Gone in a Splash? Temporal Dynamics of Flukeprint Environmental DNA (eDNA) Detection for Common Coastal Northeast Pacific Cetacean Species
Funding: This work was supported by Ocean Wise Killer Whale Adoption Program, Prince Rupert Port Authority, and Prince of Whales Whale & Marine Wildlife Adventures.
ABSTRACT
Environmental DNA (eDNA) analytical techniques have been developed and applied to a small subset of cetacean species worldwide. Direct or “flukeprint” eDNA sampling has previously been proven as an effective approach for detecting DNA from target cetacean species including harbor porpoises (Phocoena phocoena), humpback whales (Megaptera novaeangliae), and killer whales (Orcinus orca). These common species are under increasing pressure from coastal anthropogenic activities throughout their respective Northeast Pacific ranges, and there is an increasing urgency to employ less invasive tools to address data gaps to inform conservation measures. However, it is unclear how long DNA shed in flukeprints persists for each of these species in the dynamic ocean environment. In this study, we opportunistically collected seawater (2–3 L) from flukeprints when individual harbor porpoise (n = 3), humpback whales (n = 3), and killer whales (n = 3) were observed diving. We collected samples across a series of five time points after each flukeprint was first observed: 30 s, 1 min 30 s, 2 min, 5 min 30 s, and 10 min 30 s. A total of 45 samples were collected across three species and analyzed with previously published species-specific quantitative PCR (qPCR) assays to assess the persistence of eDNA over time. We found a variation in the detectability across the five time points between both target species and individuals sampled, with a general trend consisting of reduced eDNA detectability over time. These findings yield insights into the temporal dynamics of cetacean eDNA in marine environments, demonstrating the importance of species- and context-specific considerations when designing effective monitoring strategies.
1 Introduction
The Northeast Pacific, including the coastal waters of British Columbia (BC), is home to many cetacean species. Commonly found cetaceans in this region include humpback whales (Megaptera novaeangliae), Pacific harbor porpoises (Phocoena phocoena vomerina), and killer whales (Orcinus orca; Williams and Thomas 2007; Best et al. 2015; Wright et al. 2021). North Pacific populations for all three of these species have been assessed by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) as either “Special Concern,” “Threatened,” or “Endangered” (COSEWIC 2003, 2008, 2022). Coastal ecosystems across BC are areas of concentrated anthropogenic development, where vessel, industrial, and commercial activities are prevalent (Halpern et al. 2008). As such, cetacean species in near-shore coastal regions are impacted by vessel noise (Lusseau et al. 2009), ship strike and entanglement (Neilson et al. 2009; Nichol et al. 2017; Keen et al. 2023; Storlund et al. 2024), declines in prey availability (Ayres et al. 2012), exposure to contaminants (Kim et al. 2023), and the effects of human-mediated climate change (Cheeseman et al. 2024; Raverty et al. 2024).
With growing anthropogenic activity along coastal BC comes the need for effective monitoring of at-risk cetaceans. Estimates of the distribution, abundance, population demographics, prey preference, and genetic structure of cetacean populations are crucial for informing conservation policy and decision-making (Clapham et al. 1999; Parsons et al. 2015; IJsseldijk et al. 2020; Sahri et al. 2021). Methods used to generate this data include land and boat-based visual surveys (Kaschner et al. 2012; Wright et al. 2021; Frouin-Mouy et al. 2022), opportunistic observation data (Olson et al. 2024; Scott et al. 2024), acoustic surveys (Gavrilchuk et al. 2021; Frouin-Mouy et al. 2022; Pilkington et al. 2023), aerial surveys (Fearnbach et al. 2018; Boulent et al. 2023), and DNA collection via biopsies (Mathews et al. 1988; Calambokidis et al. 2008; Hunt et al. 2013), fecal plumes (Ford et al. 2016; Reidy et al. 2022) blow sampling (Apprill et al. 2017; O'Mahony et al. 2024), and, more recently, environmental DNA (eDNA) sampling (Baker et al. 2018; Suarez-Bregua et al. 2022; Robinson et al. 2023, 2024).
