Trametinib alters contractility of paediatric Noonan syndrome-associated hypertrophic myocardial tissue slices
Abstract
Aims
No curative treatment is available for RASopathy-associated childhood-onset hypertrophic cardiomyopathy (RAS-CM). Preclinical data and individual reports suggest a beneficial effect of small molecules targeting the RAS–mitogen-activated protein (MAP) kinase (MAPK) pathway in severely affected RAS-CM patients. The aim of this study was to evaluate the biophysical effects of trametinib, rapamycin and dasatinib on cultivated myocardial tissue slices of a paediatric RAS-CM patient using biomimetic cultivation chambers (BMCCs) and to correlate the findings with clinical data.
Methods
Contracting right ventricular (RV) tissue slices were prepared from resected myocardium, cultivated in BMCCs and treated with distinct molecules directly and indirectly targeting the RAS–MAPK pathway (trametinib, rapamycin and dasatinib) or dimethyl sulfoxide (DMSO). Tissue biophysical properties were assessed using electrical stimulation protocols. Contractile function, force–frequency relationship and post-pause potentiation were compared before and after treatment. These parameters correlated to L-type Ca2+ channel function and sarcoplasmic Ca2+ loading.
Results
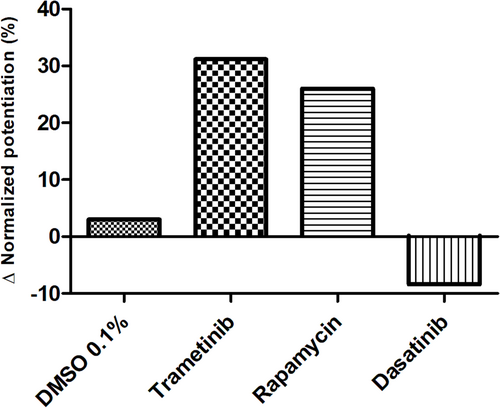
In vivo, off-label treatment with MAPK kinase (MEK) inhibitor trametinib of a child with severe RAS-CM resulted in a modest reduction of RV outflow tract (RVOT) obstruction (RVOT 151 to 122 mmHg after 11 weeks) and improved diastolic function (E/A 0.68 to 1.09 after 11 weeks) and myocardial strain [RV global radial strain (RV-GRS) 25.94 to 42.76; RV global circumferential strain (RV-GCS) −15.26 to −18.61; and RV global longitudinal strain (RV-GLS) −10.31 to −16.78 at 11 weeks], as determined by echocardiography and cardiac magnetic resonance tomography. In cultivated RV myocardial tissue slices, contraction force decreased after addition of trametinib and rapamycin but not after addition of DMSO and dasatinib. Improvement of Ca2+ handling, as depicted by a more positive force–frequency relationship and enhanced post-pause potentiation (31.2%), was noted in the trametinib-treated slice. The increase in post-pause potentiation was less pronounced in rapamycin-treated (26%) and absent in dasatinib-treated (<1%) slices.
Conclusions
Ex vivo analysis of cultivated and electrically stimulated RV myocardial tissue slices of a patient with RAS-CM showed decreased contractility and improved sarcoplasmic reticulum function after addition of trametinib and in part after addition of rapamycin, but not after addition of dasatinib.
Introduction
Paediatric hypertrophic cardiomyopathy (HCM) is often associated with Noonan syndrome (NS), the most common disease within the RASopathy spectrum. NS is prevalent in 1:1000 to 1:2500 children.1 Children affected by NS often suffer from developmental disorders and congenital heart disease (CHD). Early-onset HCM occurs in ~20% of NS patients and is associated with significant morbidity and mortality.2, 3 NS is caused by a wide range of autosomal-dominant variants within genes in the RAS–mitogen-activated protein kinase (MAPK) transduction cascade and therefore falls under the umbrella term of RASopathies. Common mutated genes are PTPN11, SOS1, KRAS, LZTR1 and RAF1.4, 5 RAF1 pathogenic variants are accountable for 3%–17% of the NS cases.6 NS-causing variants of the RAF1 gene are associated with a gain of its in vitro phosphatase activity.7 Contrary to other NS-inducing pathogenic variants, HCM is more characteristic for patients with RAF1 variants than pulmonary valve stenosis.8
In the past decade, inhibitors for the RAS–MAPK pathway have been investigated as remedies for RASopathy-associated childhood-onset hypertrophic cardiomyopathy (RAS-CM). Rapamycin9 and dasatinib10 reverted HCM in RAS-CM mouse models. Results have not been conclusive for rapamycin in the clinical setting. In two cases of the same PTPN11 variant, rapamycin treatment did not lead to a reduction in myocardial fibrosis or hypertrophy and only led to a reduction of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels in one case.11, 12 Conversely, in vitro,13 in vivo preclinical14 and clinical studies15 have shown mitigating effects of the MAPK kinase (MEK) inhibitor trametinib on RAS-CM (reviewed by Gelb et al. and observed by others16-19). Trametinib is a reversible and highly selective MEK1/2 inhibitor (MEKi), which has been Food and Drug Administration (FDA) approved for the treatment of metastatic melanomas20 and other carcinomas.21, 22 However, the mechanisms underlying the beneficial effects of trametinib on cardiomyocyte function are not yet fully understood.
