Water Interaction Type Affects Environmental DNA Shedding Rates of Terrestrial Mammal eDNA Into Surface Water Bodies
Funding: This work was supported by Austrian Science Fund, 10.55776/P35059.
ABSTRACT
The analysis of environmental DNA (eDNA) has become a non-invasive, cost-efficient, and universal biomonitoring tool, widely applied across the globe. Most eDNA research focuses on aquatic organisms in freshwater and marine environments. eDNA shedding rates are key to interpreting eDNA-based results, such as for abundance estimations or detection probabilities. Shedding rates have been estimated for several species and life stages; however, virtually all of them are aquatic. As eDNA-based biomonitoring expands to terrestrial systems, waterborne eDNA from freshwater is increasingly used to assess species presence of terrestrial mammals. When interacting with the water, terrestrial mammals deposit their DNA into the water body, with the amount deposited presumably heavily depending on the interaction type. Here we quantify eDNA shedding rates from domestic dogs during various interactions with water bodies, including “passing by”, “drinking”, “crossing through”, “standing still” and “defecating.” “Crossing through” and “defecating” had the highest DNA shedding rates (both approx. 4 × 107 pg/h/ind). All direct water interactions led to eDNA shedding rates several orders of magnitude higher than those of the indirect interaction (“passing by”), resulting in higher eDNA concentrations and, consequently, higher eDNA detection probabilities. This has important implications for interpretations of eDNA-based data from such water bodies. We also highlight the high variability of eDNA concentrations across experimental replicates, which needs to be accounted for when designing eDNA sampling schemes.
1 Introduction
Assessing species presence is essential for virtually every ecological survey, including biomonitoring and conservation efforts (Chiarucci et al. 2011; Hambler and Canney 2013). With the ongoing biodiversity crisis, the need for efficient monitoring techniques is more urgent than ever—whether to identify declining species ranges, collapsing populations, invasive species, or to measure the success of conservation efforts (Johnson et al. 2017; Leadley et al. 2022). The analysis of environmental DNA (eDNA) has evolved into such a valuable tool, praised for its non-invasiveness, cost-efficiency, and universality, and is now widely applied for species assessments (Deiner et al. 2017; Compson et al. 2020; Beng and Corlett 2020). Environmental DNA is defined as genetic material that is being deposited into the environment by target organisms and that can be collected from environmental samples, such as water, soil, or air, without capturing or handling the target organism itself (Taberlet et al. 2012). Although the source of the genetic material can be manifold, for larger animals it is most commonly assumed to stem from sloughed skin cells, hair, mucus, gametes, or feces (Deiner et al. 2017; Shaw et al. 2017). The specific source/tissue from which the eDNA originates is assumed to influence the dynamics of the eDNA, such as transport, deposition, or resuspension (depending on the particle size, Snyder et al. 2023; Brandão-Dias et al. 2023) or degradation rates (Sedlmayr and Schenekar 2024) and therefore affect eDNA persistence in the environment and eDNA detection rates in any given eDNA study. However, to date, little information is available on the specific tissues of target organisms that contribute to environmental DNA and in what quantities within a given system. Hiki et al. (2024) concluded that environmental RNA (eRNA) has a tissue-biased origin, and Zhao et al. (2023) suggested, based on DNA methylation patterns, that eDNA is primarily released from dysfunctional, inactive cells.