As the early application of eDNA for detecting harbor porpoises in a captive environment (Foote et al. 2012), this minimally invasive tool has shown promise globally for DNA monitoring of cetaceans (Suarez-Bregua et al. 2022). eDNA-based methods have been used for detecting small cetacean species including harbor porpoise (Parsons et al. 2018), Dall's porpoise (Phocoenoides dalli; Deeg et al. 2023) and finless porpoise (Neophocaena asiaeorientalis; Ma et al. 2016; Qu and Stewart 2019; Hashimoto et al. 2024), deep-diving species including Cuvier's beaked whale (Ziphius cavirostris; Boldrocchi et al. 2024) and Blainville's beaked whale (Mesoplodon densirostris; Baker et al. 2023), Arctic species including bowhead whale (Balaena mysticetus; Székely et al. 2021), beluga whale (Delphinapterus leucas), and narwhal (Monodon monoceros; Sevellec et al. 2021), rare species including Dwarf sperm whale (Kogia sima; Juhel et al. 2021) and Bryde's whale (Balaenoptera brydei; Zhang et al. 2023), and more common species including bottlenose dolphin (Tursiops truncates; Valsecchi et al. 2021; Alter et al. 2022; Boyse et al. 2024), humpback whales (Andruszkiewicz et al. 2020; Alter et al. 2022; Robinson et al. 2024), striped dolphin (Stenella coeruleoalba), sperm whale (Physeter macrocephalus), fin whale (Balaenoptera physalus; Valsecchi et al. 2021), common minke whale (Balaenoptera acutorostrata; Boyse et al. 2024), and killer whale (Baker et al. 2018; Pinfield et al. 2019). These studies have applied a mix of both DNA metabarcoding and species-specific quantitative PCR (qPCR) approaches, both of which have strengths and weaknesses for detecting species in aquatic environments (Wood et al. 2019; Yu et al. 2022; McCarthy et al. 2023; McColl-Gausden et al. 2023; Johnson et al. 2024).
In addition to detecting the presence of cetaceans through indirect sampling approaches, such as collecting seawater from specific GPS locations (e.g., Qu and Stewart 2019; Székely et al. 2021; Robinson et al. 2023), direct or flukeprint sampling has also proven to be a successful method of targeting individual cetaceans for eDNA analysis (Parsons et al. 2018; Alter et al. 2022; Székely et al. 2022; Baker et al. 2023; Robinson et al. 2024). Flukeprint sampling offers the potential to glean population demographic information, including genetic diversity (Parsons et al. 2018; Székely et al. 2021) and sex (Robinson et al. n.d., in review) from individuals and populations, which is not able to be obtained from indirect eDNA sampling. Due to the targeted nature of flukeprint sampling, qPCR is more likely to offer a more rapid and cost-effective means of determining species presence compared to DNA metabarcoding approaches (Smart et al. 2016; Harper et al. 2018). Despite this previous application of eDNA for cetaceans, there remains a need to understand DNA detectability with respect to the effect of time, tides, and other environmental parameters (Suarez-Bregua et al. 2022). For some cetacean species, estimates of DNA persistence from individual flukeprints collected in the Atlantic and Arctic oceans have been evaluated (Székely et al. 2021; Alter et al. 2022); however, for most common coastal Northeast Pacific cetacean species, the persistence of eDNA in the environment is largely unknown. Understanding the persistence of eDNA from cetacean flukeprints is essential for implementing effective sampling strategies that maximize DNA quantity and quality, ensuring the success of downstream analyses (Alter et al. 2022; Suarez-Bregua et al. 2022; Székely et al. 2022). The objective of this study was, therefore, to assess changes in the detectability of Northeast Pacific cetacean eDNA over time using qPCR assays.
2 Materials and Methods
2.1 Study Area and Sample Collection
Three species were sampled for eDNA collection in this study: harbor porpoise, humpback whale, and killer whale. These species are the most encountered coastal cetacean species in BC waters and are all listed under COSEWIC (COSEWIC 2003, 2008, 2022). Flukeprint sampling efforts occurred between August 8, 2023 and July 4, 2024, in coastal waters between northern and southern British Columbia (Canada; Table S1, Figure 1). Harbor porpoises and humpback whales were sampled in northern BC within Chatham Sound, including locations in Prince Rupert Harbor and Metlakatla Pass, and Brown Pass (Table S1; Figure 1). Sample collection in southern BC occurred during February, which is a period of the year when killer whales are the most common species encountered, specifically transient (Bigg's) killer whales. Samples were collected within the Salish Sea, in locations including Trincomali Channel and Haro Strait (Table S1; Figure 1). Sampling was conducted from two different small motor vessels (~8 and ~5.5 m long) under permit MML18.

