Prognostic value of advanced lung cancer inflammation index in heart failure patients: A comprehensive analysis
Abstract
Aims
The prognosis of heart failure (HF) is closely linked to inflammation and nutritional status. The advanced lung cancer inflammation index (ALI) is a composite indicator consisting of several parameters used to assess inflammation and nutritional status. Our study aimed to investigate the prognostic value of ALI in HF patients.
Methods
The data from Study 1, which included 1359 HF patients, were extracted from the National Health and Nutrition Examination Survey (NHANES) database spanning the years 1999 to 2018. Study 2 analysed data from patients with HF who underwent cardiac magnetic resonance imaging examinations from 2020 to 2023. Kaplan–Meier curve analysis, Cox proportional hazard model, time-dependent receiver operating characteristic (ROC) curve and restricted cubic spline (RCS) were used to evaluate the relationship between ALI and long-term prognosis of patients with HF in Study 1. Logistic regression analysis was used to evaluate the correlation between ALI and left ventricular reverse remodelling, and RCS was used to determine any dose–response relationship. Spearman correlation was used to evaluate the relationship between ALI and indicators of cardiac structural changes.
Results
Study 1 found that the average age of the patients was 68 years [inter-quartile range (IQR) 58–76], the proportion of males was 54.3%, and there were 699 all-cause mortality and 293 cardiovascular mortality cases. After adjusted by multivariable Cox regression analysis, elevated ALI levels were significantly associated with increased risks of all-cause [hazard ratio (HR) = 0.58, 95% confidence interval (CI) = 0.42–0.79, P < 0.001] and cardiovascular mortality (HR = 0.61, 95% CI = 0.38–0.97, P = 0.036) in patients with HF. A linear negative correlation was observed between ALI and both all-cause (P = 0.0011 and P < 0.001, P for nonlinear = 0.3993) and cardiovascular mortality (P = 0.0011, P for nonlinear = 0.5198). Time-dependent ROC curves showed the predictive value of ALI for all-cause mortality [area under the curve (AUC) = 0.678 in 3 years, AUC = 0.674 in 5 years and AUC = 0.683 in 10 years] and cardiovascular mortality (AUC = 0.694 in 3 years, AUC = 0.685 in 5 years and AUC = 0.697 in 10 years). Study 2 included 79 patients; the average age of the patients was 44 years (IQR 35–55); and the proportion of males was 74.7%. Adjusted multivariable logistic regression analysis indicated that high ALI levels were associated with left ventricular reverse remodelling (LVRR) in patients with HF following discharge from the hospital [odds ratio (OR) = 3.16, 95% CI = 1.06–10.8, P = 0.049]. Spearman analysis revealed a correlation between ALI and extracellular volume (ECV) (r = −0.25, P = 0.023).
Conclusion
ALI is associated with all-cause and cardiovascular mortality risk and structural changes in the heart in patients with HF.
Background
Heart failure is a multifaceted clinical syndrome that affects various organ systems, presenting a considerable obstacle in the management of cardiovascular disease.1 As a global epidemic, it imposes substantial medical burdens on public health worldwide.2 The prognosis of patients with heart failure is influenced by disease aetiology, treatment approach and the patient's overall condition, among others.3 Therefore, identifying reliable prognostic indicators for heart failure is crucial for guiding standardized clinical management.
The advanced lung cancer inflammation index (ALI), which is calculated as the product of body mass index (BMI) and serum albumin (Alb) divided by the neutrophil-to-lymphocyte ratio (NLR), was initially introduced and employed for the assessment of prognostic outcomes in individuals diagnosed with metastatic non-small cell lung cancer.4 Subsequently, this index was found to be associated with the prognosis of other cancer types and cardiovascular diseases.5-8 Prior research has demonstrated a correlation between ALI and unfavourable prognostic outcomes in individuals diagnosed with heart failure.9, 10 However, the association of ALI with adverse cardiovascular outcomes and cardiac structural changes remains uncertain. Considering the increasing prevalence of heart failure in younger patients, it is crucial to investigate the ALI and poor prognostic outcomes in various heart failure populations.11, 12 This study initially examined the correlation between ALI and both all-cause mortality and cardiovascular mortality in individuals diagnosed with heart failure. Subsequently, following the establishment of its connection to adverse outcomes, the investigation delved into the potential association between ALI and cardiac remodelling in this patient population.
In this study, data from the National Health and Nutrition Examination Survey (NHANES) database were employed, followed by a local cohort study to examine the potential association between ALI and cardiac remodelling in heart failure patients.
This study utilized data from the NHANES database, followed by a local cohort study, to investigate the potential association between ALI and cardiac remodelling in patients with heart failure. The NHANES database was used to analyse the association of ALI with overall mortality and cardiovascular mortality risk in heart failure patients. A local cohort study was then conducted to explore the potential correlation between ALI and cardiac structural changes in individuals with heart failure. Our objective was to investigate the correlation between ALI and both all-cause mortality and cardiovascular mortality risk in patients with heart failure, as well as to explore its relationship with cardiac structural alterations. We posit that our results may provide valuable insights for enhancing the clinical management and prognosis of heart failure.
