradient (TTPG) calculated by the modified Bernoulli equation.
2.3 Isometric handgrip testing
Following echocardiographic examination at rest, handgrip exercise was conducted according to a standardized protocol with a commercially available handgrip dynamometer (Jamar® Hydraulic Hand Dynamometer, Sammons Preston Inc.) while the patient lay on his left side. After recording of heart rate and blood pressure at rest, the patient was asked to push the dynamometer with one hand with maximum effort, to assess maximal handgrip strength. Then handgrip exercise was performed out at 30% maximal force for 3 to 5 min as has been described previously.14, 16 Medical therapy (including beta-blockers) was not stopped for the exercise test.
2.4 Follow-up
The clinical course was monitored by follow-up examinations and phone calls to the referring cardiologists, primary physicians, or the patients themselves. We assessed a composite of all-cause mortality, HF-associated hospitalizations, MV surgery, transcatheter edge-to-edge repair (TEER), left ventricular assist device implantation and heart transplantation during follow-up as the combined endpoint.
2.5 Statistical analysis
Percentages were reported to describe categorical variables and median with interquartile range or mean ± standard deviation was reported for continuous variables. Normality distribution of continuous variables was tested with the Kolmogorov–Smirnov test. Comparison between two groups were made using the chi-square test (or Fisher's exact test if the expected count was less than five per cell) for categorical variables. Differences in continuous variables between two groups were compared for significance with a two-tailed paired t test. To assess differences between the three groups, analysis of variance (ANOVA) or Kruskal–Wallis test was performed for continuous variables. Cox regression analysis was used to assess the predictive value of echocardiographic parameters regarding event-free survival. Baseline and echocardiographic variables were tested in univariate and multivariate Cox regression analysis. Multivariate analysis includes age, sex, LVEF, LAVi, exercise TAPSE and exercise MR grade. Variables were checked for multicollinearity using variance inflation factors. MR grade, MR EROA and MR volume showed multicollinearity; thus, only MR grade was included into final analysis. Hazard ratios and 95% confidence intervals are presented. Kaplan–Meier analysis was used to evaluate the event-free rate. For all analyses, p-values of <0.05 were considered to be statistically significant. All analyses were performed using Sigma Plot (Version 11.0; Systat Software Ltd. Inpixon GmbH, Duesseldorf, Germany) and GraphPad Prism (Version 7; Graphpad Software, San Diego, California, USA).
3 Results
3.1 Study population
We screened 539 patients that underwent echocardiography at rest and during handgrip exercise. After excluding 94 patients [because of image quality (n = 50), handgrip exercise physically not feasible (n = 22), severe aortic stenosis (n = 2), severe mitral stenosis (n = 1), previous mitral valve repair (n = 19)], the study cohort included 445 patients. Of these patients, 133 patients with ischaemic cardiomyopathy and reduced LV function (LVEF <50%) were identified that were enrolled in final analysis.
Patient characteristics are given in Table 1. Mean age was 75 ± 10 years, 21% were female. Mean LVEF was 35 ± 9%. In this regard, 50 patients (38%) presented with HFmrEF, and 83 patients (62%) presented with HFrEF. One hundred twenty-one patients (91%) underwent percutaneous coronary intervention and 44 patients (33%) underwent CABG.