Regardless of the exact origin of eDNA, the amounts of DNA deposited into the environment per individual over time (hereafter called “eDNA shedding rates”) plays a crucial role in interpreting eDNA concentrations found in the environment. Together with eDNA removal rates and interaction frequencies, shedding rates determine the current eDNA concentration of a target organism in the environment, which can be informative for abundance estimations, assuming that the amount of eDNA shed per individual is identical among individuals and therefore the overall concentration of the eDNA correlates to the amount of individuals (Lacoursière-Roussel et al. 2016; Doi et al. 2017). Furthermore, eDNA concentration of a target organism in a sampled system also determines detection probabilities of qualitative species presence assessments (Piggott 2016; Troth et al. 2021). In addition to poor sampling design, false negatives can be a result of eDNA concentrations in the environment being below the limit of detection of the respective eDNA assay. eDNA shedding rates have been shown to depend on a multitude of factors, including species, organism size, skin/scale properties, stress and water temperature (Sassoubre et al. 2016; Allan et al. 2020 and references therein). Previous studies have quantified eDNA shedding rates of various organisms. Thereby, the eDNA shedding rates have been shown to vary across several orders of magnitude within but also among studies (Allan et al. 2020, Table 1), documenting that eDNA shedding rates do not only depend on the specific target species but also the environmental conditions of the specific system. These varying eDNA shedding rates presumably result in drastically differing DNA concentrations in the systems, strongly affecting eDNA detection probabilities and potential abundance estimations. Notably, nearly all the studies studying eDNA shedding rates focused on aquatic organisms, whether freshwater or marine (reviewed by Allan et al. 2020), a pattern that hasn't changed until now (Table 1). The only exception is the Idaho giant salamander (Dicamptodon aterrimus) studied by Pilliod et al. (2014), being semi-aquatic. This highlights a significant knowledge gap in the study of eDNA shedding rates in terrestrial mammals.
| Study | Study system | Organism class | Shedding rate (order of magnitude) |
|---|---|---|---|
| Wilder et al. (2023) | Freshwater | Actinopterygii | 103–106 copies/h/ind |
| Kwong et al. (2021) | Marine | Asteroidea |
103–105 copies/h/g 102–104 copies/h/ind |
| Allan et al. (2020) | Marine |
Actinopterygii, Malacostraca, Scyphozoa |
103–105 pg/h 102–103 pg/h/g 10–104 pg/h/ind |
| Sassoubre et al. (2016) | Marine | Actinopterygii |
105–107 pg/h 102–103 pg/h/g 103–105 pg/h/ind |
| Klymus et al. (2015) | Freshwater | Actinopterygii | 104–109 copies/h |
| Maruyama et al. (2014) | Freshwater | Actinopterygii |
105–106 copies/h/g 106–107 copies/h/ind |
| Sansom and Sassoubre (2017) | Freshwater | Bivalvia |
105–107 copies/h 1 copies/h/g 104–105 copies/h/ind |
| Nevers et al. (2018) | Freshwater | Actinopterygii |
106 copies/h 105 copies/h/g 106 copies/h/ind |
| Jo et al. (2019, 2020) | Marine | Actinopterygii |
105–109 copies/h 105–107 copies/h/g |
| Minamoto et al. (2017) | Marine | Scyphozoa | 108 copies/h/ind |
| Pilliod et al. (2014) | Freshwater | Amphibia | 104 pg/h/ind |
Although the large majority of eDNA studies target aquatic systems and organisms in general (Beng and Corlett 2020), eDNA studies have been expanding to terrestrial target organisms, most notably mammals. For these studies, surface water bodies (mostly freshwater) are frequently used as a source of eDNA, as most terrestrial organisms need to regularly visit those for drinking, hunting, or grooming (Table 2). Hereby, the DNA of the terrestrial mammals accumulates in the water while they interact with it and can be captured and concentrated for genetic analysis.
