Guideline-directed medical therapy rates in heart failure patients with reduced ejection fraction in a diverse cohort
Abstract
Aims
Guideline-directed medical therapy (GDMT) is recommended for all patients with heart failure with reduced ejection fraction (HFrEF). Despite this, little data exist describing GDMT use in diverse, real-world populations including the use of vasodilators, prescribed primarily to Black populations. We sought, among a diverse population of HFrEF patients, to determine (1) GDMT use rates and target dosing by medication class and (2) predictors of GDMT use and target dosing by medication class.
Methods
We utilized electronic health records (EHRs) from Kaiser Permanente (KP) Mid-Atlantic States, a large integrated health system. Included patients had heart failure and left ventricular ejection fraction (EF) of ≤40% between 2015 and 2021. GDMT was defined by five medication classes—angiotensin-converting enzyme (ACE) inhibitors (ACEis)/angiotensin receptor blockers (ARBs)/angiotensin receptor–neprilysin inhibitors (ARNis), beta-blockers (BBs), mineralocorticoid receptor antagonists (MRAs), sodium–glucose cotransporter 2 inhibitors (SGLT2is) and vasodilators (Black patients only). Proportions of patients on GDMT and target dose rates were examined. Logistic regression determined, within each class, predictors of medication use and being at ≥80% of the target dose.
Results
A total of 3154 patients were included. Among the 93.8% on some form of GDMT, 82.8%, 81.4%, 23.5%, 3.6% and 13.4% were on ACEis/ARBs/ARNis, BBs, MRAs, SGLT2is and vasodilators (Black patients only), respectively. Among treated patients, 45.8%, 21.4%, 77.6%, 100% and 14.7% were treated at ≥80% of the target dose for ACEis/ARBs/ARNis, BBs, MRAs, SGLT2is and vasodilators, respectively. Overall, increasing age, higher EF, atrial fibrillation/flutter, chronic obstructive pulmonary disease (COPD), prior stroke and dementia were associated with decreased odds of GDMT use. Conversely, higher body mass index (BMI), Black race, higher glomerular filtration rate (GFR), recent echo and cardiac defibrillator were associated with increased odds of GDMT use. Among treated, higher BMI, higher systolic blood pressure, haemoglobin A1C ≥ 6.5% and cardiac defibrillator were associated with higher odds of being at ≥80% of the target dose.
Conclusions
Our study using real-world data from a diverse health system demonstrated gaps in GDMT use among patients with HFrEF, specifically older patients with more comorbidities.
Introduction
Heart failure (HF) with reduced ejection fraction (HFrEF), defined as HF with a left ventricular ejection fraction (LVEF) ≤40%, presents a significant burden to patients and society at large, affecting nearly 6.5 million American adults and with >1 million hospitalizations annually.1, 2 Medications such as beta-blockers,3-5 angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs),6-8 and aldosterone antagonists,9-11 have been the historical mainstay of therapy for reducing mortality and hospitalization events.1 More recently, the addition of angiotensin receptor–neprilysin inhibitors (ARNis),12 as well as sodium–glucose cotransporter 2 inhibitors (SGLT2is),13, 14 has been shown to also reduce hospitalizations and improve clinical outcomes in patients with HFrEF. Among Black patients, the additional use of vasodilator therapy with hydralazine and nitrates is recommended.15, 16 These medications make up what is commonly accepted as guideline-directed medical therapy (GDMT) in patients with HFrEF. They are a Class 1 recommendation for the treatment of HFrEF by the American Heart Association and American College of Cardiology (ACC)17, 18 and are further emphasized in a recent ACC Expert Consensus Decision Pathway for Treatment of Heart Failure With Reduced Ejection Fraction.18
Despite the robust evidence from randomized clinical trials that the medications comprising GDMT can improve HF outcomes, significant gaps in the use of GDMT have been described in a large outpatient US HF registry.19 In the CHAMP-HF study, a registry-based prospective cohort study of adult outpatients with HFrEF, the authors reported suboptimal rates of GDMT use and treatment to target dose for three classes of GDMT medications.20
To this end, we conducted an expansive, real-world retrospective cohort study using comprehensive patient electronic health records (EHRs) from a large, diverse integrated health system. The objectives of our study were to assess, among HFrEF patients within the Kaiser Permanente Mid-Atlantic States (KPMAS) health system, (1) the proportion of patients taking each of the five classes of GDMT; (2) the proportion of patients receiving the recommended target dose of GDMT, by medication class; (3) predictors of GDMT use, by medication class; and (4) predictors of treatment to GDMT target dose, by medication class.