| Demographics | All patients, 133 (100.0) |
|---|---|
| Sex female, n (%) | 28 (21.1) |
| BMI (kg/m2) | 26.6 (23.6–29.4) |
| Age (years) | 75 (67–82) |
| Diabetes mellitus, n (%) | 50 (38.2) |
| Hypertension, n (%) | 105 (79.6) |
| Smoking, n (%) | 39 (29.8) |
| CAD, n (%) | 133 (100.0) |
| 1 vessel disease, n (%) | 15 (11.4) |
| 2 vessel disease, n (%) | 15 (11.4) |
| 3 vessel disease, n (%) | 103 (78.0) |
| History of MI, n (%) | 76 (57.1) |
| History of PCI, n (%) | 121 (91.0) |
| RIVA PCI, n (%) | 91 (68.4) |
| RCX PCI, n (%) | 34 (35.6) |
| RCA PCI, n (%) | 54 (40.6) |
| History of CABG, n (%) | 44 (33.1) |
| History of VS, n (%) | 10 (7.5) |
| NYHA I, n (%) | 21 (15.8) |
| NYHA II, n (%) | 32 (24.1) |
| NYHA III, n (%) | 80 (60.2) |
| Atrial fibrillation, n (%) | 75 (56.4) |
| Medication | |
| Beta-blocker, n (%) | 118 (88.7) |
| ACE-I/AT-1 blocker, n (%) | 84 (63.2) |
| Aldosteron antagonist, n (%) | 63 (47.4) |
| Sacubitril/valsartan, n (%) | 32 (24.1) |
| Diuretic, n (%) | 114 (85.7) |
| Laboratory markers | |
| Serum creatinine (mg/dL) | 1.3 (1.0–1.8) |
| eGFR (mg/dL/1.73 m2) | 49 (35–70) |
| Haemoglobin, n (mg/dL) | 12.7 (11.5–14.0) |
| NT-proBNP (ng/mL) | 3391 (1536–8457) |
- Abbreviations: BMI, body mass index; CAD, coronary artery disease; CAGB, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; ICD, internal cardiac defibrillator; MI, myocardial infarction; NT-proBNP, N-terminal pro brain natriuretic peptide; PCI, percutaneous coronary intervention; RCA, right coronary artery; RCX, ramus circumflexus; RIVA, ramus interventricularis anterior; VS, valve surgery.
3.2 Echocardiographic parameters at rest and during exercise
Seventy patients (53%) presented with mild MR, 54 patients had moderate MR (41%), and 9 patients (7%) showed severe MR at rest. Detailed data of mitral valve parameters are shown in Table 2. Approximately one third of patients (28.6%) presented with concomitant moderate or severe TR. Mean SPAP was 40 ± 12 mmHg.
| Variable | Rest | Exercise | p-value |
|---|---|---|---|
| Heart rate (b.p.m.) | 73 ± 14 | 83 ± 19 | <0.001 |
| Systolic BP (mmHg) | 115 ± 21 | 129 ± 23 | <0.001 |
| Diastolic BP (mmHg) | 67 ± 15 | 73 ± 17 | 0.002 |
| RPP (mmHg*b.p.m.) | 8368 ± 2147 | 10,816 ± 3063 | <0.001 |
| LAVi (mL/m2) | 48 ± 17 | 50 ± 18 | 0.398 |
| LVEDVi (mL/m2) | 85 ± 30 | 92 ± 32 | 0.103 |
| LVESVi (mL/m2) | 57 ± 26 | 60 ± 28 | 0.390 |
| LVSVi (mL/m2) | 28 ± 9 | 32 ± 12 | 0.006 |
| LVEF (%) | 35 ± 9 | 37 ± 12 | 0.299 |
| Wall motion score index | 1.5 ± 0.3 | - | |
| LV sphericity index | 1.41 ± 0.2 | - | |
| LV forward flow index (mL/m2) | 28 ± 9 | 27 ± 10 | 0.695 |
| Cardiac index (L/min/m2) | 2.0 ± 0.7 | 2.3 ± 1.0 | 0.091 |
| SVRi (dyn*s/cm5/m2) | 905 ± 394 | 926 ± 417 | 0.731 |
| RAVi (mL/m2) | 36 ± 18 | 38 ± 18 | 0.333 |
| RVEDDi (mm/m2) | 21 ± 4 | 22 ± 4 | 0.087 |
| TAPSE (mm) | 17 ± 4 | 16 ± 5 | 0.583 |
| FAC (%) | 37 ± 11 | 35 ± 12 | 0.269 |