In addition to the sample location, for each sample we obtained information on the tidal cycle (ebb—when water is flowing away from the shore/slack—when there is no movement of water due to tides/flood—when water is flowing toward the shore) at the time of collection (Canadian Hydrographic Service 2024a, 2024b) to assess any trends in species detection across the time points in relation to the tide status.
Flukeprint eDNA samples were collected from three individuals for each species where possible in a sea state not exceeding Beaufort 3 (Table S1). During sample collection, an individual cetacean (or an individual traveling separate from the rest of a larger group of conspecifics) was approached from a parallel position at speeds not exceeding seven knots. A distance of approximately 100 m was maintained in a parallel position until the individual made its terminal dive. The vessel was then maneuvered to pass behind the animals' last position and to collect the sample. A GPS location was marked on the vessels' navigation device at the time of initial flukeprint collection. Samples were collected over five time points: instantly (~30 s) after the animal dove and produced the flukeprint (Time 1; T1), 30 s after T1 sample collection (Time 2; T2), 90 s after T1 sample collection (Time 3; T3), 5 min after T1 sample collection (Time 4; T4), and 10 min after T1 sample collection (Time 5; T5). The vessel remained at the initial sampling location after collection, and each subsequent time series sample was taken from the same general area as the vessel was maneuvered to combat wind and currents. Different volumes of water were collected per species: 3 L for harbor porpoise, 2.5 L for killer whales, and 2 L for humpback whales following limited success with lower volumes in a previous study (Robinson et al. 2023, 2024) and complementing existing literature for the same or similar sized species (Parsons et al. 2018; Székely et al. 2021; Alter et al. 2022). Water samples were taken from the surface (~30 cm depth) following previous approaches (Baker et al. 2023; Robinson et al. 2024). All samples were collected using previously decontaminated 3.75 L pitchers while wearing nitrile gloves, following steps previously adopted for flukeprint eDNA sampling aimed at reducing the risk of sample contamination (Robinson et al. 2023, 2024). Samples were filtered immediately on board the vessel using 0.45 μM polyethersulfone (PES) filters in self-preserving eDNA filter packs (Smith-Root, USA) using an eDNA Citizen Scientist Sampler (Smith-Root, USA). Filter packs were then labeled and stored on board the vessel in a cool and dark location. Once on land, eDNA filters were shipped to the [organization lab], where they were stored at −20°C until processing. All eDNA samples were processed in a dedicated secondary lab space separate from PCR and post-PCR areas in the main laboratory.
2.2 DNA Extraction and Quantification
Prior to DNA extraction, all tools were sterilized using 10% bleach, UltraPure DEPC-treated Water (Invitrogen), and 80% EtOH rinses. For extraction, eDNA filters were cut in half and then chopped up into ~3 mm pieces. A DNeasy Blood & Tissue Kit (Qiagen) DNA extraction protocol was followed (Qiagen 2020), with a modification of a reduced final elution volume. Pieces from each half of the filter were placed into a 1.5 mL tube with 300 μL Buffer ATL and 340 μL phosphate-buffered saline (PBS). Samples were shaken at 30.0 hertz for 45 s in a Restch MM 400 Mixer Mill with a sterile metal bead to break up the filter. Then 30 μL of Proteinase K was added, and the samples were incubated overnight at 56°C in a rotating hybridization oven. To remove the filter pieces, the tubes were centrifuged at 20,000 × g for 1 min, and the supernatant was transferred to a new tube (twice). During the spin column step, the supernatant from both tubes was spun through a single spin column. eDNA was eluted using 50 μL of Buffer AE. A negative extraction control consisting of UltraPure DEPC-treated water in place of a filter was also performed once per set of extractions for each species. DNA extracts were stored at −20°C until quantification and amplification.
eDNA purity was measured using a NanoDrop 2000 Spectrophotometer, and then eDNA sample concentration was measured using a Qubit 4 Fluorometer (Invitrogen) with a High Sensitivity Qubit dsDNA Quantification Assay Kit. All extraction blanks had DNA concentrations below the detection threshold of the high-sensitivity assay.