Methods
Study population
This study examines two separate research cohorts. The initial cohort was sourced from the 1999–2018 NHANES public database and was employed to explore the correlation between ALI and the likelihood of all-cause mortality and cardiovascular mortality in individuals with heart failure. Comprehensive information regarding data collection techniques and protocols can be accessed on the official NHANES website (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm). Inclusion criteria for Study Cohort 1 were as follows: (1) patients diagnosed with heart failure (primarily identified by an affirmative response to the questionnaire item: ‘Have you previously had congestive heart failure?’) and (2) subjects aged 20 years or older. Exclusion criteria included the following: (1) incomplete data on follow-up time or death and (2) lack of data on BMI, Alb, lymphocyte count or neutrophil count. Ultimately, the study included a total of 1359 patients.
Cohort 2 of the study comprised individuals diagnosed with heart failure with reduced ejection fraction (HFrEF) who were hospitalized at Qilu Hospital of Shandong University and underwent cardiac magnetic resonance (CMR) imaging examinations from 2020 to 2023. The research protocol was conducted in accordance with the principles outlined in the Declaration of Helsinki and was approved by the Ethics Review Committee of Qilu Hospital of Shandong University (approval number: KYLL-202309-035-2). Inclusion criteria for Study Cohort 2 were as follows: (1) confirmed diagnosis of HFrEF based on guidelines required clinical history and relevant examination results13 and (2) patient age not <20 years. Exclusion criteria were as follows: (1) incomplete cardiac magnetic resonance imaging (MRI) data; (2) insufficient BMI, Alb, lymphocyte count or neutrophil count data; (3) lack of follow-up information; and (4) presence of renal insufficiency, claustrophobia or other conditions preventing completion of cardiac MRI examinations. Ultimately, 79 patients were included in the study.
Measurement of ALI
The primary formula for ALI is BMI (kg/m2) × Alb level (g/dL)/NLR. The specific testing procedures for Study Cohort 1 can be accessed on the NHANES website, while all tests for Study Cohort 2 were carried out during hospital visits at Qilu Hospital of Shandong University. Given the significant variability of the ALI value and its overall right-skewed distribution, for accuracy and validity of subsequent statistical analysis, a natural logarithm transformation was applied for further analysis.14 Subsequently, natural log-transformed ALI (InALI) levels were categorized into three groups based on tertiles: Tertile 1, Tertile 2 and Tertile 3.
Covariate definitions
Study Population 1
Using standardized questionnaires and laboratory measurements from the NHANES database, we extracted the following data: (1) demographic characteristics, including age, gender, race (categorized as non-Hispanic White, non-Hispanic Black, Mexican American or other), smoking history and drinking history; (2) physical examination details, such as BMI and waist circumference; (3) comorbidities such as hypertension, diabetes mellitus (DM), coronary heart disease and myocardial infarction; (4) laboratory tests encompassing white blood cell counts, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), serum creatinine and serum uric acid (SUA); and (5) medication records for angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta-blockers, mineralocorticoid receptor antagonists, diuretics, antidiabetic drugs and lipid-lowering drugs. The estimated glomerular filtration rate (eGFR) was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI 2021) creatinine equation.15
Study Population 2
The hospitalization system of Qilu Hospital of Shandong University was used to collect relevant patient examination information during hospitalization.
Outcome data extraction
Mortality data, including cardiovascular deaths of Study Cohort 1, were collected from the National Death Index up to 31 December 2019. Causes of death were classified according to the 10th edition of the International Classification of Diseases. The follow-up period was from the NHANES examination date to the date of death or 31 December 2019.
The Study Cohort 2 comprised patients who underwent echocardiography at the cardiology department of our hospital 6 months after discharge, with subsequent data on cardiac structure obtained 6 months later. Left ventricular reverse remodelling (LVRR) was defined as an increase of more than 10% in left ventricular ejection fraction and a decrease of more than 10% in left ventricular end-diastolic diameter compared with baseline echocardiography results obtained prior to the 6 month period.16
In accordance with the research methodology outlined by the NHANES official website, sample weights, clustering and stratified analysis were utilized to evaluate the normality of continuous variables. Descriptive statistics were used to present normally distributed data as mean ± standard deviation, and non-normally distributed data were presented using the inter-quartile range. An analysis of variance was performed to assess baseline differences for continuous variables, while differences for categorical variables were evaluated using χ2 tests and reported as counts (percentages). The relationship between ALI levels and all-cause mortality as well as cardiovascular mortality rates in heart failure patients was first investigated through the Kaplan–Meier method. Subsequent analysis using time-dependent receiver operating characteristic (ROC) curves indicated that ALI exhibited superior diagnostic performance compared with Alb, BMI and NLR. After controlling for various confounding variables, we examined the influence of ALI levels on the rates of all-cause mortality and cardiovascular mortality in individuals with heart failure through single-factor and multivariable Cox regression analyses, presenting outcomes as hazard ratios (HRs) and 95% confidence intervals (CIs). Furthermore, we employed restricted cubic spline (RCS) to investigate the dose–response association between ALI and all-cause mortality and cardiovascular mortality, considering both linear and nonlinear relationships. The RCS models were optimized using a four-node configuration in accordance with the minimum Akaike information criterion standards. Statistical significance was determined at a threshold of P < 0.05.