| Study | Study area | Target organism(s)/scope | Water body |
|---|---|---|---|
| Schenekar et al. (2024) | Southern Africa | Mammalia | Semi-natural waterholes |
| Croose et al. (2023) | Europe | European mink Mustela lutreola | Rivers |
| Holm et al. (2023) | Europe | Vertebrata | Rivers |
| McDonald et al. (2023) | Australia | Mammalia | Granite rock-pools and cattle troughs |
| Farrell et al. (2022) | Southern Africa | Mammalia | Artificial waterholes |
| Suryobroto et al. (2022) | South-East Asia | Mammalia | Saltlicks |
| Mas-Carrió et al. (2022) | Central Asia & Southern Africa | Mammalia | Stagnant water bodies/waterholes |
| Wilcox et al. (2021) | North America | Jaguar Panthera onca | Ponds |
| Lyet et al. (2021) | North America | Mammalia | Rivers |
| Mena et al. (2021) | South America | Mammalia | Stagnant and running water bodies |
| Broadhurst et al. (2021) | Europe | Mammalia | Rivers |
| Macher et al. (2021) | Europe | Vertebrata | Rivers |
| Furlan et al. (2020) | Australia | Vertebrata | Waterholes |
| Sales et al. (2020a) | South America | Mammalia | Rivers |
| Sales et al. (2020b) | Europe | Mammalia | Rivers |
| Seeber et al. (2019) | Southern Africa | Mammalia | Natural and artificial waterholes |
| Davis et al. (2018) | North America | Wild pigs Sus scrofa | Natural ponds, streams, and wildlife guzzlers |
| Williams et al. (2017, 2018) | North America | Wild pigs Sus scrofa | Artificial wallows |
| Ushio et al. (2017) | East Asia | Mammalia | Forest ponds |
| Klymus et al. (2017) | North America | Vertebrata | Natural ponds and cattle tanks |
| Rodgers and Mock (2015) | North America | Coyotes Canis latrans | Artificial water sources (buckets) |
Given this growing application of aquatic eDNA deposited by terrestrial mammals, basic information is urgently needed about eDNA shedding rates via animal-water interactions. A major difference to the previously conducted experiments of aquatic organisms is that terrestrial mammals do not permanently live in the sampled substrate (water) but interact with it for a limited amount of time. The interaction can be manifold, ranging from very limited interaction through drinking to full body immersion, such as when wallowing or swimming. With this variation in contact, eDNA shedding rates are expected to vary drastically. However, to date there has not been an effort to quantify these.
In this study, we conducted the first ever attempt to quantify eDNA shedding rates from a terrestrial mammal while interacting with freshwater. We specifically assessed eDNA deposition of domestic dogs in water bodies from five different interactions: (A) passing by, (B) drinking, (C) crossing through, (D) standing still, (E) defecating. The results reveal how different interaction types affect the amount of eDNA deposited in surface water bodies, and we estimate the eDNA shedding rates for several water interactions.
2 Methods
2.1 Experimental Setup
Experiments were carried on five different trial days to produce five independent replicates for each dataset and run between June 26th and August 24th, 2023. Therefore, 5 × 5 = 25 (five dogs, five interactions each) interactions were tested each day, with an overall of 125 interactions across all five trial days. Hereby, the order of dogs was kept the same across all 5 days as this proved easier for dog handling. On each trial day, all five interaction types were assessed by setting up one water body per interaction type (Figure S1). As water bodies, dog pools (Ø 80 cm, height 30 cm) were utilized. Pools were placed on a 2 × 2 m plastic cover and evenly spread out across the experimental area (separated at least 5 m apart from each other) and were filled up with 50 L of well water. The only exception to this was the water body for “B-drinking” (see below), for which a 48 × 30 × 26 cm polypropylene plastic box was utilized and filled with 13 L of well water. One additional pool was set up as a control pool in the centre of the experimental area, with no interaction, to quantify airborne dog eDNA deposition during the timespan of an entire trial day from dog presence in close vicinity.
The five interactions tested were (Figure 1): A—passing by: The dog was walked around the pool within 1 m distance to the pool for 2 min. B—drinking: The dog was allowed to drink ad-libitum for approx. 5 min, measuring the time of actual snout-to-water contact and targeting at least 30 s of snout-to-water contact per run. To encourage drinking, up to 10 mL of diluted carrot juice was dispensed into a (bleached) feeding bowl that was submerged in the water. C—crossing through: The dog jumped into the pool and out on the other side. The estimated interaction time per crossing was 2 s. D—standing still: The dog entered the water and stood still for 2 min. E—defecating: A freshly (< 5 h) deposited dog scat was placed in the pool and incubated for 2 min. The interaction times were chosen (1) to reflect real-world interaction times but also (2) what proved feasible to be repeated in a consistent manner by the dogs.

