Design and rationale of the eLym™ System for Decompensation of Excess Lymphatic Fluid via the Thoracic Duct in Acute Heart Failure (DELTA-HF)
Abstract
Aims
The interstitial space is the major compartment in which the excess fluid is located, forming peripheral congestion in acute decompensated heart failure (ADHF). The lymphatic system is responsible for the constant drainage of the compartment. In ADHF, the inefficiency of this system causes extravascular fluid accumulation, underscoring the crucial role of lymphatic system failure in ADHF's pathophysiology. The eLym™ System is a transcutaneous device designed to facilitate lymph drainage by creating a low-pressure zone in the thoracic duct area, theoretically allowing more efficient decompression of the lymphatic system.
Methods and Results
The safety and feasibility of the eLym™ System for the Decongestion of Excess Lymphatic Fluid via the Thoracic Duct in Acute Decompensated Heart Failure: DELTA-HF Study is a prospective, multicentre, single-arm study designed to evaluate the safety and feasibility of the WhiteSwell eLym™ System in the treatment of fluid overload in ADHF. A maximum of 50 subjects may be enrolled and undergo the treatment. Inclusion criteria include the presence of congestion, a home diuretic dose ≥80 mg furosemide (or equivalent) and elevated natriuretic peptides. The key exclusion criteria include anatomical abnormalities and the inability to undergo systemic anticoagulation. The study endpoints include the safety (short- and long-term) and feasibility of the procedure. Several congestion indexes will be prospectively assessed. Descriptive statistics will summarize the study results. The study was registered in clinicaltrial.gov (NCT05747196).
Conclusions
The results of the DELTA-HF study will provide information about the safety and feasibility of the eLym™ System and will provide first-in-human clinical signals of its use in ADHF patients.
Introduction
Despite decades of continued efforts to improve decongestive therapies, up to 50% of heart failure (HF) patients are discharged from the hospital with residual congestion, which is associated with an increased risk of rehospitalization and mortality.1-3 Patients are also at risk of worsening renal function, defined by a more than transient increase of at least 0.3 mg/dL in serum creatinine, which affects over 30% of patients and also carries a poor post-discharge prognosis. The pathophysiology of congestion is much more complex than has been recognized, with different mechanisms responsible for its development.4-9 In simplification, there are two major mechanisms of congestion in HF that conspire together and almost always coexist, namely, fluid accumulation and redistribution.5, 10 Importantly, most of the excess fluid in HF accumulates in the interstitial compartment, impacting organ function (including the kidneys) and clinically forming oedema.11-13
The lymphatic system is responsible for maintaining interstitial space homeostasis and buffering changes in the intravascular space. The lymphatics constantly drain the interstitial compartment and return the drained volume to the central veins, allowing it to stay balanced and preventing oedema formation.
The first line of decongestive therapies used in almost all acute decompensated HF (ADHF) is loop diuretics.14-16 Importantly, however, diuretics facilitate the removal of volume directly from the intravascular space (plasma) only. Thus, to achieve complete decongestion, all the excessive volume from the interstitial space must be drained back into the circulation and then excreted. The body depends upon the lymphatic system to maintain this part of fluid balance; however, in ADHF, the lymphatic system can be overwhelmed when the fluid influx outpaces lymphatic drainage or when elevated central venous pressures increase the work required by the lymphatics to drain excess fluid.8, 13, 17-21 Therefore, the active involvement of lymphatic system failure in the pathophysiology of ADHF seems to be both a true and unmet need of the current HF treatment.22, 23
Lymphatic decompression in ADHF
Supporting lymphatic drainage during HF decompensation may result in more effective and complete tissue decongestion, potentially positively impacting the patient's well-being, organ function, inflammation and clinical outcomes.24-26 To date, no therapies have been approved for use that directly target the interstitial compartment to enhance decongestion. The early attempts aimed at the thoracic duct (TD) cannulation and drainage resulted in substantial resolution in signs and symptoms of congestion but were related to unacceptable risks of periprocedural complications and massive loss of lymph (with all its components) that is not desirable or even harmful.27, 28
The WhiteSwell eLym™ System (WhiteSwell, Ltd., Galway, Ireland) is a catheter-based device designed to support and facilitate the decompression of the TD in ADHF. The idea behind the device is based on the reasoning that it creates a localized lower-pressure zone in the central vein to which the TD is connected, and therefore, it should facilitate the movement of lymph from the tissues back into the intravascular compartment. The concept has been tested and was supported by early data from an animal model.29 The system used concurrently with parenteral diuretic therapy is meant to facilitate simultaneous decongestion of both the interstitial and intravascular compartments and restore the plasma's physiological refilling during forced diuresis.