Following this, subgroup analyses were conducted based on gender, age, smoking history, hypertension, diabetes and coronary heart disease to investigate the specific association of ALI with all-cause mortality and cardiovascular mortality in different subgroups, as well as their interaction effects. Additionally, a sensitivity analysis was carried out by excluding patients who died within the first 2 years of follow-up to further validate the reliability of our results.
In Study Cohort 2, patients were categorized according to the presence of LVRR, and comparisons between LVRR-positive and LVRR-negative groups were conducted utilizing statistical methods including the χ2 test, Fisher's exact test for categorical variables and independent t test for continuous variables. Logistic regression analysis was employed to ascertain the predictive significance of InALI in LVRR, while RCS analysis was utilized to evaluate potential nonlinear relationships. Spearman's rank correlation coefficient was employed to assess the relationships between ALI and native T1 mapping, extracellular volume (ECV) and the extent of late gadolinium enhancement (LGE).
This study was conducted using R software (Version 4.4.0) for statistical analysis.
Results
Baseline characteristics
We conducted a study involving 1359 patients diagnosed with heart failure, where Table 1 illustrates the baseline characteristics of each group categorized according to InALI tertile classification. The average age of the patients was 68 years [inter-quartile range (IQR) 58–76]. In comparison with the Tertile 1 group, both Tertile 2 and Tertile 3 groups displayed a younger average age, a lower proportion of non-Hispanic White individuals and a reduced prevalence of coronary heart disease. Furthermore, Tertile 2 and Tertile 3 exhibited higher levels of ALT, waist circumference, eGFR and TC, while lower levels of HDL-c and creatinine were observed in these groups as opposed to Tertile 1.
| Characteristics |
Total n = 1359 |
Tertile 1 n = 454 |
Tertile 2 n = 442 |
Tertile 3 n = 463 |
P |
|---|---|---|---|---|---|
| Age (years) | 68.00 [58.00–76.00] | 73.00 [65.00–80.00] | 66.00 [57.00–76.00] | 63.00 [52.00–71.00] | <0.001 |
| Sex, n (%) | 0.241 | ||||
| Male | 784 (54.3) | 283 (57.9) | 254 (54.6) | 247 (50.3) | |
| Female | 575 (45.7) | 171 (42.1) | 188 (45.4) | 216 (49.7) | |
| Race, n (%) | <0.001 | ||||
| Mexican American | 152 (4.0) | 48 (3.9) | 50 (3.9) | 54 (4.3) | |
| Other Hispanic | 90 (4.5) | 18 (3.3) | 31 (4.3) | 41 (6.0) | |
| Non-Hispanic White | 749 (74.0) | 304 (82.6) | 253 (75.6) | 192 (63.6) | |
| Non-Hispanic Black | 302 (12.8) | 67 (7.6) | 82 (10.2) | 153 (20.7) | |
| Other | 66 (4.7) | 17 (2.6) | 26 (6.1) | 23 (5.5) | |
| History of smoking, n (%) | 843 (63.6) | 298 (65.8) | 268 (61.1) | 277 (63.9) | 0.499 |
| History of drinking, n (%) | 752 (56.5) | 255 (56.2) | 243 (55.4) | 254 (58.1) | 0.808 |
| Hypertension, n (%) | 1047 (73.8) | 339 (72.2) | 332 (74.3) | 376 (75.1) | 0.657 |
| DM, n (%) | 512 (34.5) | 172 (34.7) | 170 (38.7) | 170 (29.9) | 0.232 |
| Coronary artery disease, n (%) | 561 (40.9) | 190 (44.3) | 192 (43.5) | 179 (34.9) | 0.039 |
| Heart attack, n (%) | 602 (43.8) | 214 (45.5) | 201 (45.2) | 187 (40.7) | 0.464 |
| Alb (g/dL) | 4.10 [3.90–4.30] | 4.00 [3.80–4.30] | 4.10 [3.90–4.40] | 4.10 [4.00–4.40] | 0.005 |
| ALT (U/L) | 20.00 [15.00–26.00] | 19.00 [14.00–23.00] | 20.00 [15.00–27.00] | 22.00 [17.00–28.00] | <0.001 |
| AST (U/L) | 23.00 [19.00–28.00] | 22.00 [19.00–27.00] | 23.00 [19.00–28.00] | 24.00 [19.00–28.00] | 0.209 |
| WC (cm) | 107.20 [96.60–119.57] | 101.25 [91.00–112.20] | 107.50 [98.05–120.93] | 111.70 [100.21–127.2] | <0.001 |