Methods
Study design
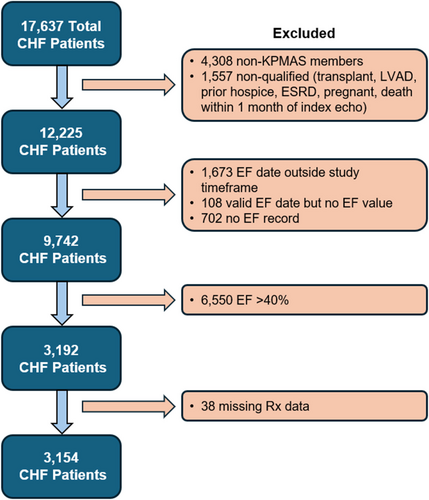
This was a retrospective, observational study of the KPMAS population (>18 years of age) with congestive HF (CHF) between 1 January 2015 and 31 December 2022. The study inclusion criteria were patients with (1) CHF defined by a widely validated method using one or more hospitalizations with a primary diagnosis of HF and/or having ≥3 ambulatory visits coded for HF21-25 (see Methods in the supporting information for greater detail) and (2) LVEF ≤ 40%, which we used to define HFrEF. We defined the study index date as the first occurrence of LVEF ≤ 40% as assessed by echocardiogram or other imaging modality. Only patients with continuous 12 months of continuous membership prior to the index date were included. Excluded patients were patients with a history of end-stage renal disease (ESRD), heart transplantation, left ventricular assist device and hospice status indicated within the EHR prior to the index date. In addition, patients were excluded from the analysis if they were pregnant at the time of index date, died within 1 month of the index date, had a contraindication to a particular class of GDMT (contraindications are listed by GDMT class in Table S3) and had no medication fill record whatsoever. The baseline period was defined as the 36 months preceding the index date for each patient.
Study variables
Patient baseline measures were extracted from the EHR and are listed in Table S1. In instances of multiple measurements, laboratory values and vital signs were averaged. Total counts of cardiology-related encounters were computed. The history of comorbidities and cardiac procedures was ascertained through the presence or absence of corresponding International Classification of Diseases (ICD)-9/10 and Current Procedural Terminology (CPT) codes.
Study outcomes
Medication fill data for five classes of GDMT were obtained during the 12 months following the index date. The medication classes included ACEs/ARBs/ARNis, beta-blockers, mineralocorticoid receptor antagonists (MRAs) and SGLT2is in all patients and vasodilators among Black patients only. The primary outcome measures were (1) a medication fill for each of the GDMT medication classes and (2) a dosing regimen achieving at least 80% of the target dose for each of the GDMT classes. The percentage of target dose was calculated by dividing the average daily dose per patient by the established target dose for a given medication class and weighted by the number of days on that medication. Established target doses by medication are detailed further in Table S2. In the target dose analysis, we chose a percentage of the target dose threshold of ≥80% to define the target dose. This threshold was chosen based on mean doses achieved in the majority of clinical trials for GDMT, as well as to allow for a period of uptitration in the weighted average dose and to accommodate realistic prescribing patterns used in real-world practice.