| SPAP (mmHg) | 40 ± 12 | 47 ± 14 | <0.001 |
| Mitral regurgitation | 0.001 | ||
| Mild, n (%) | 70 (52.6) | 51 (38.3) | |
| Moderate, n (%) | 54 (40.6) | 48 (36.1) | |
| Severe, n (%) | 9 (6.8) | 34 (25.6) | |
| Vena contracta (mm) | 5.2 ± 1.4 | 5.9 ± 1.7 | 0.002 |
| MR EROA (mm2) | 15.4 ± 6.4 | 20.1 ± 8.5 | <0.001 |
| MR vol (mL) | 24.7 ± 10.8 | 31.6 ± 13.4 | <0.001 |
| Annulus diameter systole (mm) | 36.1 ± 4.1 | 36.3 ± 4.5 | 0.692 |
| Annulus diameter diastole (mm) | 38.1 ± 3.6 | 38.0 ± 3.8 | 0.736 |
| Tenting height (mm) | 8.5 ± 2.4 | 9.4 ± 3.0 | 0.011 |
| Tenting area (cm2) | 2.1 ± 0.8 | 2.4 ± 0.8 | 0.007 |
| IPM distance systole (mm) | 24.0 ± 7.1 | - | |
| IPM distance diastole (mm) | 32.0 ± 7.2 | - | |
| Change IPM distance (%) | 25.8 ± 10.2 | - | |
| Tricuspid regurgitation | 0.978 | ||
| No TR, n (%) | 21 (15.8) | 21 (15.8) | |
| Mild, n (%) | 73 (54.9) | 71 (53.4) | |
| Moderate, n (%) | 24 (18.0) | 27 (20.3) | |
| Severe, n (%) | 14 (10.5) | 14 (10.5) | |
- Abbreviations: BP, blood pressure; EROA, effective regurgitant orifice area; FAC, fractional area change; IPM Distance, interpapillary muscle distance; LAVi, left atrial volume index; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume index; LVSVi, left ventricular stroke volume index; RAVi, right atrial volume index; RPP, rate pressure product; RVEDDi, right ventricular end-diastolic diameter index; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; Vol, volume.
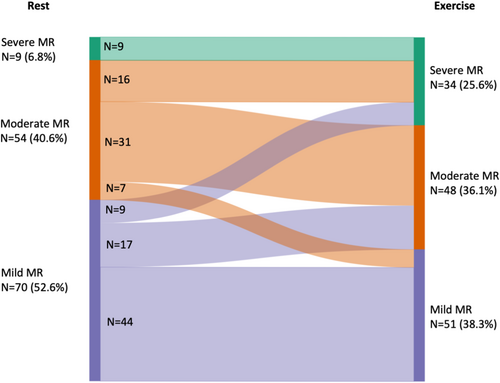
Handgrip exercise led to a re-classification of MR severity in 49 patients (36.8%) (Figure 1). Twenty-five patients (20%) with non-severe MR at rest, developed severe MR during handgrip exercise (Figure 1). Together, 99 patients showed non-severe MR neither at rest nor during exercise, 25 patients developed dynamic severe MR, and 9 patients had severe MR even at rest. In the overall cohort, there were no changes in LV and RV volumes and function, despite an increase in LVSVi during exercise. SPAP increased by 7 ± 13 mmHg from rest to exercise. Detailed changes of echocardiographic parameters from rest to exercise were shown in Table 2.
3.3 Mechanisms and haemodynamic consequences of dynamic MR
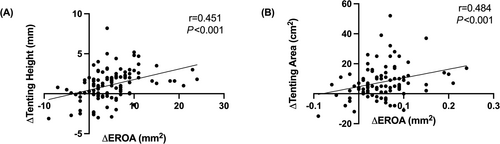
To assess predictors of dynamic MR, patients were divided into tertiles according to the change in EROA from rest to exercise (Table S1): Patients with advanced exercise-induced increases in EROA (3rd tertile) had larger left atrial dimensions, increased wall motion score index and larger tenting area at rest (Table S1). During exercise, the increase in tenting area and tenting height was more pronounced in patients with marked exercise-induced increases in EROA (3rd tertile) compared with the other groups (Table S2). In this regard, there was a correlation between the change in EROA and the change in tenting area (r = 0.484; P < 0.001) and tenting height (r = 0.451; P < 0.001) (Figure 2).