2.3 Species-Specific qPCR Screening
To assess the detectability of target species DNA across each time point, quantitative PCR (qPCR) was performed using species-specific primers (Baker et al. 2018; Parsons et al. 2018; Robinson et al. 2023, 2024; Table S2). A total reaction volume of 10 μL was used, including 0.25 μL each of the forward and reverse primers (Table S2), 5 μL PowerUp SYBR Green Master Mix (Applied Biosystems), 2.5 μL water, and 2 μL DNA. qPCR was run on a StepOnePlus Real-Time PCR System (Applied Biosystems). The cycling conditions included 95°C for 10 min, followed by 50 cycles of 95°C for 30 s, species-specific primer annealing temperature (Table S2) for 20 s, and 72°C for 30 s. A melt curve step was run from 65°C to 95°C which increased by 0.1°C increments every 10 s.
2.4 Statistical Analyses
The goodness-of-fit was assessed using the residual sum of squares (RSS) and 𝑅2 values. We used the ANCOVA statistical test to compare the significance of the resulting decay rates between species (Kirtane et al. 2021), followed by the Tukey Honest Significant Difference (HSD) test to determine where any significant differences lie.
In addition, we tested the change in mean relative DNA quantity (response variable) against several predictor variables: Species (categorical variable with levels “Harbor porpoise,” “Humpback whale,” and “Killer whale”), Time (a continuous variable presented in seconds), and Tide (a categorical variable with levels “Flood,” “Slack,” and “Ebb”). We first tested the normality of the relative DNA quantity (Shapiro–Wilk test) (Shapiro and Wilk 1965), which showed non-normal data with high levels of skewness (3.847) and kurtosis (17.254). Subsequently, we used the zeroinfl() function from the pscl (Jackman et al. 2024) to assess the relationship between predictors and change in mean relative DNA quantity using a zero-inflated negative binomial (ZINB) regression with a log link function, to account for excess zeros and over-dispersion. We selected the best model through comparing Akaike information criterion (AIC) values for each model. All statistical analyses on resulting data were carried out in R Studio version 2024.04.2 (Posit Team 2024) using R version 4.3.2.
3 Results
In total, 45 eDNA samples were collected from three different species (Table S1). Three harbor porpoise, killer whale, and humpback whale individuals were sampled for eDNA collection and analysis across the five time points. For killer whale and humpback whale eDNA samples, all flukeprints were collected from known individuals, including adult male transient (Bigg's) killer whales (T101A, T060E, and T087; Figure S1) and adult unknown sex humpback whales (BCX1944, BCX2217, and BCX1682; Figure S2). For harbor porpoises, samples were collected from unidentified individuals; due to a lack of an existing ID catalogue, they were not able to be identified to the individual level (Table S1; Figure S3). The majority of individuals were sampled while traveling slowly, with the exception of some surface-active harbor porpoises and a potentially deep feeding humpback whale (HW3; Table S1). For all three species, DNA was successfully detected for the first time point (30 s; Table S4). Across the five time points, DNA detectability varied across species (Table S4). The number of positive replicates per species varied across the different individuals sampled, with the highest number of positive replicates for all species occurring at time point one (30 s; Figure 2). The highest relative DNA quantity was detected in harbor porpoise eDNA at time point five (10 min 30 s, mean = 52,345, SD = 5438; Table S5). For all species, there was no significant difference in the number of positive replicates per time point (Table S6).