The aim of this study was therefore to evaluate the biophysical effects of small molecules directly or indirectly targeting the RAS–MAPK signalling pathway (trametinib, rapamycin and dasatinib) on cultivated right ventricular (RV) myocardial tissue slices of a paediatric RAS-CM patient using biomimetic cultivation chambers (BMCCs) and to correlate the findings with clinical data. Sarcoplasmic reticulum (SR) function and calcium ion handling were assessed during cultivation by particular stimulation sequences. Contraction data of sequential RV slices before and after treatment were analysed, and trametinib treatment was compared with rapamycin and dasatinib treatment.
Methods
Tissue was obtained, and the study was performed according to the Declaration of Helsinki guidelines. The patient or their legal guardian provided their written informed consent prior to tissue collection {384/15 and 732/20S by the Ethics Committee of the Technical University of Munich, Germany [NS-HCM patient]; 063-12 by the LMU Clinic, Munich, Germany [heart transplant (HTx) patient]}. Original data for these studies are available upon reasonable request.
Myocardial slice preparation
Hypertrophic RV tissue was obtained via a resection procedure of the RV outflow tract (RVOT) in a 7-year-old male patient diagnosed with NS and severe RVOT obstruction warranting resection. Tissue was immediately transferred into a closed cardioplegia (4°C)-filled container and kept at 4°C. After transfer into a flow hood, four 0.5 × 0.5-cm-sized and 300-μm-thick tissue slices were produced using a vibratome according to the protocol developed by Fischer et al.23 and described in detail by Hamers et al.24 Briefly, tissue was embedded in 4% low-melt agarose and cut in 300-μm-thick slices using a vibratome (VT1200s, Leica AG, Wetzlar, Germany). Plastic laser-cut triangles (made in-house) were attached to the slices and placed in a commercially available BMCC on a rocker (60 r.p.m.) (InVitroSys GmbH, Gräfelfing, Germany) in a CO2 incubator (5%; CB240, Binder GmbH, Tuttlingen, Germany) at 37°C. BMCCs contained medium M199 (31150-022, Thermo Fisher, Bremen, Germany) [supplemented with 5% Insulin-Transferrin-Selenium 100× (5150056, Thermo Fisher, Bremen, Germany), 5% Penicillin–Streptomycin (P0781-100ML, Sigma, Taufkirchen, Germany) and 50 mM β-mercaptoethanol (A1108.0100, AppliChem, Darmstadt, Germany)]. The tissue slices were continuously electrically stimulated to contract at 30 b.p.m. Additionally, tissue slices were also prepared, cultivated and treated (n = 2) from the RV of the explanted heart of an HTx patient, following the same protocol as for the paediatric tissue slices. The 51-year-old patient was diagnosed with dilated cardiomyopathy after a viral myocarditis (diagnosed in 2019) and transmural fibrosis.
Cultivation and treatment in BMCCs
Tissue slices were initially cultivated for 180 h to allow for the original trametinib treatment of the patient to wash out and to equilibrate to the ex vivo environment. After this period, four slices were treated with trametinib (GSK1120212, Selleckchem GmbH, Cologne, Germany), rapamycin (S1039, Selleckchem GmbH, Cologne, Germany), dasatinib (SML2589, Merck KGaA, Darmstadt, Germany) (all 100 nM) or dimethyl sulfoxide (DMSO) vehicle (0.1%) as control with medium change (every 2 days). Contraction force was analysed using LabChart Pro 8 (ADInstruments, Dunedin, New Zealand) and measured by an in-house developed macro, which averaged the contraction of the slice in a 5 min block for each hour of the cultivation. The average contraction force of the slices was normalized to the contraction force at the start of the cultivation.