| BMI (kg/m2) | 30.20 [26.16–35.75] | 27.61 [24.00–31.28] | 30.44 [27.01–35.80] | 33.23 [28.36–40.33] | <0.001 |
| TC (mmol/L) | 4.63 [3.85–5.43] | 4.34 [3.62–5.21] | 4.63 [3.88–5.56] | 4.81 [4.11–5.56] | <0.001 |
| WBCs (1000 cells/μL) | 7.40 [6.20–8.80] | 7.80 [6.30–9.20] | 7.60 [6.28–9.10] | 7.00 [5.90–8.16] | 0.002 |
| HDL-c (mmol/L) | 1.16 [0.98–1.45] | 1.24 [1.01–1.63] | 1.16 [0.96–1.45] | 1.14 [0.98–1.40] | 0.033 |
| Neutrophil (1000 cells/μL) | 4.50 [3.60–5.60] | 5.30 [4.31–6.60] | 4.70 [3.70–5.70] | 3.70 [2.85–4.50] | <0.001 |
| Lymphocyte (1000 cells/μL) | 2.45 [1.75–3.41] | 1.34 [1.00–1.70] | 1.90 [1.60–2.40] | 2.40 [1.90–2.90] | <0.001 |
| NLR | 1.90 [1.40–2.40] | 3.86 [3.20–4.97] | 2.43 [2.08–2.85] | 1.52 [1.20–1.88] | <0.001 |
| Serum creatinine (mg/dL) | 1.00 [0.81–1.29] | 1.16 [0.90–1.44] | 1.00 [0.80–1.22] | 0.95 [0.80–1.11] | <0.001 |
| InALI | 3.95 [3.58–4.32] | 3.39 [3.13–3.58] | 3.95 [3.84–4.08] | 4.48 [4.33–4.70] | <0.001 |
| eGFR (mL/min/1.73 m2) | 72.75 [53.60–93.09] | 63.20 [43.57–83.63] | 73.69 [56.43–94.82] | 81.43 [62.15–95.77] | <0.001 |
| Uric acid (μmol/L) | 368.80 [297.40–440.20] | 356.90 [291.50–458.00] | 362.80 [291.50–431.42] | 380.70 [321.20–440.20] | 0.076 |
| ACEI/ARB, n (%) | 724 (52.5) | 227 (50.3) | 253 (56.0) | 244 (51.3) | 0.299 |
| Beta-blockers, n (%) | 746 (54.7) | 270 (60.5) | 236 (54.3) | 240 (49.2) | 0.017 |
| Hypoglycaemic agents, n (%) | 437 (29.7) | 142 (29.8) | 149 (33.2) | 146 (26.0) | 0.236 |
| Antilipemic agents, n (%) | 733 (53.9) | 253 (58.2) | 244 (54.9) | 236 (48.5) | 0.105 |
| Diuretics, n (%) | 639 (46.2) | 234 (48.9) | 220 (49.4) | 185 (40.2) | 0.054 |
| MRA, n (%) | 146 (10.4) | 52 (10.9) | 54 (12.3) | 40 (7.9) | 0.241 |
- Note: Values are weighted mean (IQR) for continuous variables or numbers (weighted %) for categorical variables.
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; Alb, albumin; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL-c, high-density lipoprotein cholesterol; InALI, natural log-transformed advanced lung cancer inflammation index; IQR, inter-quartile range; MRA, mineralocorticoid receptor antagonist; NLR, neutrophil-to-lymphocyte ratio; TC, total cholesterol; WBCs, white blood cells; WC, waist circumference.
Kaplan–Meier analysis
Kaplan–Meier analysis was employed to evaluate the relationship between InALI and the risk of all-cause mortality and cardiovascular mortality. The results depicted in Figure 1 indicate a decreased incidence of both types of mortality in Tertiles 2 and 3 compared with Tertile 1 (log-rank P < 0.001). Additionally, a negative correlation was observed between lower levels of InALI and the likelihood of experiencing all-cause mortality and cardiovascular mortality.
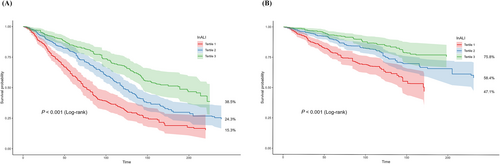
The association between ALI and mortality
A Cox regression analysis was conducted to further investigate the relationship between InALI levels and the risk of all-cause mortality and cardiovascular mortality. The results presented in Table 2 demonstrate that higher InALI levels were associated with a decreased risk of both all-cause mortality and cardiovascular mortality. In adjusted Model 2, the HRs for all-cause mortality were 0.74 (0.58–0.93) for Tertile 2 and 0.58 (0.42–0.79) for Tertile 3, while the HRs for cardiovascular mortality were 0.72 (0.52–0.99) for Tertile 2 and 0.61 (0.38–0.97) for Tertile 3. Additionally, InALI showed a significant association with reduced risk of all-cause mortality in the unadjusted model (HR = 0.48, 95% CI = 0.40–0.58). These findings remained robust and statistically significant in the multivariable-adjusted models—Model 1 (HR = 0.62, 95% CI = 0.51–0.76) and Model 2 (HR = 0.66, 95% CI = 0.54–0.81)—with similar trends observed for cardiovascular mortality risk (Table 2).