Study design
The safety and feasibility of the eLym™ System for the Decongestion of Excess Lymphatic Fluid via the Thoracic Duct in Acute Decompensated Heart Failure: DELTA-HF Study is a prospective, multicentre, single-arm study designed to evaluate the safety and feasibility of the WhiteSwell eLym System in the treatment of fluid overload in adult patients with acute decompensated heart failure (ADHF). Descriptive statistics will be used to summarize study results and describe several decongestion metrics among study participants. No formal hypothesis testing is planned. The study data will guide the design and sample size of future studies. The study was registered in clinicaltrial.gov under the number NCT05747196.
Study population
A maximum of 50 modified intent-to-treat (mITT) subjects may be enrolled and undergo WhiteSwell eLym treatment worldwide. Table 1 lists key study eligibility requirements and complete inclusion and exclusion criteria, which may be found at ClinicalTrials.gov. Hospitalized ADHF patients requiring intravenous diuretic therapy will be enrolled. Inclusion criteria include confirmation of congestion based on signs and symptoms. A home diuretic dose prior to admission of at least 80 mg furosemide or equivalent is required, as well as elevated natriuretic peptides and preserved kidney function based on estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2. The key exclusion criteria include anatomical abnormalities and vein diameters outside the size range of the eLym System, inability to be put on systemic anticoagulation and systolic blood pressure <90 mmHg at the time of enrolment. The study protocol indicates that subjects willing to participate and who signed the study's consent form will be ‘enrolled’. However, the number of subjects treated with the investigational device will be lower due to screening for inclusion/exclusion.
| Inclusion | Exclusion |
|---|---|
|
1 Age ≥ 18 years 2 Subject is admitted to the hospital with a primary diagnosis of ADHF 3 Subjects receiving intravenous (IV) diuretic for decompensated heart failure and demonstrating fluid overload. This includes a minimum of two of the following: a Peripheral oedema ≥2+ (on a 0 to 4+ scale); b Jugular venous distension ≥8 cm H2O; c Pulmonary oedema as determined by auscultation or chest radiography; d Enlarged liver or ascites; e Paroxysmal nocturnal dyspnoea or more than two-pillow orthopnoea; f Dyspnoea at rest with respiration rate ≥20 per minute; g ≥4.5 kg (10 pounds) weight gain in previous week(s)/month(s) attributable to fluid accumulation. 4 Total DAILY diuretic dose prior to admission of ≥80 mg Lasix or equivalent 5 Renal function parameters as measured by estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2 6 Elevated NT pro B-type natriuretic peptide (NT-proBNP) or BNP: a NT-proBNP >500 pg/mL, no atrial fibrillation b NT-proBNP >800 pg/mL, persistent or permanent atrial fibrillation c BNP >100 pg/mL, no atrial fibrillation d BNP >150 pg/mL, persistent or permanent atrial fibrillation 7 Albumin >3.0 g/dL 8 Subject must be able to be enrolled into the trial ≤72 h of their admission to the hospital and still be demonstrating fluid overload at the time of device placement 9 Subject agrees to comply with all follow-up evaluations 10 Subject has provided written informed consent |
1 Ultrasound screening assessment exclusions: a Subject has anatomical abnormalities that would affect the insertion and deployment of the eLym System b Subject has vein diameters at anticipated sites of deployment balloons that are not within the following specifications: i Proximal restriction balloon site must have a venous diameter of 8–18 mm ii Distal balloon site must have a diameter of 12–20 mm 2 Subjects requiring intravenous vasoactive therapies (e.g., vasodilators, inodilators and inotropes), mechanical ventilation or MCS 3 Subject has experienced a thromboembolic event [e.g., pulmonary embolism (PE), deep vein thrombosis (DVT), transient ischemic attack (TIA) or cerebrovascular events (CVA)] within the previous 6 months 4 Subject has contraindications to systemic anticoagulation 5 Subject currently on Dabigatran 6 Subject with international normalized ratio (INR) > 2.2 or on novel anticoagulants (NOACs) that cannot be held for a minimum of 48 h prior to eLym System placement Note: If subject's INR is >2.2 at the screening and baseline assessment, it may be repeated to assess eligibility up to the time of the procedure. 7 Subject with systolic blood pressure <90 mmHg at time of enrolment 8 Subject has evidence of active blood stream infection or pneumonia 9 Malignant arrhythmias [e.g., ventricular tachycardia/fibrillation, atrial fibrillation with a rapid ventricular response (sustained HR > 130 bpm) in the last 90 days] 10 Subject with acute coronary syndrome (ACS) in the last 3 months 11 Subject with severe concomitant disease expected to prolong hospitalization or expected to cause death in ≤90 days 12 Subject with a rhythm management device implanted within the last 45 days (i.e., cardioverter/defibrillator, pacemaker and cardiac resynchronization device) 13 Subject is pregnant or lactating. Women of childbearing age who are not post-menopausal or not surgically sterile will need to demonstrate a negative urine or serum pregnancy test. 14 Physician discretion |
- Abbreviation: ADHF, acute decompensated heart failure.