Electrical stimulation protocols
Before and during treatment cultivation, distinct stimulation protocols were run to assess the contractile and electrophysiological status of the slices. Stimulation protocols consisted of post-pause potentiation (PPP) and force–frequency relationship (FFR). During PPP, electrical stimulation was halted for 120 s. This allows for Ca2+ loading of the SR. The contraction force induced by the first electrical stimulation after the pause was calculated as a percentage of the average twitch force of the five last cycles before the pause. This was done before, at 4 days and at 8 days after treatment. During FFR assessment, the electrical stimulation frequency is increased in a stepwise fashion, while the duration of the respective step is shortened to keep the number of induced contractions constant (20–40 beats/step). After 380 h of cultivation, of which 180 h with treatment, the slices were removed from the BMCCs.
Statistics
To compare the contractility over time after treatment start (Figures 4A and S2A), a two-way ANOVA with Dunnett correction for multiple comparisons was performed. For each contractility comparison before and after medium change (Figure 4B), a Mann–Whitney U test was performed. To compare the potentiation of the HTx slices (Figure S2B), an ordinary one-way ANOVA with Dunnett correction for multiple comparisons was performed. All statistics were performed, and graphs were made using GraphPad Prism (Version 9.3.1, Boston, USA).
Results
Clinical patient characteristics
At 8 weeks of age, a male patient presented for the first time with hypertrophic obstructive cardiomyopathy, extensive left ventricular outflow tract obstruction and pulmonary hypertension resulting in heart failure with tachydyspnoea and the need for high-flow oxygen therapy. Electrocardiogram showed signs of ventricular hypertrophy without arrhythmias (Figure S1). The patient's mother and sister had previously been diagnosed with NS prior to the current patient and also showed HCM. NS was clinically diagnosed and confirmed by a genetic panel showing a pathogenic variant in the RAF1 gene [c.775T>A (p.Ser259Thr)]. Phosphorylation of residue Ser259 results in autoinhibition of RAF1, while the pathogenic variant Thr259 leads to a gain-of-function phenotype by facilitating binding of GTP-bound RAS.25 The prenatal and immediate perinatal course of this patient had been uneventful. After initial treatment with high-dose beta-blocker and disopyramide (at the time of treatment, institution guidelines contraindicated the use of calcium channel antagonists to avoid hypotension and deteriorating outflow tract obstruction), the current patient underwent open heart surgery for left ventricular outflow tract resection at the age of 14 months.
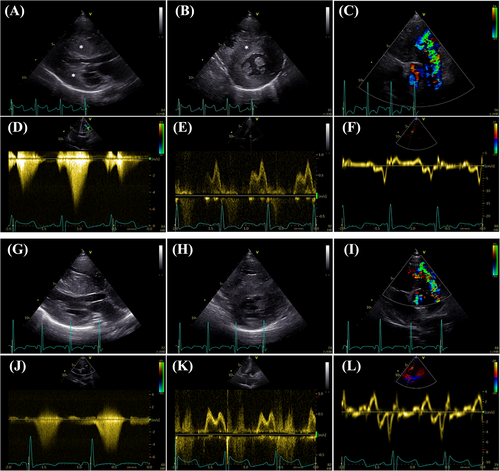
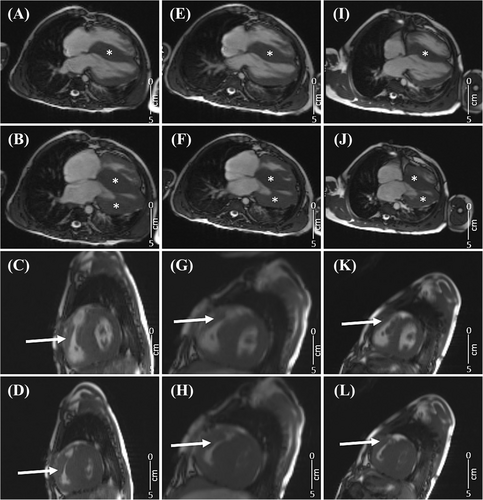
At 7.5 years of age, the patient (22.2 kg) was re-admitted due to aggravation of hypertrophy and severe obstruction in the RVOT in the absence of significant left ventricular outflow tract obstruction. An off-label treatment with the MEKi trametinib (Novartis Germany AG, Nuremberg) was initiated (0.011 mg/kg/day at start; increased to 0.023 mg/kg/day after 7 days), which resulted in a decrease of the peak RVOT gradient from initial 151 to 124 mmHg (Table 1 and Figure 1A), a reduction of the cardiac insufficiency marker NT-proBNP z-scores from 6.5 to 5.2 (Figure 1B), improvement of diastolic dysfunction as depicted by reversal of the abnormal mitral valve E to A ratio on echocardiography (Figure 2), and an improvement of right and left ventricular strain parameters on cardiac magnetic resonance (CMR). The left ventricular ejection fraction remained unchanged and hyperdynamic throughout the course of trametinib (Table 1). Representative echocardiographic and CMR images from the time points prior and after initiation of MEKi treatment are presented in Figures 2 and 3.