| All-cause mortality | ||||
|---|---|---|---|---|
| Death |
Crude HR (95% CI) |
Adjusted Model 1 HR (95% CI) |
Adjusted Model 2 HR (95% CI) |
|
| Tertile 1 | 296 (59.5) | Ref | Ref | Ref |
| Tertile 2 | 234 (49.3) | 0.59 (0.45–0.76)** | 0.72 (0.58–0.91)* | 0.74 (0.58–0.93)* |
| Tertile 3 | 169 (35.1) | 0.36 (0.27–0.49)** | 0.54 (0.40–0.72)** | 0.58 (0.42–0.79)** |
| InALI | 0.48 (0.40–0.58)*** | 0.62 (0.51–0.76)*** | 0.66 (0.54–0.81)*** | |
| P for trend | <0.001 | <0.001 | <0.001 | |
| CVD mortality | ||||
|---|---|---|---|---|
| InALI | Death |
Crude HR (95% CI) |
Adjusted Model 1 HR (95% CI) |
Adjusted Model 2 HR (95% CI) |
| Tertile 1 | 136 (25.3) | Ref | Ref | Ref |
| Tertile 2 | 96 (19.0) | 0.55 (0.40–0.76)** | 0.68 (0.51–0.92)* | 0.72 (0.52–0.99)* |
| Tertile 3 | 61 (13.1) | 0.33 (0.22–0.51)** | 0.50 (0.33–0.76)* | 0.61 (0.38–0.97)* |
| InALI | 0.44 (0.34–0.56)** | 0.58 (0.44–0.76)** | 0.64 (0.47–0.87)** | |
| P for trend | <0.001 | <0.001 | 0.028 | |
- Note: Values are n (weighted %) or weighted HR (95% CI). Model 1: adjusted for age, sex and race. Model 2: adjusted for age, sex, race, coronary artery diseases, ALT, TC, HDL-c, serum creatinine, WBCs, WC, uric acid, eGFR, antilipemic agents, beta-blockers and diuretics.
- Abbreviations: ALI, advanced lung cancer inflammation index; ALT, alanine aminotransferase; CI, confidence interval; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL-c, high-density lipoprotein cholesterol; HR, hazard ratio; InALI, natural log-transformed advanced lung cancer inflammation index; TC, total cholesterol; WBCs, white blood cells; WC, waist circumference; Ref, reference.
- * P < 0.05.
- ** P < 0.01.
- *** P < 0.001.
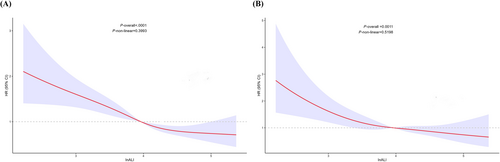
Figure 2 depicts the results of the RCS analysis, demonstrating a statistically significant correlation between levels of InALI in patients with heart failure and both all-cause mortality (P < 0.001) and cardiovascular mortality (P = 0.0011). Nevertheless, there is no indication of a nonlinear relationship between InALI levels and either overall mortality (P for nonlinear = 0.3993) or cardiovascular mortality (P for nonlinear = 0.5198).
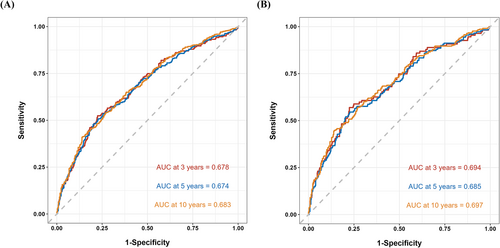
In order to further evaluate the prognostic value and predictive accuracy of InALI, we conducted time-dependent ROC analyses for both all-cause mortality and cardiovascular mortality. The results depicted in Figure 3 indicate that the area under the curve (AUC) for the cumulative incidence of all-cause mortality was 0.678 at 3 years, 0.674 at 5 years and 0.683 at 10 years. Similarly, for cardiovascular mortality, the AUC was 0.694 at 3 years, 0.685 at 5 years and 0.697 at 10 years. Notably, the predictive performance of InALI exceeded that of Alb, BMI, NLR and prognostic nutritional index [PNI, Alb (g/L) + 5 × total lymphocyte count (109/L)]17 as illustrated in Figure S1.
Subgroup analysis
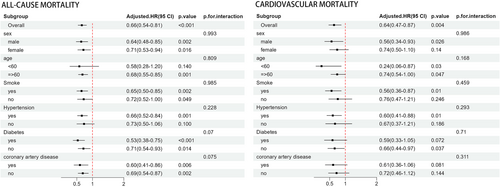
Subgroup analyses were performed to explore the relationship between InALI and both all-cause mortality and cardiovascular mortality, taking into account variables such as gender, age, smoking status, hypertension, diabetes and history of coronary heart disease. The results, depicted in Figure 4, revealed that there was no significant interaction between these factors and InALI in relation to the risks of all-cause mortality and cardiovascular mortality. There was no statistically significant difference in all-cause mortality risk among individuals under the age of 60 with no history of hypertension. Similarly, for cardiovascular mortality risk, no statistically significant difference was observed among females, nonsmokers, individuals without a history of hypertension or coronary heart disease, and those with diabetes.
We also conducted sensitivity analyses by excluding patients who died within the first 2 years of follow-up (Table S1). The results did not show any significant changes compared with the previous ones.