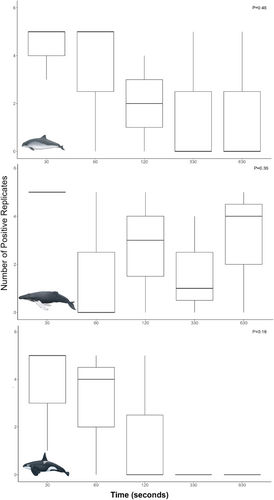
3.1 DNA Detectability Across Species, Time Points, and Tides
For all species with more than one individual sampled, DNA detectability from flukeprints over time varied across individuals (Figure 3). Harbor porpoise DNA was detected across all five time points for HP3 (Table 1), with DNA from HP1 and HP2 not being detected beyond the first time point (30 s) for the first individual and the third time point (2 min) respectively Tables S4 and S5. The sample with the most harbor porpoise DNA was collected during the fifth time point (10 min) across all replicates (mean = 17,448 molecules/L, SD = 25,707; Table 1, Tables S4 and S5), representing over a 5-fold increase from time one (30s) to time five (10 min 30 s) for HP3. For humpback whales, overall DNA was detected across all time points; however, DNA was not consistently detected between HW1 to HW3 across the time series (Table 1; Tables S4 and S5). The highest relative DNA quantity for humpback whales was observed in the time one (30 s) samples (mean = 3541 molecules/L, SD = 5188; Table 1), with an eightfold decrease in the amount of DNA over time until time point five (10 min 30 s), where there was a slight increase in relative DNA quantity from a mean of 40 molecules/L (SD = 72) to 473 molecules/L (SD = 526) between time points four (5 min 30 s) and five (10 min 30 s; Table 1). Killer whale eDNA samples exhibited a decrease in relative DNA quantity over time (3-fold decrease), with no DNA being detected at either time point four (5 min 30 s) or five (10 min 30 s) for KW1, KW2, or KW3 (Table 1, Tables S4 and S5). Individual variability in DNA detection across the time points was high, with KW3 only being successfully detected at time point one (30 s; mean = 21,718 molecules/L, SD = 3366; Table S4).

| Species | Time point | Mean relative DNA quantity (molecules/L) | Standard deviation (±) |
|---|---|---|---|
| Harbor porpoise | T1 | 3053 | 3319 |
| T2 | 7756 | 6748 | |
| T3 | 197 | 287 | |
| T4 | 7651 | 11,353 | |
| T5 | 17,448 | 25,707 | |
| Humpback whale | T1 | 3541 | 5188 |
| T2 | 461 | 699 | |
| T3 | 282 | 319 | |
| T4 | 40 | 72 | |
| T5 | 473 | 526 | |
| Killer whale | T1 | 10,226 | 9184 |
| T2 | 4634 | 4464 | |
| T3 | 3840 | 5970 | |
| T4 | 0 | 0 | |
| T5 | 0 | 0 |
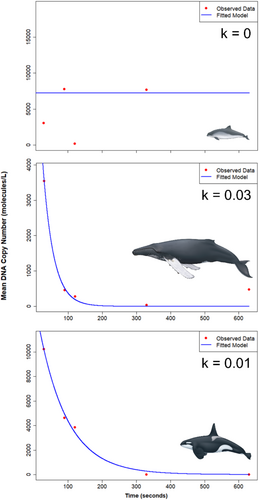
The decay rate constant was significantly different between species (ANCOVA, p < 0.05) and the interaction of species with time (ANCOVA, p < 0.01; Table S7), with post hoc tests revealing a significant difference in the decay rates between humpback whale and harbor porpoise samples (Tukey HSD, p < 0.05) For all species, ebb tides resulted in a higher relative DNA quantities across all time points, with the lowest relative DNA quantities observed for flood tide samples (Table 2; Figure 4), though this difference was not significant. Lastly, the top zero-inflated regression model revealed significant effects of only species on relative DNA quantity (Table S8). Though time was not a significant predictor (p = 0.147), a lower AIC was returned by retaining time within the model (Table S9). For species, relative DNA quantity was significantly higher in harbor porpoise samples compared to humpback whale (p < 0.001), but not killer whale (p = 0.806; Table S8). Species-specific decay constants (k) were estimated at between 0.01 for killer whales and 0.03 for humpback whales (Figure 4; Table S10), indicating that DNA decays at a rate of ~3% per time unit for humpback whales and ~1% per time unit for killer whales, representing 1.8 min−1 and 0.6 min−1 for humpback and killer whales respectively (Table S10). The decay rate for harbor porpoise returned a zero, indicating stable DNA levels over time (Table S10).
| Tide cycle | Number of samples | Mean copy number (molecules/L) | Standard deviation (±) |
|---|---|---|---|
| Ebb | 15 | 7662 | 13,836 |
| Flood | 25 | 1985 | 5187 |
| Slack | 5 | 2866 | 3797 |
4 Discussion
Overall, we found a general, non-significant trend of decline in copy number from the first (30 s) to the fifth (10 min 30 s) time point across all species. For some species, the higher relative DNA quantity at later time points may reflect the influence of factors including species being sampled, proximity of conspecifics, and presence of fecal DNA contributing to the overall amount of DNA detected.