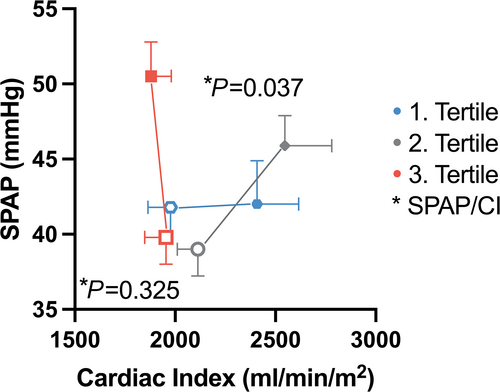
Patients with marked increases in EROA during exercise (3rd tertile) showed a similar increase in LVEDVi (+7 ± 21 mL/m2) and LVESVi (+7 ± 17 mL/m2) during exercise, and thus, no change in LVEF (−1 ± 9%). In contrast, patients without dynamic MR showed a slight improvement in LVEF (1st tertile: +4 ± 10%; 2nd tertile: +4 ± 10%; P = 0.014). Moreover, patients with dynamic MR (3rd tertile) showed reduced LV forward flow (forward LVSVi) compared with the other groups (P = 0.033), as well as no increase in cardiac index during exercise (P = 0.029). At rest SPAP was similar in patients with and without increases in EROA (Table S1). Patients with marked increases in EROA (3rd tertile) showed advanced increases in SPAP during exercise (+10 ± 13 mmHg), compared with the other groups (P = 0.009). Thus, the SPAP/cardiac index ratio got worse in patients with dynamic MR (3rd tertile), compared with patients without dynamic MR (Figure 3). The change in MR severity from rest to exercise did not with changes in RA and RV diameter or function (Table 3).
| Variable | Non-severe MR (n = 99) | Dynamic severe MR (n = 25) | Severe MR at rest (n = 9) | p-value |
|---|---|---|---|---|
| Heart rate (b.p.m.) | 73 ± 14 | 73 ± 14 | 75 ± 11 | 0.123 |
| Systolic BP (mmHg) | 116 ± 23 | 113 ± 17 | 111 ± 18 | 0.771 |
| Diastolic BP (mmHg) | 66 ± 15 | 67 ± 14 | 74 ± 19 | 0.289 |
| RPP (mmHg/min) | 8431 ± 2367 | 8165 ± 1357 | 8267 ± 1510 | 0.857 |
| LAVi (mL/m2) | 46 ± 16 | 57 ± 21 | 47 ± 14 | 0.009* |
| LVEDVi (mL/m2) | 82 ± 29 | 98 ± 29 | 90 ± 39 | 0.056 |
| LVESVi (mL/m2) | 54 ± 26 | 66 ± 25 | 60 ± 36 | 0.106 |
| LVSVi (mL/m2) | 28 ± 9 | 29 ± 10 | 27 ± 8 | 0.770 |
| LVEF (%) | 36 ± 9 | 34 ± 9 | 36 ± 11 | 0.932 |
| Forward LVSVi (mL/m2) | 29 ± 9 | 27 ± 10 | 31 ± 10 | 0.582 |
| Cardiac index (L/min/m2) | 2.0 ± 0.7 | 1.9 ± 0.5 | 2.2 ± 0.6 | 0.493 |
| Wall motion score index | 1.5 ± 0.3 | 1.6 ± 0.3 | 1.5 ± 0.5 | 0.309 |