Statistical analysis
Continuous variables were reported as means and standard deviations (SDs), while categorical variables were summarized using frequencies and percentages for the full cohort as well as separately for Black patients. Missing data were not a significant concern; the variables with the most missing data were National Deprivation Index (NDI) quartile (8 patients or 0.3%) and glomerular filtration rate (GFR; 76 patients or 2.4%) for demographic and standard laboratory values, respectively.
Logistic regression with backward elimination was used to assess which baseline predictors were associated with GDMT medication use and achieving GDMT target dose. Certain predictors that demonstrated high correlation with other predictors were eliminated from models prior to running the backward elimination to avoid collinearity.26 All data analyses were conducted using Python 3.10 with statsmodels 0.14.1,27, 28 Pandas 2.2.1,29 Matplotlib 3.8.430 and Seaborn 0.13.231 packages.
Ethics
This study was conducted in accordance with the Declaration of Helsinki. This study was approved by the KPMAS Institutional Review Board with a waiver of informed consent.
Results
Cohort characteristics
A total of 3154 HFrEF patients were included in the final analytic cohort after exclusion criteria were applied (Figure 1). Overall, the mean age of patients in the cohort was 67.5 ± 12.8 years, and 65.8% were male (Table 1). Patients were predominantly of Black race (54.9%), followed by White (34.7%), Asian (4.7%) and other/unknown (5.7%) race. Only 4.3% of the study population was Hispanic. The mean LVEF was 30.0 ± 8.0% in this population of HFrEF patients (Table 1). Common comorbidities among patients in our study included hypertension (82.5%), dyslipidaemia/hyperlipidaemia (69.1%), coronary artery disease (53.8%), atrial fibrillation/flutter (35.1%), prior stroke/transient ischaemic attack (TIA) (37.7%) and chronic obstructive pulmonary disease (COPD) (32.3%). Average systolic blood pressure was 131.4 mmHg, average heart rate was 79.7 b.p.m., and average GFR was 54.4 mL/min/1.73 m2. Baseline characteristics of the vasodilator cohort are also displayed in Table 1. Compared with the full cohort, the vasodilator cohort trended younger (mean age 64.8 years), with a higher proportion of females (38.6%), lower socioeconomic status, similar ejection fraction (EF), and equal or lower rates of comorbidities.
| Clinical characteristics |
Full study cohort (N = 3154) |
Vasodilator cohort (N = 1730) |
|---|---|---|
| Demographics | ||
| Age (years), mean (SD) | 67.5 (12.8) | 64.8 (12.7) |
| Sex, n (%) | ||
| Male | 2074 (65.8) | 1062 (61.4) |
| Female | 1080 (34.2) | 668 (38.6) |
| Race, n (%) | ||
| Black/African American | 1730 (54.9) | 1730 (100) |
| White | 1095 (34.7) | |
| Asian/Pacific Islander | 148 (4.7) | |
| Other | 45 (1.4) | |
| Unknown | 136 (4.3) | |
| Ethnicity, n (%) | ||
| Non-Hispanic | 3018 (95.7) | |
| Hispanic | 136 (4.3) | |
| Language | ||
| English | 3038 (96.3) | |
| Other | 116 (3.7) | |
| NDI, n (%) | ||