| Prior to treatment with MEKi trametinib | 1 week after treatment with MEKi trametinib | 11 weeks after treatment with MEKi trametinib | |
|---|---|---|---|
| TTE | |||
| Peak dP LVOT (mmHg) | 12 (no LVOTO) | 9 (no LVOTO) | 7 (no LVOTO) |
| Peak dP RVOT (mmHg) | 151 | 124 | 122 |
| IVSd max (mm) | 27 | 26 | 27 |
| IVSd max (z-score; Detroit) | 7.4 | 7.2 | 7.4 |
| LVPWd max (mm) | 18 | 18 | 16 |
| LVPWd max (z-score; Detroit) | 6.7 | 6.7 | 6.0 |
| LVEF (%) | 91 | 94 | 94 |
| E (m/s) | 0.63 | 0.82 | 0.62 |
| A (m/s) | 0.92 | 0.86 | 0.57 |
| E/A | 0.68 | 0.95 | 1.09 |
| e′ (cm/s) | 0.04 | 0.08 | 0.06 |
| Septal E/e′ | 16 | 10 | 11 |
| E deceleration time (ms) | 304 | 289 | 181 |
| IVRT (ms) (measured on pulsed-wave Doppler) | 143 | 110 | 72 |
| IVCT (ms) (measured on pulsed-wave Doppler) | 63 | 49 | 82 |
| E-wave duration (ms) | 124 | 180 | 168 |
| A-wave duration (ms) | 121 | 131 | 164 |
| Comment | TR velocity not applicable given severe RVOTO | TR velocity not applicable given severe RVOTO | TR velocity not applicable given severe RVOTO |
| Heart rate (min−1) | 85 | 88 | 74 |
| CMR | |||
| CI (L/min/m2) | 3.2 | 3.5 | 3.0 |
| RVEDV:LVEDV | 1:1 | 1:1 | 0.8:1 |
| RVEF (%) | 66 | 69 | 73 |
| RVEDV (mL) | 49 | 53 | 45 |
| RVEDVI (mL/m2) | 64 | 70 | 58 |
| LVEF (%) | 74 | 72 | 72 |
| LVEDV (mL) | 51 | 51 | 58 |
| LVEDVI (mL/m2) | 67 | 67 | 74 |
| LV mass (g/m2) | 158 | 152 | 146 |
| RV-GRS | 25.94 | 22.84 | 42.76 |
| RV-GCS | −15.26 | −11.84 | −18.61 |
| RV-GLS | −10.31 | −11.18 | −16.78 |
| LV-GRS | 20.5 | 23.52 | 29.12 |
| LV-GCS | −13.56 | −15.07 | −17.57 |
| LV-GLS | −10.32 | −9.46 | −13.89 |
- Abbreviations: CI, cardiac index; IVCT, isovolumic contraction time; IVRT, isovolumic relaxation time; IVSd, interventricular septal thickness; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LV-GCS, left ventricular global circumferential strain; LV-GLS, left ventricular global longitudinal strain; LV-GRS, left ventricular global radial strain; LVOT, left ventricular outflow tract; LVOTO, left ventricular outflow tract obstruction; LVPWd, left ventricular posterior wall thickness; MEKi, MEK1/2 inhibitor; RVEDV, right ventricular end-diastolic volume; RVEDVI, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; RV-GCS, right ventricular global circumferential strain; RV-GLS, right ventricular global longitudinal strain; RV-GRS, right ventricular global radial strain; RVOT, right ventricular outflow tract; RVOTO, right ventricular outflow tract obstruction; TR, tricuspid regurgitant.
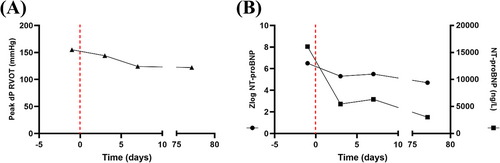
Despite an initial RVOT gradient reduction under trametinib treatment, severe RVOT obstruction remained with gradients between 110 and 120 mmHg after 5 months of MEKi treatment (Figures 1B and 3K,L). This prompted cardiac surgery for the resection of the RVOT obstruction. The resected RV myocardial tissue was further processed in an experimental setting, where four RV myectomy slices were generated. Slices were cultured for 380 h in BMCCs on a mechanical rocker at 37°C (5% CO2).