ALI and cardiac remodelling
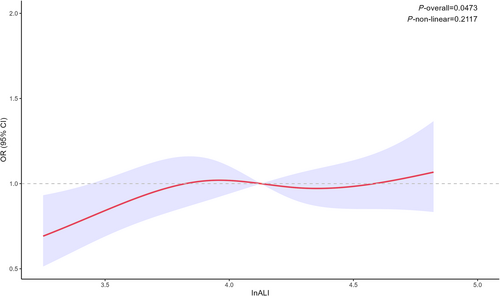
The baseline data for Study Group 2 are presented in Table 3. This reveals that the InALI value of the group exhibiting LVRR is higher than that of the nonremodelling group. Logistic analysis indicated a positive correlation between InALI and LVRR (Table 4), with an odds ratio (OR) of 3.63 (1.32–11.6). Even after adjusting for ECV and heart rate, the model remained stable and statistically significant, with an OR of 3.16 (1.06–10.8). RCS analysis showed no nonlinear relationship between InALI and LVRR (P for nonlinear = 0.2117) (Figure 5). Lastly, Spearman correlation analysis revealed a correlation between InALI and ECV (r = −0.25, P = 0.023), while no correlations were found with native T1 or LGE (Figure S2).
| Characteristics |
Total n = 79 |
LVRR (−) n = 38 |
LVRR (+) n = 41 |
P |
|---|---|---|---|---|
| Sex, n (%) | 0.95 | |||
| Male | 59 (74.7) | 29 (76.3) | 30 (73.2) | |
| Female | 20 (25.3) | 9 (23.7) | 11 (26.8) | |
| Age (years) | 44.00 [35.00–55.50] | 46.50 [37.00–54.75] | 39.00 [32.00–57.00] | 0.579 |
| Systolic BP (mmHg) | 118.00 [108.00–128.00] | 117.50 [104.00–124.75] | 118.00 [109.00–129.00] | 0.319 |
| Diastolic BP (mmHg) | 73.00 [65.00–82.50] | 72.00 [63.25–79.75] | 74.00 [68.00–86.00] | 0.195 |
| Heart rate (b.p.m.) | 77.00 [66.50–90.00] | 72.00 [63.25–82.00] | 83.00 [72.00–95.00] | 0.005 |
| Smoke, n (%) | 34 (43) | 17 (44.7) | 17 (41.5) | 0.591 |
| Drink, n (%) | 42 (53.2) | 19 (50.0) | 23 (56.1) | 0.502 |
| DM, n (%) | 9 (11.4) | 2 (5.3) | 7 (17.1) | 0.195 |
| OSAS, n (%) | 27 (34.2) | 11 (28.9) | 16 (39.0) | 0.48 |
| Coronary artery disease, n (%) | 9 (11.4) | 3 (7.9) | 6 (14.6) | 0.557 |
| NYHA, n (%) | 0.532 | |||
| I | 1 (1.3) | 0 (0.0) | 1 (2.4) | |
| II | 31 (39.2) | 16 (42.1) | 15 (36.6) | |
| III | 41 (51.9) | 18 (47.4) | 23 (56.1) | |
| IV | 6 (7.6) | 4 (10.5) | 2 (4.9) | |
| BMI (kg/m2) | 26.06 [24.41–28.01] | 25.73 [24.19–28.26] | 26.47 [24.69–27.89] | 0.825 |
| Alb (g/dL) | 4.54 ± 0.42 | 4.56 ± 0.42 | 4.51 ± 0.42 | 0.586 |
| WBCs (× 109/L) | 6.60 [5.60–7.70] | 6.62 [5.66–7.70] | 6.52 [5.52–7.61] | 0.864 |
| NLR | 2.01 [1.44–2.67] | 2.24 [1.53–3.15] | 1.80 [1.35–2.26] | 0.009 |
| RBCs (× 1012/L) | 4.89 [4.52–5.38] | 4.87 [4.54–5.30] | 4.95 [4.49–5.49] | 0.825 |
| HGB (g/L) | 150.11 ± 20.04 | 149.76 ± 17.52 | 150.44 ± 22.34 | 0.882 |
| PLT (× 1012/L) | 234.00 [198.00–272.00] | 231.00 [197.00–264.75] | 247.00 [201.00–281.00] | 0.382 |
| ALT (U/L) | 22.00 [14.95–35.00] | 22.00 [14.93–30.75] | 22.00 [15.00–44.00] | 0.527 |
| AST (U/L) | 20.00 [16.70–26.00] | 20.00 [17.00–23.75] | 21.00 [16.00–29.00] | 0.565 |
| TBIL (μmol/L) | 14.00 [10.70–19.80] | 14.05 [10.38–22.78] | 13.90 [10.80–18.90] | 0.68 |
| TC (mmol/L) | 4.44 ± 1.12 | 4.36 ± 1.06 | 4.51 ± 1.19 | 0.554 |
| HDL-c (mmol/L) | 1.06 ± 0.25 | 1.07 ± 0.27 | 1.06 ± 0.23 | 0.81 |
| LDL-c (mmol/L) | 2.76 [2.24–3.22] | 2.74 [2.38–3.15] | 2.81 [1.87–3.35] | 0.776 |
| TG (mmol/L) | 1.42 [1.08–2.14] | 1.40 [0.98–2.07] | 1.53 [1.15–2.13] | 0.227 |
| FPG (mmol/L) | 5.16 [4.74–5.77] | 5.18 [4.62–5.72] | 5.16 [4.87–5.87] | 0.339 |
| Serum creatinine (μmol/L) | 78.00 [64.50–91.00] | 79.00 [65.50–92.05] | 76.00 [64.00–89.00] | 0.806 |
| Serum potassium (mmol/L) | 4.21 [3.96–4.44] | 4.22 [4.04–4.48] | 4.19 [3.94–4.39] | 0.459 |
| Serum sodium (mmol/L) | 141.50 [140.00–143.00] | 141.00 [139.25–142.75] | 142.00 [141.00–144.00] | 0.103 |
| Uric acid (μmol/L) | 365.00 [291.50–480.50] | 372.50 [318.35–481.75] | 361.00 [256.00–474.00] | 0.41 |
| BUN (mmol/L) | 6.00 [4.80–7.40] | 6.04 [4.79–7.40] | 6.00 [5.10–7.40] | 0.753 |
| eGFR (mL/min/1.73 m2) | 111.76 [91.02–119.27] | 111.76 [92.46–120.15] | 111.62 [89.87–118.32] | 0.891 |
| NT-proBNP (pg/mL) | 768.60 [370.15–1525.50] | 613.65 [291.05–1125.25] | 899.10 [377.80–1782.00] | 0.179 |
| Neutrophil counts (× 1012/L) | 3.98 [3.10–4.87] | 4.17 [3.28–5.10] | 3.81 [2.87–4.63] | 0.189 |
| Lymphocyte counts (× 1012/L) | 2.06 ± 0.63 | 1.87 ± 0.60 | 2.23 ± 0.62 | 0.011 |
| InALI | 4.13 [3.76–4.38] | 3.94 [3.58–4.33] | 4.16 [3.97–4.39] | 0.044 |