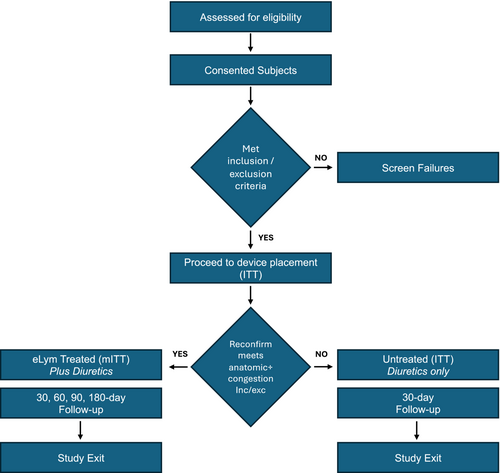
Patients are noninvasively screened with ultrasound to assess anatomic eligibility. Confirmation of anatomic compatibility is performed via fluoroscopic road mapping prior to the procedure. Patients no longer meeting inclusion/exclusion criteria but who have had jugular access with a standard sheath are in the intent-to-treat (ITT) group and are followed for 30 days for safety assessments. Patients who meet all criteria and undergo a procedure for treatment with the eLym System are in the mITT group and are followed for 180 days (Figure 1).
Clinical assessments
A series of clinical and laboratory assessments will be performed before, during and after treatment, including out-of-hospital follow-up. These include medical history, concomitant medications, physical exam, fluid intake/output balance, laboratory tests (urine sodium, chemistry panel, liver function, complete blood count, N-terminal pro B-type natriuretic peptide/B-type natriuretic peptide (NT pro-BNP/BNP), Cystatin C, CA125 and plasma free haemoglobin), coagulation monitoring, ultrasound, extravascular lung water assessments (assessed by remote dielectric sensing), additional biomarker assessment and patient-reported outcomes (KCCQ, EQ-5D, Likert Scale for Dyspnea and Patient Global Assessment). Follow-up extends out to 180 days, with visits at 30, 60, 90 and 180 days.
The WhiteSwell eLym™ System
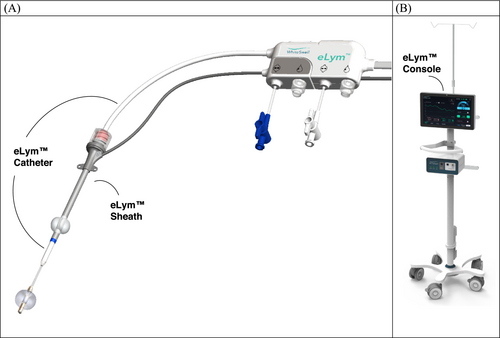
The WhiteSwell eLym™ System is a percutaneous device designed to treat congestion in ADHF patients. The device comprises two endovascular components, a catheter and a sheath, connected to an external WhiteSwell eLym™ System console (Figure 2). The device is designed to create a controllable lower-pressure zone at the thoracic duct (TD) outflow, which is located adjacent to the venous angle (junction of the left subclavian vein and left internal jugular vein). The pressure sensors on the sheath and catheter allow the monitoring of jugular zone and TD zone pressures in real time, respectively. The treatment system is configured by connecting the sheath and catheter cable connectors to the WhiteSwell Console controller, which powers and controls the pump.
Procedure and therapy with the WhiteSwell eLym™ System
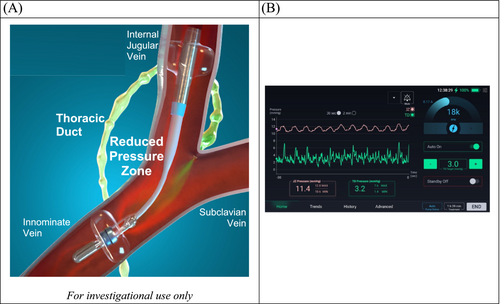
The eLym System is placed, per the instructions for use, by a trained physician in a procedure room that has fluoroscopy and ultrasound assessments available. The eLym sheath is introduced via the left internal jugular vein and remains in place for the duration of therapy. The eLym catheter is delivered through the sheath and advanced to the innominate vein. Balloons in the jugular and innominate veins are inflated, combined with pump activation, to create a low-pressure zone encompassing where the TD returns lymph to the venous system. The eLym System, controlled by a console, reduces the pressure within this zone (TD zone) to facilitate lymph drainage from the interstitial compartment. An automated algorithm on the console can be used to control the eLym System to maintain the desired target TD zone pressure (Figure 3). Alternatively, the treating physician can control the device settings in manual mode, adjusting the pump speed. The pressure gradient between the TD zone and jugular venous zone can be managed and adjusted in real time. After deployment, the patient is managed in the cardiac care unit. The patient is anticoagulated both for the procedure and during treatment with close monitoring of the activated clotting time. Treatment may continue for up to 3 days. At the end of treatment, the eLym System may be removed at the bedside.