Contraction force development during cultivation
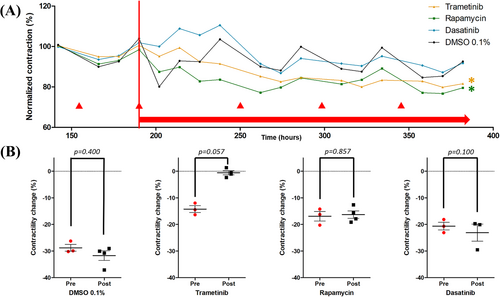
After placement of the prepared myocardial tissue slices in BMCCs on the rocking device, the contraction force of the slices stabilized and was normalized to its value at 6 days (i.e., 140 h) of culture (pre-treatment baseline) (Figure 4). In all slices, every medium change resulted in a temporary depression of contraction force (▴, Figure 4), and therefore, these data points are omitted from the timelines. After 190 h of cultivation, treatment with rapamycin, dasatinib or trametinib was initiated. When comparing the contractile force of each slice after treatment start (Figure 4), the trametinib-treated (P = 0.023) and rapamycin-treated (P ≤ 0.001) slices showed a significantly decreased contraction force compared with DMSO 0.1%-treated control. Intriguingly, treatment with trametinib, but not any other compound, tended to abrogate the temporary depression of contraction force induced by the medium change (P = 0.057; Figure 4B). In a separate RV myocardial slice preparation and cultivation from a 51-year-old female HTx patient, a similar significant effect of trametinib (P < 0.001) and rapamycin (P = 0.016) on contraction force was observed after an identical ex vivo treatment regimen compared with vehicle control (Figure S2A).
Assessment of SR function by PPP and FFR
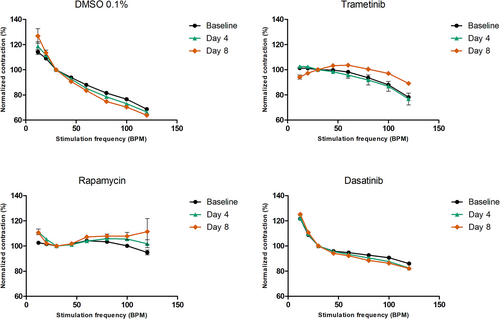
PPP correlating to sarcoplasmic Ca2+ loading was assessed before and after treatment. Vehicle treatment (DMSO 0.1%) did not affect PPP. Trametinib and rapamycin treatment resulted in a respectively 31.2% (173.3% pre-treatment to 204.5% post-treatment) and 26% (181% pre-treatment to 207.4% post-treatment) increase of potentiation compared with pre-treatment (Figure 5), reflecting increased sarcoplasmic Ca2+ loading. Dasatinib treatment lowered the potentiation by 9% (202.6% pre-treatment to 193.6% post-treatment). In the separate RV HTx preparation, trametinib showed the strongest potentiation increase (P = 0.195) (Figure S2B). The FFRs of the four individual NS patient tissue slices [reflecting L-type Ca2+ channel (LTCC) function] were slightly different in shape prior to treatment (Figure 6). However, 4 and 8 days of vehicle and dasatinib treatment did not alter the shape of the FFR. Conversely, trametinib treatment lowered the contraction force at low frequencies and increased the contraction force at higher frequencies after 8 days of treatment. Rapamycin treatment increased the contraction force at both lower and higher frequencies. The RV tissue slices from the HTx patient did not show any noteworthy changes in FFR before and after treatment.
Discussion
This is the first report to describe the effect of trametinib on myocardial properties both in vivo and ex vivo in a paediatric patient with NS carrying a RAF1 pathogenic variant. The child suffered from severe RAS-CM and received off-label treatment with trametinib in the hope to avoid cardiac surgery for severe RVOT obstruction. While improvement of echocardiographic diastolic function and CMR global strain parameters suggested beneficial treatment effect, the decrease in the RVOT gradient was not sufficient so that surgical resection was warranted. Resected RV myocardial tissue was obtained for further ex vivo investigations. Using an established myocardial slice cultivation technique, as described by Fischer et al.,23 the direct effects of trametinib, rapamycin and dasatinib were subsequently studied on electrically stimulated, contracting slices. The most important findings included a significant down-regulation of contractile function observed in trametinib- and rapamycin-treated slices from both the paediatric NS-HCM and HTx patients. SR function improved as well, as indicated by the increased PPP effect after trametinib treatment. The FFR of the trametinib-treated slice showed the strongest change towards an all-positive slope in the NS-HCM slices, but not in the slices of the HTx patient.