| CMR imaging | ||||
| LVEF (%) | 23.11 [15.61–28.94] | 22.55 [15.46–28.91] | 23.99 [16.26–28.80] | 0.498 |
| LVMI (g/m2) | 68.25 (10.98) | 69.93 (10.99) | 66.69 (10.87) | 0.191 |
| Native T1 (ms) | 1056.00 [1028.90–1104.80] | 1063.50 [1038.07–1109.97] | 1047.00 [1018.80–1079.50] | 0.184 |
| LGE presence | 59 (74.7) | 30 (78.9) | 29 (70.7) | 0.562 |
| LGE extent (%) | 24.93 [18.30–32.91] | 24.59 [18.85–31.96] | 25.86 [17.20–33.21] | 0.814 |
| ECV (%) | 29.90 [27.35–32.60] | 31.60 [28.73–37.05] | 28.70 [26.20–31.00] | 0.002 |
- Note: Values are mean (IQR) or mean ± SD for continuous variables and numbers (%) for categorical variables.
- Abbreviations: Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CMR, cardiovascular magnetic resonance; DM, diabetes mellitus; ECV, extracellular volume; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL-c, high-density lipoprotein cholesterol; HGB, haemoglobin; InALI, natural log-transformed advanced lung cancer inflammation index; IQR, inter-quartile range; LDL-c, low-density lipoprotein cholesterol; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; LVRR, left ventricular reverse remodelling; NLR, neutrophil-to-lymphocyte ratio; NT-proBNP, N-terminal B-type natriuretic peptide; NYHA, New York Heart Association; OSAS, obstructive sleep apnoea syndrome; PLT, platelet count; RBCs, red blood cells; TBIL, total bilirubin; TC, total cholesterol; TG, triglyceride; WBCs, white blood cells.
|
Crude OR (95% CI) |
P value |
Adjust model OR (95% CI) |
P value | |
|---|---|---|---|---|
| InALI | 3.63 (1.32–11.6) | 0.019 | 3.16 (1.06–10.8) | 0.049 |
- Note: Adjusted model: adjusted for ECV and heart rate.
- Abbreviations: ALI, advanced lung cancer inflammation index; CI, confidence interval; ECV, extracellular volume; HR, heart rate; InALI, natural log-transformed advanced lung cancer inflammation index; LVRR, left ventricular reverse remodelling; OR, odds ratio.
Discussion
Our study indicates a linear correlation of InALI with all-cause mortality and cardiovascular mortality in patients with heart failure. Elevated levels of InALI have been linked to a reduced likelihood of mortality in individuals suffering from heart failure. This is consistent with the results of previous studies on ALI in patients with heart failure that ALI is a prognostic marker for patients with heart failure.18 Compared with BMI, Alb, NLR and PNI, InALI demonstrated superior predictive capability for long-term prognosis in patients with heart failure. Furthermore, InALI is associated with left ventricular remodelling in patients with HFrEF. To our knowledge, this is the first study to explicitly demonstrate the association of ALI with the risk of all-cause mortality and cardiovascular mortality in patients with heart failure, as well as the first to suggest a potential link between ALI and left ventricular remodelling in these patients.
Heart failure remains a public health challenge and a significant obstacle in cardiovascular disease management, with its prognosis influenced by various factors, including underlying disease and treatment regimen.19, 20 Inflammation and nutritional status have been identified as significant prognostic markers for individuals diagnosed with heart failure.21, 22 Numerous studies have shown that patients with heart failure exhibit elevated levels of systemic inflammation,23 which involves excessive activation of immune cells that release numerous inflammatory mediators. This leads to structural changes in the heart and subsequent decline in cardiac systolic or diastolic function.24 Additionally, the nutritional status of patients with heart failure is closely linked to their prognosis due to its association with malnutrition.25, 26 Poor nutritional status can exacerbate inflammation in patients with heart failure, creating a detrimental cycle.27 Hence, there is a necessity for a thorough index that considers both nutritional levels and inflammatory status in order to evaluate the prognosis of individuals suffering from heart failure.