| 1st quartile | 799 (25.4) | 181 (10.5) |
| 2nd quartile | 794 (25.2) | 404 (23.4) |
| 3rd quartile | 757 (24.1) | 498 (28.9) |
| 4th quartile | 796 (25.3) | 643 (37.3) |
| Left ventricular ejection fraction, mean (SD) | 30.0 (8.0) | 28.9 (8.2) |
| Medical history, n (%) | ||
| History of atrial fibrillation or flutter | 1108 (35.1) | 464 (26.8) |
| History of ischaemic stroke or TIA | 1190 (37.7) | 670 (38.7) |
| History of coronary artery disease | 1696 (53.8) | 798 (46.1) |
| Hypertension | 2601 (82.5) | 1443 (83.4) |
| Dyslipidaemia/hyperlipidaemia | 2179 (69.1) | 1128 (65.2) |
| Hyperthyroidism | 76 (2.4) | 52 (3.0) |
| Hypothyroidism | 370 (11.7) | 121 (7.0) |
| Chronic liver disease/cirrhosis | 410 (13.0) | 235 (13.6) |
| Asthma | 520 (16.5) | 305 (17.6) |
| COPD | 1017 (32.2) | 539 (31.2) |
| Home oxygen therapy | 103 (3.3) | 42 (2.4) |
| Depression | 583 (18.5) | 260 (15.0) |
| Dementia/cognitive conditions | 447 (14.2) | 230 (13.3) |
| Current or prior smoker | 575 (18.2) | 360 (20.8) |
| Vital signs | ||
| Systolic blood pressure (mmHg), mean (SD) | 131.4 (14.7) | 133.9 (14.9) |
| Body mass index (kg/m2), mean (SD) | 31.2 (7.4) | 32.2 (7.9) |
| Heart rate (b.p.m.), mean (SD) | 79.7 (11) | 80.8 (10.7) |
| Laboratory results | ||
| Glomerular filtration rate (mL/min/1.73 m2), mean (SD) | 54.4 (10.2) | 53.6 (10.7) |
| HDL, n (%)a | ||
| Very low (<40 mg/dL) | 638 (20.2) | 282 (16.3) |
| Low (40–59 mg/dL) | 1318 (41.8) | 720 (41.6) |
| Optimal (≥60 mg/dL) | 712 (22.6) | 444 (25.7) |
| LDL, n (%)b | ||
| Very high (≥160 mg/dL) | 176 (5.6) | 113 (6.5) |
| High (130–159 mg/dL) | 304 (9.6) | 187 (10.8) |
| Normal (100–129 mg/dL) | 557 (17.7) | 315 (18.2) |
| Optimal (≤100 mg/dL) | 1749 (55.5) | 905 (52.3) |
| Haemoglobin, n (%)c | ||
| High (>18 g/dL males, >16 g/dL females) | 10 (0.3) | 2 (0.1) |
| Normal (14–18 g/dL males, 12–16 g/dL females) | 1371 (43.5) | 702 (40.6) |
| Low (<14 g/dL males, <12 g/dL females) | 1623 (51.5) | 948 (54.8) |
| Haemoglobin A1C, n (%)d | ||
| High (≥6.5%) | 1052 (33.4) | 597 (34.5) |
| Elevated (5.7–6.4%) | 749 (23.8) | 402 (23.2) |
| Normal (<5.7%) | 433 (13.7) | 219 (12.7) |
| Albumin, n (%)e | ||
| Low (<3.4 g/dL) | 171 (5.4) | 107 (6.2) |
| Normal (3.4–5.4 g/dL) | 2118 (67.2) | 1116 (64.5) |
| Brain natriuretic peptide, n (%)f | ||
| High (≥100 pg/mL) | 1635 (51.8) | 943 (54.5) |
| Normal (<100 pg/mL) | 193 (6.1) | 114 (6.6) |
| Utilization | ||
| Encounters, mean (SD) | ||
| Cardiology visits | 11.8 (15.8) | 10.4 (14.1) |
| Primary care visits | 14.7 (24.1) | 13.8 (23.3) |
| Inpatient hospitalizations | 0.3 (0.7) | 0.3 (0.7) |
| Procedures | ||
| Echocardiogram visits, mean (SD) | 1.6 (1.3) | 1.6 (1.3) |
| Automated implantable defibrillator, n (%) | 351 (11.1) | 179 (10.4) |
| Cardiac catheterization, n (%) | 581 (18.4) | 280 (16.2) |
| Cardiac resynchronization therapy, n (%) | 85 (2.7) | 37 (2.1) |
- Abbreviations: COPD, chronic obstructive pulmonary disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NDI, National Deprivation Index; TIA, transient ischaemic attack.