Downregulation of contractility by trametinib and rapamycin
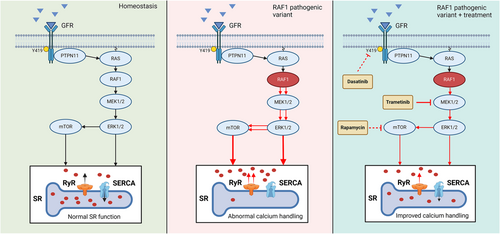
In vivo treatment of a paediatric NS-HCM patient led to a reduction in RVOT gradient and ameliorated radial strain, which is suggestive of reduced contractility. Similarly, an ex vivo down-regulation of contractile force was observed in the trametinib- and rapamycin-treated slices of the same patient but not in the dasatinib-treated slice. The difference in contractile force regulation between these pharmacological treatments is possibly caused by the difference in their treatment targets. Here, we used compounds that modulate the MAPK pathway at different levels, namely, PTPN11, MEK1/2 and the mammalian target of rapamycin (mTOR) (Figure 7). It is important to note that dasatinib inhibits PTPN11, which is located upstream of the pathogenic RAF1 variant. Such upstream inhibition might not impact SR function in a patient with a gain-of-function RAF1 variant. Both trametinib and rapamycin act downstream of the pathogenic RAF1 variant at different levels. Trametinib is a highly selective MEKi. As RAF1 forms a complex with MEK1/2 and extracellular signal-regulated kinase 1/2 (ERK1/2),26 inhibition of MEK1/2 prevents the formation of this complex. Rapamycin inhibits mTOR, which is located further downstream of the pathogenic RAF1 variant within the MAPK pathway. Because both trametinib and rapamycin act downstream of the pathogenic RAF1 variant, they may aid in restoring nominal Ca2+ handling and SR loading. In the HTx slices from a patient with dilated cardiomyopathy, trametinib reduced contractile force more than rapamycin. This supports our hypothesis that trametinib influences Ca2+ handling.
Trametinib enhances PPP
To further elucidate Ca2+ handling of each treated slice, PPP and FFR stimulation protocols were run and compared with DMSO 0.1%-treated control slices. As part of a predesigned electrical stimulation protocol, electrical stimulation was temporarily halted for 120 s to assess the relative contribution of SR Ca2+ release via the ryanodine receptor (RyR) in enhancing contractility. Halting the electrical stimulation of the slices leads to Ca2+ loading of the SR. With the first stimulated contraction, the increased Ca2+ load of the SR is released through the RyR, leading to a more powerful contraction than observed during continuous electrical stimulation. The PPP can therefore be used as a parameter to assess the relative contribution of Ca2+ released from the SR.24 Analysis of the PPP revealed that trametinib and rapamycin increased the relative contribution of Ca2+ release from the SR via the RyR. Conversely, dasatinib had no effect on this parameter. Similarly, in the HTx procured slices, trametinib showed the strongest potentiation increase compared with the control-treated slice.
The increase in potentiation observed in the trametinib-treated slice could be an effect of the drug's inhibitory effect on hyperphosphorylation of the RyR located on the SR.27 The myocardial tissue in this study was resected from a patient carrying a gain-of-function RAF1 variant. RAF1 activation leads to the reduced expression of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA),26, 28 and variants of this gene in particular are associated with the activation of the Ca2+/calcineurin.28 This consequently will impair Ca2+ sequestration in the SR and cause diastolic accumulation of cytosolic Ca2+ ([Ca2+]Cyt). This hypothesis is in line with a study generating iPSC-CM of an NS-HCM patient with a similar RAF1 variant (Ser257Leu). Here, a reduction in SERCA/phospholamban (PLN) ratios compared with non-affected iPSC-CM was observed, in combination with significantly reduced Ca2+ transients between cytosol and SR.26 Furthermore, iPSC-CM generated from an NS patient with an LZTR1 variant showed a significant increased Ca2+ leak from the SR, which was attenuated with the addition of calcium channel blocker.5 An increased [Ca2+]Cyt promotes the binding of Ca2+ to calmodulin, followed by the activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII). CaMKII phosphorylates the RyR2,29 also located in the SR membrane, stimulating the release of Ca2+ from the SR,30 thereby possibly further aiding in the increase of [Ca2+]Cyt. In addition, CaMKII inhibits SERCA29 and therefore promotes Ca2+ leakage from the SR.