ALI—a composite index comprising BMI, Alb and NLR—was initially utilized for prognostication in patients with lung cancer, from which its name originated.4 However, its primary function is to evaluate the comprehensive inflammation and nutritional status of patients. Consequently, ALI has been utilized in patients other than those with lung cancer. To date, ALI has been linked to adverse outcomes in cardiovascular disease, endocrine disorders and other ailments.10, 28-31 The objective of this study was to further investigate the correlation between ALI and the likelihood of all-cause mortality and cardiovascular mortality in individuals diagnosed with heart failure. After establishing the correlation between ALI and these risks, we elucidate the relationship between ALI and cardiac structural changes. Our study initially uncovered a correlation between ALI and the risks of both all-cause mortality and cardiovascular mortality in individuals with heart failure. Subsequent studies confirmed the associations of ALI with LVRR and ECV. Previous research has indicated a connection between ECV and the extent of cardiac fibrosis,32 suggesting that ALI may also predict cardiac fibrosis severity. These findings suggest that higher ALI levels indicate a more favourable prognosis and less severe cardiac structural changes in patients with heart failure.
As a composite index integrating BMI, Alb and NLR, we believe that these results can be explained in several ways. First, NLR is a traditional inflammation marker that reflects the degree of inflammation in the body. Our results revealed that the NLR levels in the Tertile 2 and 3 groups gradually decreased compared with the Tertile 1 group, reflecting an improved prognosis in patients with heart failure by reducing inflammation. This aligns with previous research findings.33-35 Additionally, NLR levels have been associated with cardiac structural changes. Previous studies have indicated that NLR may serve as an indicator of infarct size in patients with myocardial infarction.36-38
Second, Alb, a marker of nutritional status, has recently been found to protect the cardiovascular system through anti-inflammatory and antioxidant mechanisms.39, 40 Our findings show that Alb levels in the Tertile 2 and 3 groups were significantly higher than in the Tertile 1 group. Previous studies have linked low Alb levels in patients with heart failure to poor prognoses, suggesting that Alb improves nutritional status and regulates inflammation. Moreover, increasing Alb levels in patients with heart failure helps alleviate oedema and other clinical symptoms.
Finally, the use of BMI as an indicator for evaluating the nutritional status of patients with heart failure has been a topic of ongoing debate. In obese individuals, heightened activation of the renin–angiotensin–aldosterone system and increased production of pro-inflammatory factors by adipose tissue result in chronic inflammation. Notably, this study found that BMI levels were significantly higher in the Tertile 2 and 3 groups than in the Tertile 1 group, highlighting the paradoxical relationship between obesity and heart failure.41, 42 This finding may be linked to the protective role of adiponectin secreted by obese individuals. Furthermore, it is important to note that while BMI is widely used as an assessment tool, it does not accurately reflect fat distribution and may introduce biases.43
ALI is a comprehensive indicator of nutritional status and inflammatory state in patients with heart failure. Compared with the PNI, it takes into account BMI and neutrophil count, and we believe that it can better reflect the nutritional and inflammatory status of patients. A higher ALI indicates better nutritional status and lower inflammation, promoting cardiac structure recovery and improved prognosis. A recent study found that ALI is associated with prognosis in the population after acute myocardial infarction, and the prediction effect is better than NLR, which further indicates that the combination of inflammation and nutritional status is critical to evaluating prognosis in patients with cardiovascular disease.44 Therefore, ALI can serve as a clinical composite index for assessing prognosis and changes in cardiac structure in patients with heart failure, thereby facilitating the development of appropriate treatment strategies.
This study has several strengths. First, Study Cohort 1 employed a comprehensive weighted analysis to demonstrate the generalizability of the findings to the US population and firmly established the association between ALI and mortality of patients with heart failure. Second, we utilized diverse analytical approaches to validate the robustness of our results. Third, Study Cohort 2 uniquely elucidated the correlation between ALI and cardiac remodelling for the first time. Finally, we applied various analytical methods to ensure the reliability of our findings.
Limitations
Our study has several limitations. First, it could not establish a definitive causal relationship due to its observational nature. Second, the static nature of our data collection limits our ability to assess dynamic changes in ALI. Third, incomplete adjustment for confounding factors in Study Cohort 1 may have led to the loss of some essential variables. Fourth, due to the limitation of the NHANES database, we cannot identify the specific classification of patients with heart failure. Because the pathological mechanism of patients with different types of heart failure is different, it is necessary to further study the predictive effect of ALI on the outcome of patients with different classifications of heart failure. Last, the small sample size of Study Cohort 2 may have resulted in missing relevant indicators related to ALI, highlighting the need for further expansion of clinical samples.
Conclusions
Elevated levels of ALI were found to be correlated with decreased risks of all-cause mortality and cardiovascular death in patients with heart failure, as well as with LVRR.
Acknowledgements
The authors express their gratitude for the contributions of all participants and investigators involved in the NHANES study.
Conflict of interest statement
The authors declare that they have no competing interests.
Funding
This research was supported by the Key Technologies Research and Development Program (Nos. 2021YFF0501404 and 2021YFF0501403) and the Taishan Scholars Program of Shandong Province (LQ), and the Shandong Provincial Natural Science Foundation (No. ZR2022QH056).
Open Research
Data availability statement
Data are unavailable.




