Refractory heart failure due to acquired aortic coarctation after total arch replacement: find the right antidote!
Yi Xie and Chen Lu contributed equally to this article.
Abstract
A 31-year-old male presented with unexplainable symptoms of heart failure including recurrent fatigue and orthopnoea after total arch replacement for type A aortic dissection 2 months ago. Computed tomography angiography detected a severe intra-luminal stenosis, and we successfully implanted a balloon-expandable stent to dilate the stenosis. The patient with improved haemodynamics after endovascular reintervention remains stable at 2 years. The case provides a feasible endovascular therapy for heart failure caused by post-surgery aortic coarctation.
Introduction
Aortic coarctation with a discrete narrowing of the aorta, accounting for ~5% of congenital heart diseases,1 is an uncommon cause of heart failure. Thereinto, adult-onset acquired aortic coarctation, usually secondary to trauma and atherosclerotic/atheroembolic disease, is much rarer and idiopathic to lead to acute heart failure, and there are only sporadic reports.2-4 Endovascular therapy has been reported in post-traumatic aortic coarctation. Here, we describe a case with post-surgery aortic coarctation treated using endovascular therapy and document long-term outcome.
Case report
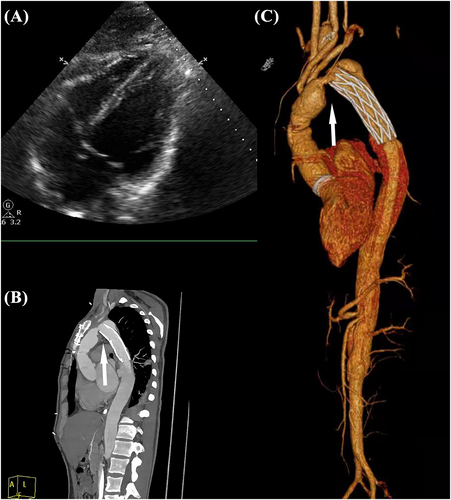
A 31-year-old male, who was discharged uneventfully after a total arch replacement (TAR) using a tetrafurcate graft with frozen elephant trunk (FET) for acute type A aortic dissection 2 months ago, was urgently transferred from a suburb hospital due to refractory heart failure. The patient did not exhibit any forms of vascular vulnerability, including heritable thoracic aortic diseases such as Marfan syndrome, Loeys–Dietz syndrome, and vascular Ehlers–Danlos syndrome, or inflammatory aortitis conditions like Takayasu arteritis and giant cell arteritis, nor did the patient have aortic atherosclerosis. Prior to this re-admission, the patient had already received 1 week of intensive pharmacotherapy in suburb hospital, containing intravenous isosorbide, inotropes, intravenous furosemide, and oral digoxin. However, he remained fatigued, had worsening dyspnoea, and was coughing with pink frothy sputum, and his haemodynamic condition progressively deteriorated in recent 24 h. Thorough physical examination revealed high blood pressure from both arms (160s/80s), contrasting with lower limb measurements at 80s/50s after admission. Echocardiogram (Figure 1) demonstrated a left ventricular ejection fraction of 25.6% with severe global hypokinesis, and blood test of N-terminal fragment brain natriuretic peptides was 17 737 ng/L. Work-up for coronary issues, endocarditis, and valvular diseases was unremarkable. Computed tomography angiography (CTA) (Figure 1) detected a twist between the proximal vascular graft (mobile portion) and the distal stented elephant trunk (relatively fixed portion), causing the severe intra-luminal stenosis.
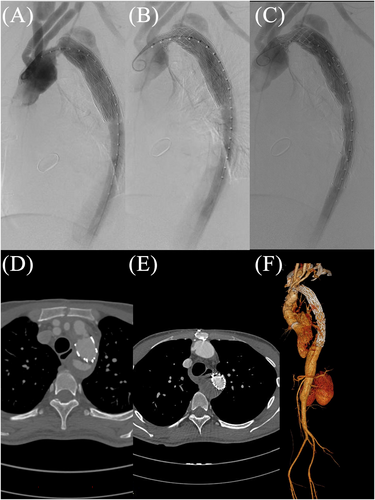
After a multidisciplinary discussion with the heart team, we took a novel endovascular strategy and implanted a balloon-expandable Cheatham Platinum stent (NuMED, Hopkinton, NY, USA) to dilate the stenosis (Figure 2), with transcatheter pressure gradient reduced from 49 to 10 mmHg. A covered stent (Medtronic, Minneapolis, MN, USA) was then deployed (Figure 2) at the distal part of the Cheatham Platinum stent to exclude the residual communicating false lumen within the arch and descending aorta. After intervention, the haemodynamic parameters and clinical condition of this patient improved dramatically. Post-operative CTA showed that the diameter of the stenosis was widened from 6 to 20 mm. At 3 months of follow-up, the patient reported New York Heart Association class I symptoms, and echocardiogram showed an ejection fraction of 51%. There was blood flow in the false lumen proximal to the FET (Figure 2), which may be attributed to the fact that the FET does not completely conform to the aorta. However, it is important to note that this did not affect the aortic coarctation, and throughout the 2 year follow-up, there has been no recurrence of re-coarctation and the thrombosis of false lumen (Figure 2).