- a Full unknown: N = 486 (15.4%); vasodilator unknown: N = 284 (16.4%).
- b Full unknown: N = 368 (11.7%); vasodilator unknown: N = 210 (12.1%).
- c Full unknown: N = 150 (4.8%); vasodilator unknown: N = 78 (4.5%).
- d Full unknown: N = 920 (29.2%); vasodilator unknown: N = 512 (29.6%).
- e Full unknown: N = 865 (27.4%); vasodilator unknown: N = 507 (29.3%).
- f Full unknown: N = 1326 (42.0%); vasodilator unknown: N = 673 (38.9%).
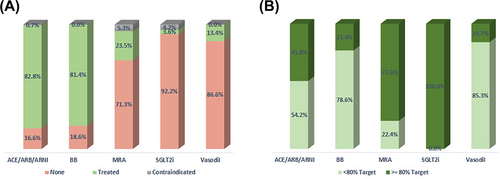
Of the 3154 patients, 2959 (93.8%) were on some form of GDMT in the 12 months after index date, and the remaining 195 (6.2%) were not on any GDMT. For each medication class, the percentage of patients treated, not treated and with a contraindication are displayed in Figure 2A, and the percent at target dose among those treated is reported in Figure 2B. Among treatment-eligible patients, 2610 (82.8%) were taking an ACE/ARB/ARNi, 2567 (81.4%) were taking a beta-blocker, 740 (23.5%) were taking an MRA, and 113 (3.6%) were on SGLT2i. Among the GDMT-treated patients, 1136 (45.8%), 504 (21.4%), 574 (77.6%) and 113 (100%) were treated at ≥80% of the recommended target dose for ACEi/ARB/ARNi, beta-blocker, MRA and SGLT2i, respectively (Figure 2A). Among 1730 Black patients eligible for vasodilator therapy, 231 (13.4%) were on some form of vasodilator therapy (Figure 2A). Specifically, 66 (3.8%) were on a nitrate, 194 (11.2%) were on hydralazine, and 32 (1.9%) were on the combination hydralazine/nitrate medication (not shown). Of the patients treated with vasodilators, 34 (14.7%) were treated at target (Figure 2B).
Characteristics of patients were also reported by GDMT use, target dose threshold and medication class (Tables S4–S8).
Predictors of GDMT use and target dose by medication class
ACEi/ARB/ARNi
Logistic regression models showed that higher body mass index (BMI), higher systolic blood pressure, higher GFR and haemoglobin A1C ≥ 6.5% were significantly associated with treatment versus non-treatment with ACEi/ARB/ARNi (Figure 3A). Older age, increased heart rate, higher EF, atrial fibrillation or flutter, COPD, depression, dementia, home oxygen therapy and albumin <3.4 g/dL were all inversely associated with being treated with ACEi/ARB/ARNi.
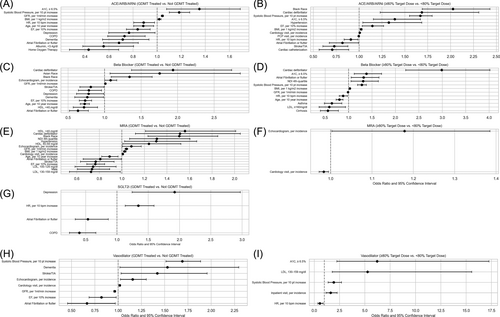
Among treated patients, increased BMI, systolic blood pressure and haemoglobin A1C ≥ 6.5%, as well as Black race, hypertension and presence of cardiac defibrillator, were associated with higher odds of being at the target dose (Figure 3B). Increased heart rate, higher EF, history of stroke/TIA and prior cardiac catheterization within 36 months were inversely associated with being at the target dose.