| RAVi (mL/m2) | 34 ± 17 | 43 ± 22 | 38 ± 18 | 0.118 |
| RVEDDi (mm/m2) | 21 ± 4 | 22 ± 5 | 21 ± 4 | 0.361 |
| TAPSE (mm) | 17 ± 4 | 18 ± 4 | 15 ± 6 | 0.256 |
| FAC (%) | 38 ± 12 | 34 ± 7 | 34 ± 8 | 0.363 |
| SPAP (mmHg) | 39 ± 13 | 43 ± 11 | 41 ± 10 | 0.301 |
| Mitral regurgitation | 0.190 | |||
| Mild, n (%) | 61 (61.6) | 9 (36.0) | 0 (0) | |
| Moderate, n (%) | 38 (41.8) | 16 (64.0) | 0 (0) | |
| Severe, n (%) | 0 (0) | 0 (0) | 9 (100) | |
| MR vena contracta (mm) | 4.9 ± 1.3 | 5.6 ± 1.3 | 7.2 ± 1.2 | <0.001*# |
| MR EROA (mm2) | 13.3 ± 4.7 | 18.4 ± 4.6 | 30.0 ± 8.1 | <0.001*# |
| MR vol (mL) | 21 ± 7 | 29 ± 8 | 49 ± 15 | <0.001*# |
| Annulus systole (mm) | 36 ± 4 | 37 ± 3 | 38 ± 6 | 0.056 |
| Annulus diastole (mm) | 38 ± 4 | 40 ± 4 | 39 ± 3 | 0.029* |
| AML length (mm) | 28 ± 5 | 30 ± 4 | 28 ± 4 | 0.163 |
| PML length (mm) | 16 ± 3 | 18 ± 5 | 15 ± 3 | 0.061 |
| Tenting height (mm) | 8.3 ± 2.4 | 9.2 ± 2.6 | 9.1 ± 0.9 | 0.172 |
| Tenting area (cm2) | 2.0 ± 0.7 | 2.1 ± 1.0 | 2.2 ± 0.4 | 0.021* |
| IPM distance systole (mm) | 24 ± 8 | 25 ± 5 | 23 ± 7 | 0.716 |
| IPM distance diastole (mm) | 32 ± 7 | 33 ± 6 | 31 ± 5 | 0.269 |
| Change IPM (%) | 27 ± 11 | 23 ± 8 | 25 ± 7 | 0.861 |
| TR grade (n) | 0.718 | |||
| No TR, n (%) | 19 (19.2) | 1 (4.0) | 1 (11.1) | |
| Mild, n (%) | 53 (53.5) | 16 (64.0) | 5 (55.6) | |
| Moderate, n (%) | 17 (17.2) | 5 (20.0) | 2 (22.2) | |
| Severe, n (%) | 10 (10.1) | 3 (12.0) | 1 (11.1) |
- Abbreviations: BP, blood pressure; EROA, effective regurgitant orifice area; FAC, fractional area change; IPM Distance, interpapillary muscle distance; LAVi, left atrial volume index; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume index; LVSVi, left ventricular stroke volume index; RAVi, right atrial volume index; RPP, rate pressure product; RVEDDi, right ventricular end-diastolic diameter index; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; Vol, volume.
- * Non-severe MR vs. dynamic severe MR.
- # Dynamic severe MR vs. Severe MR at rest.