Trametinib increases contraction force at higher beating frequencies
The FFR assesses the entry of Ca2+ into the myocardial cells as a function of stimulation frequency and contractility and aids in the assessment of excitation–contraction coupling.31 [Ca2+]Cyt is maintained by SR Ca2+ loading, LTCC and Ca2+/Na+ exchanger.31 With an increasing stimulation frequency, the [Ca2+]Cyt increases.31 Hence, the contraction force increases directly to the increase in stimulation frequency. Literature suggests that healthy myocardial tissues show a positive FFR.32 A flat or negative FFR is characteristic for a failing heart.32 The FFRs of the four slices were already different at baseline, which may reflect a difference in composition and morphology as has previously been observed.32 However, prior to initiation of the treatment, repeated measurements of the FFR within one slice yielded highly similar results, as indicated by the small error bars (SEM). Previous studies have described increased intracellular Ca2+ levels and currents in HCM patients.33 This is in line with the previously described observation in NS patients that a pathogenic RAF1 variant is associated with reduced SERCA expression and promoted activation of Ca2+/calcineurin. Increased [Ca2+]Cyt is observed to activate Ca2+/calmodulin, which subsequently facilitates the phosphorylation of LTCCs. This leads to an increased flux of Ca2+ into the cytosol, increasing the [Ca2+]Cyt further.34 Dysregulation of Ca2+ handling leads to an altered contraction of the myocardium.34
The baseline FFR of all four cultivated slices showed a negative or flat form. This observation was to be expected as the patient was diagnosed with CHD, which resulted in heart failure. Similar FFR forms are typical for heart failure patients and are associated with alterations in cardiac mineral handling.35 Previous research using the identical scientific set-up observed a positive FFR or negative FFR when human diseased left ventricular tissue slices had been cultivated at lower [0.12 Hz (7 b.p.m.)] or higher [0.5 Hz (30 b.p.m.)] baseline stimulation frequencies.23, 24 The plateau phase of the FFR diminished after long-term cultivation of 4–16 weeks.23 When increasing the [Ca2+]Cyt by adding Bay K8644, the FFR transitioned into an exclusively negative FFR as well.24 In the present study, trametinib treatment changed the FFR from negative to mixed positive/negative FFR. The molecular mechanism behind the observation seen after treatment of trametinib is similar in the FFR as in the PPP. Trametinib inhibits MEK1/2, counteracting the gain-of-function RAF1 activity. This lowers the Ca2+ leak from the SR, decreasing the [Ca2+]Cyt and partly restoring the function of RyR and SERCA. In case there would be a full restoration of the SR-located calcium channels, a positive FFR was expected. This might be a first step towards an FFR resembling healthy myocardial tissue. Dasatinib and rapamycin treatment did not change FFR.
The cardiomyocytes of the slices used in this study presumably have high [Ca2+]Cyt due to dysfunctioning Ca2+ handling. It is speculated that at higher stimulation frequencies, Ca2+ leaks out of the SR at higher rates, preventing the necessary calcium spikes needed for proper myocardial contraction. Alternatively, another study hypothesized that LTCC and RyR are located closely together to facilitate rapid intracellular calcium release. In the failing myocardium, T-tubule remodelling causes a greater distance between LTCC and RyR, resulting in decreased cardiac contractility.36 However, literature on T-tubule composition regarding LTCC in NS hearts is lacking.
Limitations
The main limitation of our study is the limited number of samples available and the use of an adult HTx patient with dilated cardiomyopathy as a control. NS-HCM is a rare disease, and the amount of resection tissue is very limited (two 5 × 5 × 10 mm samples). With a typical tissue slice size in our culture system of 7 × 7 mm with a thickness of 300 μm, we were limited to four NS-HCM slices. As a control preparation, eight adult HTx slices were made. Compared with the four NS-HCM slices, these differed in size, biopsy location, and patient characteristics like age and gender. These slices were treated with trametinib, rapamycin or dasatinib. There is a limited availability of literature on the use of these drugs in the NS setting, and we are the first to compare the effects of these drugs on intact tissue from a patient with NS-HCM. Our results point towards aberrant Ca2+ handling in the tissue slices, but it is unfortunately not possible to perform Ca2+ measurements in our system. The study described here would have benefited from calcium measurements, supported by measurements of the activity and expression levels of the LTCC, RyR and/or SERCA. Performing those additional experiments was not possible due to the limited size of the tissue biopsy.