Beta-blocker
Patients of Black and Asian race, higher GFR, prior echocardiogram within 36 months and prior cardiac defibrillator demonstrated increased odds of beta-blocker treatment (Figure 3C). Older age, higher EF, atrial fibrillation or flutter, COPD, history of stroke/TIA, depression, dementia and high-density lipoprotein (HDL) ≤40 mg/dL were inversely associated with beta-blocker treatment.
Among treated patients, increased BMI, lowest socioeconomic status, increased systolic pressure, higher GFR, haemoglobin A1C ≥ 6.5% and prior cardiac defibrillator were associated with higher odds of being at the target dose (Figure 3D). Conversely, older age, increased heart rate, asthma, cirrhosis and low-density lipoprotein (LDL) ≥160 mg/dL were inversely associated with being at the target dose.
MRA
Black race, living in the least deprived neighbourhood, higher BMI, hypertension, increased GFR, HDL > 59 mg/dL, prior echocardiogram and cardiology encounters within 36 months, and prior cardiac defibrillator were associated with higher odds of MRA treatment (Figure 3E). Conversely, increasing age, male sex, higher EF, atrial fibrillation or flutter, prior stroke or TIA, and LDL > 100 mg/dL were inversely associated with MRA treatment (Figure 3F).
Among treated patients, prior echocardiogram and cardiology encounters within 36 months were associated with higher odds of being treated at the target dose.
SGLT2i
Higher average heart rate and depression were associated with higher odds of being treated with SGLT2i, while atrial fibrillation and COPD were inversely associated with treatment (Figure 3G). All patients treated with SGLT2i were treated at the target dose.
Vasodilators
Among Black patients, higher systolic blood pressure, dementia, history of stroke/TIA and prior echocardiogram and cardiology encounter within 36 months were associated with higher odds of vasodilator treatment, while increased GFR, higher EF and atrial fibrillation or flutter were inversely associated with treatment (Figure 3H). Among patients treated with vasodilators, haemoglobin A1C ≥ 6.5%, LDL 130–159 mg/dL, higher systolic blood pressure and prior hospitalization were associated with higher odds of being treated at the target dose, while increased heart rate was inversely associated with being treated at the target dose (Figure 3I).
Discussion
In our large, diverse, real-world population of HFrEF, there exist notable gaps in use of GDMT medications, as well as their dosing to recommended targets. Among the large proportion of Black patients in our cohort, very few of them were treated with vasodilator therapy, and only a small proportion were treated at target.
Although rates of ACEi/ARB/ARNi and beta-blocker use exceeded 80% in our HFrEF population, less than half of treated patients were dosed at target. For the MRA and SGLT2i medications, fewer than 25% of patients were treated at all; although among those treated, the majority (>75%) were on doses considered to be at or near target, which may be due to narrower dose ranges for these medications. SGLT2i rates were very low in our population, likely due to the fact that they were not adopted into the guidelines as recommended GDMT until 2022. Only 1.6% of patients were treated with four classes of GDMT medications in our overall cohort. Among a large cohort of Black patients, only 13.4% were on a vasodilator, and among these, only 14.7% were at the target dose. Logistic regression models assessing significant predictors of GDMT use demonstrated that patients with more comorbidities (atrial fibrillation/flutter, COPD, stroke and dementia in the full cohort; higher BMI in the vasodilator cohort) were associated with decreased odds of GDMT use. Trends among treated patients demonstrated that more comorbidities (higher BMI, haemoglobin A1C ≥ 6.5% and higher systolic blood pressure) were associated with higher odds of achieving the recommended target dose within both cohorts.
To date, the 2010 IMPROVE-HF and more recent CHAMP-HF are the primary large US-based CHF registries reporting rates of GDMT in HFrEF, and given the voluntary participation in such registries, the findings are potentially not representative of real-world cohorts.19, 32 While the patients in IMPROVE-HF and CHAMP-HF were >70% male and predominantly White, our cohort included a higher proportion of females and markedly higher numbers of patients of Black race. Given the registry format of CHAMP, inpatients were not included in the cohort, nor were patients with no GDMT prescriptions, and finally, no data for the novel SGLT2i class were available in CHAMP. Our analysis additionally provides more comprehensive EHR data including longitudinal medication use, laboratory values and healthcare utilization data not available in other studies.