3.4 Clinical outcomes according to the presence of dynamic MR
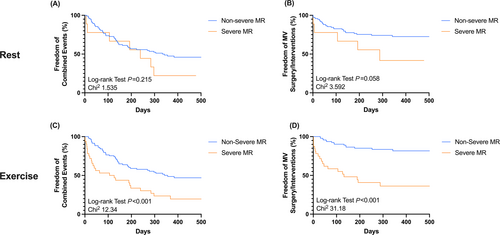
One-year follow-up (median 321 (162–427) days) was completed in 128 patients (96%). Seventy-one patients (56%) experienced the combined endpoint: 13 patients died (10%), 33 patients (26%) were re-admitted to hospital due to HF symptoms, 30 patients (24%) underwent MV TEER, six patients (5%) received MV surgery, one patient (1%) underwent LVAD implantation, and another three patients (4%) underwent heart transplantation. According to MR severity at rest, there was no difference in outcomes in patients with non-severe MR and severe MR (χ2 1.535; log-rank test P = 0.215) (Figure 4) (Table 5). As expected, patients with severe MR at rest more often underwent MV surgery/interventions compared with patients with non-severe MR (χ2 3.592; log-rank test P = 0.058) (Figure 4). In contrast, patients with severe MR during exercise showed adverse outcomes compared with those with non-severe MR (χ2 12.34; log-rank test P < 0.001) (Figure 4). Furthermore, patients with severe MR during exercise underwent MV surgery/interventions more frequently than patients with non-severe MR (χ2 31.18; log-rank test P < 0.001) (Figure 4). Excluding MV surgery/interventions from the combined endpoint, there was no difference between both groups at rest (χ2 0.262; P = 0.609) and during exercise (χ2 2.270; P = 0.132) (Figure S3). Multivariate analysis identifies MR severity during exercise (1.998 (1.367–2.938); P < 0.001), and exercise TAPSE (0.913 (0.853–0.973); P = 0.007) as predictors of the combined endpoint (Table 4). The need for MV surgery/interventions during follow-up was predicted solely by MR severity during exercise (HR 3.165 (1.832–5.768); P < 0.001) (Table 4). Patients with (dynamic) severe MR who underwent early MV surgery/interventions (<120 days from echocardiography) experienced numerically less adverse events (all-cause mortality; HF hospitalization; LVAD implantation; heart transplantation) than patients who were treated conservatively (14.3% vs. 44.4%; P = 0.072).
| Univariate analysis | Multivariate analysis | |||
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value |
| (A) Combined events | ||||
| Resting data | ||||
| Age (years) | 1.007 (0.985–1.031) | 0.529 | 1.006 (0.978–1.034) | 0.691 |
| Sex | 0.892 (0.504–1.498) | 0.679 | 0.789 (0.390–1.467) | 0.474 |
| LVEF (%) | 0.983 (0.959–1.008) | 0.183 | 1.004 (0.975–1.033) | 0.788 |
| LAVi (mL/m2) | 1.018 (1.006–1.030) | 0.002 | 1.009 (0.993–1.023) | 0.257 |
| RAVi (mL/m2) | 1.017 (1.004–1.029) | 0.008 | ||
| RVEDDi (mm/m2) | 1.083 (1.021–1.150) | 0.009 | ||
| TAPSE (mm) | 0.924 (0.867–0.985) | 0.015 | ||
| FAC (%) | 0.969 (0.944–0.995) | 0.019 | ||
| Exercise data | ||||
| LAVi (mL/m2) | ||||
| RAVi (mL/m2) | 1.016 (1.004–1.028) | 0.007 | ||
| RVEDDi (mm/m2) | 1.097 (1.037–1.162) | 0.001 | ||
| TAPSE (mm) | 0.937 (0.882–0.997) | 0.038 | 0.913 (0.853–0.973) | 0.007 |
| MR grade | 1.634 (1.212–2.205) | 0.001 | 1.998 (1.367–2.938) | <0.001 |
| MR EROA (mm) | 1.066 (1.019–1.118) | 0.007 | ||
| MR vol (mL) | 1.028 (1.012–1.044) | <0.001 | ||
| TR grade | 1.410 (1.080–1.840) | 0.011 | ||
| (B) MV surgery/TEER | ||||
| Exercise data | ||||
| MR grade | 3.165 (1.832–5.768) | <0.001 | ||
| MR EROA (mm) | 1.080 (1.030–1.137) | 0.001 | ||
| MR vol (mL) | 1.072 (1.038–1.112) | <0.001 | ||
- Abbreviations: BP, blood pressure; EROA, effective regurgitant orifice area; FAC, fractional area change; IPM Distance, interpapillary muscle distance; LAVi, left atrial volume index; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume index; LVSVi, left ventricular stroke volume index; RAVi, right atrial volume index; RPP, rate pressure product; RVEDDi, right ventricular end-diastolic diameter index; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; Vol, volume.