In terms of species, DNA was detectable for up to 2 min for killer whale, 5 min 30 s and 10 min 30 s for harbor porpoise and humpback whale. Harbor porpoise DNA detectability followed an inverse trend to the other two species, with the higher relative DNA quantities detected at 5 min 30 s and 10 min 30 s compared to the earlier time points. Interestingly, despite the overall downward trend of DNA detectability for humpback whales, we found that there was an increase in the amount of DNA at later time points for two individuals, which has previously been observed for humpback whales (Alter et al. 2022). Over a total time of 10 min, Alter et al. (2022) found that for two of the seven whales sampled, there was a later increase in the percentage of humpback whale reads. For one of these cases, this increase was most likely due to DNA from feces being present in the sample as the first whale was observed defecating after sample collection (Alter et al. 2022). While we did not see any evidence of defecation, it is still possible that the whale could have defecated but that the plume sank straight after being deposited and therefore could not be observed (Hunt et al. 2019; Reidy et al. 2022). While the Alter et al. (2022) study used DNA metabarcoding and corresponding read abundance to assess DNA persistence, our approach employed qPCR and relative DNA quantity to assess changes in eDNA signal over time. This method is comparable to theirs and has previously been applied in conjunction with DNA metabarcoding to study fish eDNA persistence (Andruszkiewicz et al. 2017). Similarly, another study which used a combination of qPCR-based approaches, total DNA concentration, and mean CT values across three time points (5, 10, and 20 min) found that detection probability was marginally reduced with increasing time since the dive or breach (Rodriguez et al. 2025). However, much like our study, there was a non-significant effect of timing as a predictor for cetacean eDNA detection (Rodriguez et al. 2025).
Despite this observed increase of DNA at T5, overall humpback whale DNA showed an overall faster DNA decay rate compared to killer whales, with a higher starting relative DNA quantity detected for killer whales compared to humpback whales. For large baleen whales, skin shedding happens more readily in warmer temperatures (Neves et al. 2024; Roman et al. 2025), as has been observed with humpback whales (Pitman et al. 2020). Toothed whales in comparison have higher skin shedding rates (Aubin et al. 1990; Hooker and Baird 2001), with ex situ studies on bottlenose dolphins (Tursiops truncatus) reporting that the outermost cell layer is typically shed ~12 times per day (Hicks et al. 1985). Despite their smaller relative size to humpback whales, killer whales likely shed more DNA into the local environment in relation to their body volume, which could explain the difference in DNA constants and decay rates for the three species (Aubin et al. 1990; Bylemans et al. 2018; Pitman et al. 2020).
4.1 DNA Persistence and Presence of Conspecifics
In addition to the influence of DNA from fecal deposits (Klymus et al. 2015; Baker et al. 2018; Alter et al. 2022), the presence of conspecifics (i.e., another individual of the same species) within close proximity to the sampling event could influence the relative DNA quantity across the time series (Baker et al. 2018; Alter et al. 2022). For sample HW1, there were two other whales documented nearby, and 5–8 whales were reported in that general area 2 h prior. For humpback whales, out of the three individuals sampled, the only humpback whale sample to have detectable DNA at 10 min 30 s was HW1. For this encounter, we noted two additional individuals ~800 m away, moving in the same direction as the sampled whale at the time of flukeprint collection. It is possible that DNA from these two individuals, combined with the remnant DNA from HW1, could have resulted in the relatively consistent relative DNA quantity detected over the five time points (Baker et al. 2018). Robinson et al. (2023) also detected humpback eDNA at a sampling station approximately 300 m away from six to seven foraging humpback whales that were documented in the greater area for over an hour. This suggests that a combination of a greater number of individuals shedding DNA and the duration of time the individuals are in the area prior to sample collection can greatly influence detection rates.
The influence of conspecific presence on DNA detectability at time of eDNA collection has also been previously described for killer whales (Baker et al. 2018). This study reported positive killer whale detections from larger groups up to 2 h after an encounter, even in areas largely influenced by tidal currents. There was a significant relationship between number of individuals and the likelihood of a positive sample, and DNA collected from larger group sizes (i.e., 3–18; Baker et al. 2018). Comparatively, Pinfield et al. (2019) were unable to conclusively amplify killer whale DNA from within 20 m from fishing vessels in Iceland. In our study, we found a consistent downward trend in detectability, despite the presence of 9–11 other killer whale individuals within the vicinity of the sampled individual.