In comparing our overall GDMT treatment rate findings with aforementioned registry data, IMPROVE-HF and CHAMP-HF reported relatively similar treatment rates with ACEi/ARB, beta-blocker and higher MRA treatment rates.19, 32 A recently published analysis of US claim data with 63 759 patients evaluated GDMT at 12 months after HFrEF diagnosis and reported lower rates of ACEi/ARB/ARNi (65.2%) and beta-blocker (64.3%) treatment and similar MRA (24.7%) treatment compared with our findings; only 6.2% of patients were on optimal GDMT at 12 months.33
With respect to dosing to recommended targets, compared with our study, IMPROVE-HF demonstrated higher target dosing of ACE/ARB (36.1%),32 while CHAMP-HF reported lower target dosing (16.8%, using 100% target dose cut-off).19 Target dosing for beta-blocker and MRA was similar in those registries to our results. EVOLUTION HF, a multinational registry of CHF patients (with reduced and preserved EF) including patients from Japan, the United States and Sweden, evaluated the initiation of GDMT following an HF hospitalization and reported low rates of target dose achievement (<10% for beta-blocker and MRA and <30% for ACE and ARNi) at 12 months.34 It is worth noting that while the aforementioned US-based (or US-inclusive) registries showed similar results, a recent report from the Netherlands TITRATE-HF included 4288 patients and demonstrated high (44%) rates of quadruple therapy among their higher risk patients.35 The treatment rates were noted to be higher in dedicated outpatient HF clinics versus general cardiology clinics, which may relate to a persistent education gap among general cardiologists.
Our finding of an association between age and lower treatment rates with GDMT has been previously described.19, 36, 37 This may be due to lower frailty burden and ability to tolerate multiple GDMT classes. Frailty among HFrEF patients has been independently assessed to be associated with not only higher rates of adverse outcomes38, 39 but also lower rates of optimal GDMT implementation (17.7% vs. 28.4% in frail vs. non-frail patients) in a post hoc analysis of the GUIDE-IT trial.40 Moreover, polypharmacy, defined as ≥5 non-GDMT medications and more commonly seen with increasing age, has been shown to be associated with lower odds of achieving optimal GDMT.41
Prior studies have noted that comorbidities such as atrial fibrillation, COPD, diabetes, depression and prior stroke were negatively correlated with optimal GDMT use,19, 33, 42 which is similar to our findings. Atrial fibrillation in particular is an important comorbidity in CHF and has been increasingly understood to drive worsening HF.43 GDMT medications maintain their beneficial effects on morbidity and mortality among HFrEF with coincident atrial fibrillation and may reduce overall atrial fibrillation burden,43 and therefore, improving GDMT implementation among atrial fibrillation patients with HFrEF is of paramount importance. It is widely reported across prior studies, including ours, that patients with increasing comorbid conditions are less likely to receive GDMT, an example of the ‘risk-treatment paradox’ where sicker patients are less likely to receive appropriate care.36
We observed in our analysis that Black patients demonstrated a higher likelihood of both being treated with GDMT and being treated at the target dose. This is similar to findings of prior studies showing higher rates of optimal GDMT prescription in Black HFrEF patients compared with White patients.33, 44, 45 It has been hypothesized that this may be due to higher prevalence of hypertension, diabetes and lower EFs among Black patients.44 This trend is of particular importance especially in light of other data suggesting that Black patients are less likely than non-Black patients to receive guideline-directed cardiovascular diagnostic and therapeutic interventions as well as being less likely to be admitted to cardiology specialty services.46-50
Importantly, real-world data on the use of vasodilators among Black patients have not been widely reported. In our analysis, vasodilator treatment rates were surprisingly low, with similarly low percentage of patients treated at target. These findings are similar to limited existing data, including data from 863 (18%) Black patients in the CHAMP-HF registry reporting 13% use of hydralazine/nitrates at 12 months among Black patients, of which only 10% were at target dose.51 Reasons for this undertreatment are not clear but likely include provider-patient and system-level reasons, including non-specialty care physicians who may not be familiar with the evidence and recommendations for vasodilator therapy.51 Black patients are diagnosed with CHF at younger ages, often a full decade earlier,51 have the highest prevalence of CHF52 and may additionally be exposed to systemic racism, which underscores the importance of establishing GDMT optimization and adherence early in the arc of disease in this important subgroup.