| Non-severe MR (n = 95) | Dynamic severe MR (n = 23) | Severe MR at rest (n = 9) | |
|---|---|---|---|
| All-cause mortality | 11 (12) | 1 (4) | 2 (22) |
| HF hospitalizations | 23 (24) | 7 (30) | 2 (22) |
| Heart transplantations | 3 (3) | 0 (0) | 0 (0) |
| LVAD implantations | 1 (1) | 1 (4) | 0 (0) |
| MV surgery/interventions | 15 (16) | 14 (61) | 5 (56) |
4 Discussion
In this study, we investigated the role of isometric handgrip exercise for the assessment of dynamic MR in patients with ischaemic cardiomyopathy. We demonstrated that (1) handgrip exercise unmasks dynamic severe MR in 20% of patients with non-severe MR at rest; (2) dynamic MR was associated with left atrial dimensions, local LV remodelling and wall motion abnormalities at rest and correlated with changes in local LV remodelling during exercise; (3) handgrip exercise puts a pressure/volume load on the LV, that cannot be compensated in patients with dynamic MR causing a reduction in LV forward flow and a pressure load on the pulmonary circulation; and (4) MR severity during exercise was associated with adverse outcomes and increased rates of MV surgery/interventions during follow-up.
4.1 Prevalence of dynamic MR
Previous studies investigated the role of exercise testing in patients with ischaemic cardiomyopathy mainly focusing on bicycle exercise testing, and reported dynamic MR in 20%–50% of patients, depending on the definition of dynamic MR and the included patient cohort.7, 8, 11, 17 Until now, there is a paucity of data regarding the effects of isometric handgrip exercise on dynamic MR.14, 18-23 The current study underscores previous findings by unmasking dynamic severe MR during handgrip exercise in 20% of patients with non-severe MR at rest. Furthermore, in our study handgrip testing led to a re-classification of MR severity in 37% of patients. Thus, our results highlight the benefit of exercise echocardiography in patients with ischaemic heart disease and non-severe MR at rest. Symptomatic patients with dynamic severe MR might benefit from intensified HF medication or early interventional strategies.24
4.2 Mechanisms of dynamic MR
Predictors of dynamic MR unmasked by dynamic bicycle exercise have been studied before.10 Now, this is the first study investigating the mechanisms of dynamic MR provoked by isometric handgrip exercise in patients with ischaemic MR. While dynamic bicycle exercise is known to decrease afterload, increase contractility and venous return (increase in preload), that together causes an increase in stroke volume, isometric handgrip exercise predominantly leads to an increase in afterload.10 These different loading conditions may translate into different LV geometric changes during exercise, and precipitate exercise-induced changes in MR severity.21 However, there might be several other factors influencing exercise-induced changes in MR as activation of the sympathetic nervous system and release of several hormones during exercise. We identified increased left atrial dimensions, parameters of local LV remodelling and wall motion score abnormalities at rest, as predictors of dynamic MR during isometric exercise. Furthermore, the change in MR severity was directly associated with the change in tenting height and tenting area. Thus, local LV remodelling rather than global LV remodelling seems to be responsible for worsening of MR during isometric exercise. Future studies comparing both exercise modalities are necessary to further elucidate these findings.