Similar to killer whales, harbor porpoises are observed in both small and large groups (Dracott et al. 2022). Harbor porpoise DNA detection was highest in the final sampling period after 10 min. While this inverse relationship seems unusual, sample collection from an individual flukeprint occurred in close proximity to a group of porpoises, which likely would have influenced the amount of DNA material present, particularly for HP3, which was collected amidst an aggregation of up to 30 animals. This was further evident when observing the species-specific decay rate of −0.659 for harbor porpoises. This finding is similar to Parsons et al. (2018) in Alaska, where flukeprints from a single porpoise were collected and the sample was influenced by nearby groups ranging from 1 to 15 individuals.
4.2 DNA Persistence and Animal Behavior
Behavior of sampled individuals at the time of sample collection is an important variable to consider with respect to DNA detectability. Harbor porpoises nearby to the HP3 individual were surface active and exhibiting sustained feeding behavior in shallow coastal waters, which could account for increased amounts of genetic material at the ocean surface (Alter et al. 2022). Porpoises feeding for a prolonged period in these waters would likely contribute to the presence of sloughed skin or fecal matter at the surface and possibly retain DNA from exhalation blows due to surface tension (Baker et al. 2018). Notably, Robinson et al. (2024) found that eDNA collected from a breaching humpback whale calf resulted in a high DNA concentration (and cycle threshold) despite the small size of the calf relative to the adult. Conversely, all killer whales sampled in our study were observed swimming slowly at the time of collection, with no other types of behaviors noted for nearby killer whales, which could account for the general downward trend in relative DNA quantity across the timepoints for this species.
4.3 DNA Persistence and Influence of Tidal Cycle
Despite the fact that near coastal waters of BC are particularly dynamic environments with large tidal fluctuations, especially Chatham Sound (Lin and Fissel 2018), we did not find a significant relationship between the amount of DNA detected and all of the tidal cycles (Figure 5; Table S6). A previous study which examined eDNA detection of chum salmon (Oncorhynchus keta) along 500 m transects from a large DNA source (> 46 million juvenile chum salmon in coastal pens) to offshore sampling locations found a significant difference in DNA concentration between ebb and flood tides (Baetscher et al. 2024). Inverse to our study, they found that ebb tides resulted in a more rapid decrease in eDNA concentration and elevated levels of PCR inhibitors (e.g., tannins) in eDNA samples (Baetscher et al. 2024). One factor that we did not account for was the influence of the vessel and water mixing induced by the propeller while maintaining position beside the flukeprint. The near-constant corrections required to maintain position throughout the sampling period likely caused mixing of the water at the sampling site.

5 Conclusion
This study demonstrated that cetacean eDNA can be consistently detected from flukeprints using qPCR between 30 s and 2 min after an individual dives. Understanding the detectability of flukeprint eDNA and species-specific decay constants is important for targeted DNA collection within time frames that are likely to yield a positive qPCR result. Our results did not find significant variation between tide cycles, but other factors including the species being sampled, the number of conspecifics in the immediate area, and the behavior of the species prior to and during eDNA collection may have influenced our detections. These factors should be carefully considered when designing flukeprint-based studies for cetaceans. Future work should focus on developing hydrodynamic models that incorporate bathymetric and flow parameters to better understand eDNA transport and persistence, as well as their influence on sample design to maximize cetacean eDNA detectability. Overall, the findings of this study contribute to a deeper understanding of eDNA persistence in the marine environment and highlight important factors to consider for successful eDNA sampling of cetaceans from flukeprints. This study underscores the value of in situ validation of eDNA methodologies, as a comprehensive understanding of DNA shedding, transport, and detectability remains an ongoing challenge.
Author Contributions
C.V.R. conceived the study; G.J.S., A.M., K.D., A.B., and C.V.R. collected samples; E.L. completed eDNA processing, and C.V.R. completed statistical analysis; C.V.R. wrote the manuscript with support from all co-authors.
Acknowledgments
This project was supported by Prince of Whales Adventures, Prince Rupert Port Authority, and the Ocean Wise Killer Whale Adoption Program (KWAP). We would like to thank Ocean Pathways participants Cora Filipetti and Colin Doepker for support with sample collection. We would also like to extend our gratitude to the two anonymous reviewers who provided valuable feedback and suggestions throughout the review process. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All raw data are provided in the supplementary material.