An alternative approach that has been used in literature is the use of induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). Various studies have looked into the interaction between trametinib and RAF1 variations in iPSC-CM.26, 37 These predominantly focus on the RAF1 Ser257Leu variation. Although this is a different genetic variant closely located to the Ser259 phosphorylation docking site, it resulted in a 44% reduction in phosphorylation.26 In this conformation, RAF1 will remain in the open, activated conformation. Trametinib treatment in iPSC-CM was shown to normalize MEK1/2 and ERK1/2.37 As discussed before, one study generating iPSC-CM observed lower SERCA/PLN ratios in RAF1 Ser257Leu variant-affected iPSC-CM in combination with significantly reduced Ca2+ transients between cytosol and SR.26 SERCA/PLN ratios were rescued with the application of MEKi. This is in line with our finding that treatment of paediatric NS-HCM myocardial tissue slices with MEKi trametinib improves SR loading, as evidenced by the increased potentiation.
As with iPSC-CM, the cultivation of myocardial tissue slices in our set-up requires regular medium change. Medium change of the BMCC tissue cultures led to changes in contractile force (Figure 4B). Here, loss of CO2 in the incubator increased the culture medium pH causing an initially positive inotropic effect.24 We hypothesize that the responses of cardiomyocyte contractility to pH changes involve the Na+/H+-exchanger 1 (NHE1), which increases Na+ influx under alkaline conditions and may be inhibited by SRC-family tyrosine kinases.38 Treatment with trametinib attenuated this effect, suggesting that this treatment protects the myocardium from adverse effects of temporarily increased pH. Even though contraction force is partly influenced by the method itself, the use of the BMCC to culture myocardial tissue slices allows for the tissue to remain intact and exposed to preload and afterload, mimicking the biophysical in vivo environment. Future studies should aim to incorporate Ca2+ measurements in cultivated myocardial tissue slices to further elucidate the role of altered SR Ca2+ handling and evaluate the effects of novel therapies on SR Ca2+ handling in NS-HCM.
Conclusions
The data presented here aid in filling the research gap between the ex vivo and in vivo use of trametinib for the treatment of RAF1 variant-caused NS-HCM. In summary, the ex vivo analysis of effects achieved by the addition of trametinib to cultivated RV myocardial tissue slices of a patient with a gain-of-function RAF1 variant is in line with the observed beneficial effects of in vivo treatment with trametinib, as well as with previous studies in iPSC-CM from patients with NS-HCM caused by pathogenic RAF1 variants.26 Improvement of diastolic and systolic function parameters was observed after treatment start, as measured via echocardiography and CMR tomography. Results of ex vivo experiments provided evidence for decreased normalized contraction force, improved SR Ca2+ loading and LTCC function resulting from the addition of trametinib to cultivated myocardial tissue slices from the same patient in BMCCs. It is unclear if the improvement in contractility seen in vivo and in vitro is a cause or effect of myocardial hypertrophy attenuation during trametinib treatment. However, given the similarity of the effect observed in myocardial slices from the HTx patient, it is likely that there is a direct effect of trametinib on contractile function. Future research should look into the question if trametinib directly lowers hypertrophy, ameliorating the RVOT gradient, which subsequently decreases contractility, or if trametinib primarily lowers myocardial contractility, leading to a lower RVOT gradient and radial strain, allowing the RV to remodel and unload as a secondary effect. Therefore, trametinib should be further investigated as a potential pharmacological treatment for NS-associated HCM in patients with RAF1 gain-of-function variants.
Acknowledgements
The authors would like to thank Mei Ping Wu, Matthias Semisch and Claudia Fahney for the preparation and maintenance of the myocardial slice cultivation. Figure 7 was made using BioRender.
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest statement
AD is a shareholder of InVitroSys GmbH, which developed the MyoDish cultivation system used for this study. CMW is a consultant for Bristol Myers Squibb, Rocket Pharmaceuticals, Day One Biopharmaceuticals, BioMarin Pharmaceutical, Adrenomed AG and Pliant Therapeutics. CMW has an ownership interest in Preventage Therapeutics.
Funding
The study was supported by the German Centre for Cardiovascular Research [Deutsches Zentrum für Herz-Kreislaufforschung (DZHK)] (81X2600253 to AD; 81Z0600207 to DM) and the Deutsche Herzstiftung e.V. (Gerd Killian Award to CMW). CMW is a member of the European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN GUARD-Heart).