4.3 Haemodynamic consequences of dynamic MR
The haemodynamic response caused by handgrip exercise translated in a similar increase in LVEDVi (+8%) and LVESVi (+5%); thus, LVEF was unchanged during exercise (Table 2). This was accompanied by a reduction in LV forward flow in patients with marked exercise-induced increases in MR (Table S2). These changes might be explained by the sudden increase in afterload that in combination with the worsening MR outweighs compensatory LV mechanisms (Frank-Starling mechanism) and leads to a reduction in LV forward flow and cardiac index (Table S2). In addition to the decrease in LV forward flow, isometric handgrip exercise leads to an increased backward transmission and causes a pressure load on the pulmonary circulation, that was more pronounced in patients with dynamic MR (SPAP + 10 ± 13 mmHg) compared with those without dynamic MR (SPAP + 2 ± 7 mmHg) (Table S2). Thus, the SPAP/cardiac index ratio got worse during handgrip testing in patients with dynamic MR (Figure 3).
4.4 Impact of handgrip echocardiography on outcomes
In our cohort, MR severity during exercise, but not at rest, was predictive for the combined endpoint (Figure 4; Table 4). Excluding MV surgery/intervention from the combined endpoint there were no difference between the groups either at rest, nor during exercise. Solely MR severity during exercise (but not at rest) independently predicted the need for MV surgery/intervention during follow-up (Figure 4; Table 4). These results are in line with previous findings from Lancellotti et al. who investigated 161 patients with ischaemic MR undergoing bicycle exercise testing.11 They found that an exercise-induced increase in EROA by >13 mm2 was associated with mortality and HF hospitalizations.11 In another study by Lancellotti et al., significant exercise-induced increases in MR during bicycle exercise unmasked patients at high risk of poor outcome.8 Peteiro et al. included 388 patients with LV dysfunction and demonstrated the incremental prognostic value of worsening MR during exercise.25 On the other hand, there are some studies denying a prognostic impact of exercise-induced dynamic MR.22, 26 In our cohort, patients with (dynamic) severe MR who underwent early MV surgery/intervention showed favourable clinical outcomes compared with those who were treated conservatively. However, we only included a small number of patients with (dynamic) severe MR (n = 33). Only nine patients had severe MR already at rest. Together, there are only a few, conflicting data on the prognostic importance of dynamic MR during exercise in patients with ischaemic cardiomyopathy. This is the first study that demonstrated an incremental value of isometric handgrip testing over echocardiography at rest in patients with ischaemic MR. Further studies are warranted that randomize patients to additional exercise testing to further elucidate the role of exercise echocardiography in this patient cohort before the widespread use of exercise testing can be recommended.
4.5 Limitations
Main limitation of the current study is that patients were not randomized to exercise testing. Thus, there might be a selection bias for patients undergoing MV surgery/interventions. Moreover, cardiologists and cardiac surgeons were not blinded to the results of the exercise tests, thus, the decision for surgery/intervention might be by part influenced by the results of exercise echocardiography. Another main limitation is the size of the study cohort (n = 133), which prohibited subgroup analysis. However, the current study is the largest so far performing handgrip exercise for the assessment of dynamic MR in patients with ischaemic cardiomyopathy In addition, follow-up was complete in 96%. Prognostic data were missing in five patients that might have impact on outcome data.
5 Conclusions
Our results demonstrate that the evaluation of ischaemic MR only at rest often underestimates the full severity of the lesion. Handgrip exercise unmasks dynamic severe MR in every fifth patient with non-severe MR at rest. Moreover, MR severity during exercise, but not at rest, was predictive of clinical outcomes. Thus, handgrip exercise might be a useful tool in symptomatic patients with suspicion of dynamic MR to help guiding further therapeutic decision making.
6 Acknowledgements
Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interest
MS received consulting fees from Edwards Lifesciences and travel support from Abbott Medical GmbH. PH has received travel support and educational grant form Abbott Medical GmbH and an unrestricted research grant from Edwards Lifesciences. RW is an employee of Abiomed GmbH. The rest of the authors declare no conflict of interest.
Funding
This work was supported by the Forschungskommission of the Medical Faculty of the Heinrich Heine University Düsseldorf (No. 2021-03 to Maximilian Spieker).