Discussion
Heart failure is a heterogeneous clinical syndrome, and it is more likely to consider inherent cardiac aetiology in clinical practice. The refractory heart failure, which failed to respond satisfactorily to optimal therapy, was caused by the stenosis of the vascular graft after Sun's procedure: using a stent graft and a four-branch prosthetic graft in TAR combined with stented elephant trunk implantation. The formation of a unique acquired aortic coarctation might be relevant with the twisting of the vascular graft because of different anatomical characteristics between the mobile vascular graft and the relatively fixed stented elephant trunk.5
Aortic coarctations share the same clinical manifestations and pathophysiology mechanism of heart failure. Whether congenital or acquired aortic coarctation, extreme upper hypertension and lower hypotension are always significant because of the wave reflection from the site of coarctation. Subsequently, profound left ventricular afterload leads to compensatory hypertrophy, which impairs systolic and diastolic function.6, 7 Besides, hypoperfusion of the kidneys, if below the site of coarctation, would activate the renin-angiotensin-mediated humoral system and exacerbate the cardiac dysfunction.8 Patients with aortic coarctation may present with symptoms of left-sided heart failure, including fatigue, dyspnoea, and pulmonary congestion. While heart failure may not always be present in individuals with aortic coarctation, lower extremity fatigue, delayed femoral pulses, and visceral ischaemia may also present.
In addition, in most cases of acquired aortic coarctation, the left ventricular myocardium does not have enough time to adopt to booming afterload and remodel. The imbalance between the increased afterload and the unmodified ventricular wall would damage the cardiac function dramatically and rapidly, and the symptoms of heart failure would appear at the early stage. Thus, timely treatment of acquired aortic coarctation is essential.
Surgical repair or endovascular repair is recommended for adults with aortic coarctation. Surgical repair can be performed by several types, including subclavian flap aortoplasty, resection with end-to-end anastomosis, and a bypass graft across the area of coarctation. However, open surgery is associated with high mortality, especially in elderly patients and those who had undergone previous cardiovascular surgery. In addition, 2020 European Society of Cardiology Guidelines for adult congenital heart disease have recommended endovascular covered stent to be first-line treatment with lower short and long complications.9 Keen and Johnson2 and Thompson et al.4 have previously reported acquired post-traumatic aortic dissection with unusual presentation of acute heart failure safely managed using endovascular repair. The literature10 has reported aortic coarctation resulting from tuberculous aortitis, which was treated with antituberculosis therapy. Acquired coarctation has also been identified in the context of severe atherosclerosis/calcification within the aorta.11 Additionally, there are other aetiologies12, 13 that can lead to acquired aortic coarctation, encompassing inflammatory aortitis, aortic dissection, iatrogenic factors, and so on. In our case, we adopted an endovascular strategy of implanting a balloon-expandable stent to dilate the stenosis of the vascular graft. In addition, our case can be regarded as post-surgery aortic coarctation complicated by type B aortic dissection. Anegawa et al.14 described a case of endovascular treatment of aortic coarctation with type B aortic dissection. But the descending aortic false lumen was rapidly dilated because of increased cardiac output after the stenosis relief, and the patient finally underwent descending aortic replacement. Thus, we also implanted another covered stent (Medtronic) anchored in the FET via the same transfemoral artery access to expand the true lumen in the distal aorta and avoid distal aortic dilation. Improved haemodynamics after endovascular reintervention and excellent echocardiographic findings in the follow-up emphasizes the significance of timely diagnosis and accurate therapy for acquired aortic coarctation. For the preventive measures for non-stented graft stenosis, selecting an appropriate graft size, employing meticulous suturing techniques, avoiding twists and sharp angles in the graft, and ensuring that the graft's diameter is precisely matched to the native aorta can effectively minimize non-stented graft stenosis.
Conclusions
In conclusion, we reported an extremely rare event of refractory heart failure aggravated by excessive left ventricular afterload due to acquired aortic coarctation after TAR. This case highlights (i) the importance of a thorough investigation that led to the discovery of the authentic aetiology of heart failure and (ii) that short-term impaired cardiac function due to acquired post-surgery aortic coarctation can be efficiently saved by appropriate endovascular treatment.
Acknowledgements
We thank all the medical staff for saving the critically ill patient.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81670327), the Sichuan Science and Technology Program (Nos 2019YJ0046, 2022YFS0358), the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. 2020HXJS015), the 1·3·5 Project for Disciplines of Excellence—Clinical Research Incubation Project, West China Hospital, Sichuan University (Nos 2019HXFH027, 2020HXFH043), and the Project of China International Medical Foundation (Z-2016-23-2101-27).
Conflict of interest
None declared.