Among treated patients in our analysis, higher BMI, higher systolic blood pressure, haemoglobin A1C ≥ 6.5% and cardiac defibrillator were associated with a higher likelihood of being treated at target. Conversely, patients with lower BMI, with lower systolic pressure and without diabetes—that is, patients who might be deemed to be ‘healthier’—were less likely to be treated at target. This trend was also noted in the vasodilator analysis among Black patients. This might reflect a provider bias of sorts, namely, that a provider seeing a healthier patient who is clinically stable or asymptomatic may be less likely to uptitrate GDMT. Our finding is consistent with an analysis of limitations for GDMT optimization from the GUIDE-IT trial, which reported that the most common reason for not uptitrating GDMT was due to the patient being ‘clinically stable’.53 It is also possible that patients with lower systolic blood pressure are less able to tolerate GDMT at higher doses; another common reason for lack of uptitration was due to the patient being ‘already at maximally tolerated therapy’.53
Taken together, our study findings would suggest that clinical practice models to improve GDMT use among HFrEF patients should focus on older patients, patients with more comorbidities and patients with higher baseline EFs. Among GDMT-treated patients, targeted uptitration efforts should be made specifically in ‘healthier’ patients—that is, patients with fewer comorbidities who demonstrated lower target treatment rates in our study. Efforts should be made to ensure frequent and standardized follow-up for all HFrEF patients, which would allow for reassessment of GDMT tolerance and regimen expansion where clinically appropriate.
There are limitations to our study that warrant mentioning. First, our study was observational, and thus, causal relationships could not be established. Second, because we used fill data instead of order data to assess use and doses of GDMT medications, our data may not capture actual provider intent and may underestimate prescribing rates and adherence. Third, patients with missing covariate data were excluded from the analysis, and this complete case analysis may have introduced bias into our results by potentially omitting systematic differences between included and excluded patients. Fourth, because our analysis was conducted in a predominantly insured population within a single health system, it may not be representative of the general US population.
Despite these limitations, our study had several strengths including the comprehensive EHR data, which provided not only standard demographic and claims data but also detailed pharmacy data, clinical measures, laboratory and healthcare utilization data. Our large sample of Black patients allowed us to provide real-world data in Black patients that, to our knowledge, have not been previously reported.
Conclusion
This analysis reports GDMT rates and predictors in a large, diverse, real-world HFrEF patient population and, to our knowledge, is the first to report vasodilator use and predictors in a large cohort of Black patients. Our results suggest a significant ongoing gap in GDMT that may be related to underprescription of GDMT in older HFrEF patients with higher EFs and more comorbidities. Further, among GDMT-treated patients, we observed reduced likelihood of target dosing in patients who are overall healthier. Additional research is needed to further understand provider prescribing patterns across the spectrum of patient illness that may contribute to the gaps in GDMT prescription rates and target dosing, and additional targeted efforts are needed to ensure optimal GDMT implementation across diverse patient populations.
Acknowledgements
We thank Michael Horberg, MD, for his assistance and support with this study. We also thank the Mid-Atlantic Permanente Medical Group (MAPMG) and the Mid-Atlantic Permanente Research Institute for supporting this work as part of the MAPMG Physician Research Scholars Program.
Conflict of interest statement
None declared.