The therapy will be conducted according to each site's standard of care. No formal diuretic protocol will be implemented.
Study endpoints
- The rate of acute adjudicated device-, treatment- and procedure-related serious adverse events (SAE) from device placement (skin puncture) to 30 days post-treatment.
- The rate of chronic adjudicated device-, treatment- and procedure-related SAE (31–180 days follow-up post-treatment).
An independent clinical events committee (CEC) will adjudicate safety and clinical outcomes.
-
Technical success:
- Initial placement success, defined as the ability to successfully deploy, activate and remove the WhiteSwell eLym System.
-
Treatment outcomes:
- Describe acute individual patient responses to treatment e.g., changes in body weight, net fluid loss/gain, changes in renal function (eGFR and serum creatinine), NT pro-BNP/BNP, predefined physical signs/symptoms of congestion, need for intensified HF therapies, assessment of extravascular lung water and changes in patient quality of life].
- Describe long-term individual patient responses to treatment 30-, 60-, 90-, and 180-days post eLym Treatment (e.g., biomarkers (BNP, CA125), improvement in congestion, quality of life, need for hospitalization for ADHF).
An independent group of physicians who are not involved in the clinical investigations will act as the CEC. The CEC is responsible for reviewing and adjudicating all adverse events. It will verify that the appropriate adverse event term has been applied to the event, its seriousness and its relationship to the device, the procedure or the treatment.
Discussion
In ADHF, central venous pressure can increase significantly, driving more fluid into the interstitial space, ‘functionally restricting’ lymph return via the TD and impeding the interstitial compartment's drainage. Interstitial/tissue congestion in ADHF is believed to be responsible for several adverse phenomena, including end-organ dysfunction, fibrosis and inflammation.9, 18, 20 On the other hand, no direct therapy is available that facilitates tissue decongestion, as both diuretics and ultrafiltration can only indirectly influence the drainage of the lymphatic system by removing the volume from the intravascular space.30 The WhiteSwell eLym™ System is a catheter-based device designed as the first device that may directly enhance lymphatic system decompression and, therefore, directly impact tissue decongestion beyond standard therapies' reach. It is designed to locally lower the venous pressure at the left venous angle where lymph drains via the TD (see Figure 3). The approach was assessed in preclinical and early clinical evaluations, showing signals of safety, feasibility, and potential effectiveness.29 When compared with direct cannulation and drainage of the TD (as previously described),27, 28 this system offers a significant potential advantage and benefit. It should facilitate decongestion by promoting TD intravascular decompression rather than removing lymph fluid directly. This is crucial because lymph contains vital substances essential for life, and their loss could have serious consequences, particularly in the long term. Moreover, the use of the WhiteSwell eLym System may potentially improve responsiveness to loop diuretics by mobilizing lymph and thus decongesting organs/peripheral tissues and promoting intravascular refill. Elevated renal interstitial pressure and intra-abdominal pressure are known to impact renal function, and lymph drainage may prove beneficial.17, 19 Improving lymph return may also help prevent intravascular depletion that may result from brisk diuresis in some patients.31 As lymphatics refill intravascular volume during diuresis, any intervention facilitating lymphatic return may also mitigate the risk of transient intravascular volume depletion. Increasing the effectiveness of diuresis and decongestion has the potential to improve symptoms, reduce hospital stays and potentially lower the risk of recurrent admissions.
It is important to appropriately emphasize limitations in assessing the clinical efficacy of WhiteSwell therapy in the DELTA-HF study. Visualizing lymphatic flow is challenging, so the DELTA-HF study focuses on important markers of decongestion (which are also more important from clinical and patient perspectives). However, it is crucial to consider that the interstitial fluid drained back into the central circulation needs to be excreted by the kidneys to ensure a lasting effect. Thus, there is still a risk that, in some patients, the unsatisfactory diuretic response may blunt the clinically detectable effect of the device despite the successful effect on lymphatic dynamics.
In summary, the DELTA-HF study is a multicentre, safety and feasibility study to examine the clinical decongestive effect of the WhiteSwell eLym System. This system is meant to facilitate the decompression of the TD and, therefore, enhance interstitial/tissue decongestion in ADHF patients.
Conflict of interest
J. B. has received honoraria from Bayer, Boehringer Ingelheim and AstraZeneca for lectures and Alleviant Medical and WhiteSwell for participating in clinical trial advisory boards. J. B. is a member of the Editorial Board of ESC Heart Failure and Heart Failure Reviews.