For each interaction, five runs were carried out consecutively, repeating the same interaction with the five different dogs. For the “defecating” interaction, an additional scat was placed after 2 min incubation each. All five dogs were females of the same breed (Lagotto Romagnolo, Figure S2), weighing between 12.4 kg and 13.5 kg, each. All scats of the “defecating” experiment were weighed (mean: 55.9 g, stdev: 19.2 g). After each run, the water of the pool was vigorously stirred and two replicate water samples, 250 mL each, were collected and stored on ice until filtration (all within 6 h). Of the control pool, one set of duplicate samples was taken before the beginning of the experimental runs and one set of duplicate samples were taken at the very end of the experimental runs.
Water samples were filtered through a 25 mm glass fiber disc filter (GF/F filters, nominal pore size of 0.7 μm, Whatman) via a 25 mm Swinnex filter holder and using 50 mL disposable hand syringes (Omnifix, B. Braun). Filters were preserved in 700 μL Longmire's solution (0.1 M Tris, 0.1 M EDTA, 10 mM NaCl, 0.5% SDS, Longmire et al. 1997; Renshaw et al. 2015) and stored at −20°C until DNA extraction. All sampling equipment (syringes, filter holders, forceps, sampling bottles etc.) were soaked in 10% bleach for 30 min, followed by soaking in deionized water for 30 min and then air-dried before being (re-)used. Large equipment (dog pools, polypropylene box, plastic covers, etc.) was rinsed with tap water, thoroughly wiped with 50% bleach, rinsed with tap water again, and air-dried. The protocol and procedures employed concerning live animals were approved by the Local Ethics Committee of the University of Graz (GZ. 39/84/63 ex 202122).
2.2 DNA Extraction, Primer Design and qPCRs
DNA was extracted from the filters using the DNeasy Power Soil Pro Kit (Qiagen) using the protocol described in Schenekar et al. (2024). Briefly, each filter was carefully removed from the sampling tube and cut into 1–2 mm pieces using sterile forceps, a scalpel, and a weighing boat. The snippets were transferred to a Power Bead Pro tube containing 800 μL buffer CD1. The filter and buffer in the Power Bead Pro tube were then homogenized at 4000 rpm for 60 s using an MP FastPrep 24 Homogenizer. The rest of the protocol followed the manufacturer's instructions.
The two sampling replicates taken after each run were pooled in the course of the extraction in order to receive one independent sample per sampling event by pooling the snippets of the two filters into one Power Bead Tube Pro tube before homogenization. DNA extracts were eluted in 100 μL buffer C6 of the extraction kit. With each extraction batch (up to 11 extractions), one extraction blank was included (14 extraction blanks in total). DNA extracts were stored at −20°C until qPCR. DNA extractions and setup of qPCRs were carried out in a dedicated low-template DNA laboratory under two separate laminar flows for DNA extractions and PCR setup. Laminar flow workstations were irradiated with UV light for 30 min between work sessions.
We designed dog-specific primers on the mitochondrial cytochrome oxidase 1 (COI) gene using the DesignPrimers function of the DECIPHER package (Wright et al. 2014; Wright 2015) by designing a primer pair with the highest priming efficiency for Canis lupus while minimizing priming efficiency with non-target species, most notably human Homo sapiens, as well as other central European mammal species known to occur in suburban areas where the experimental trials were conducted (17 species and 371 sequences in total—see Table S1 for full species and reference sequence list). Species specificity of the primer pair was tested in silico via Primer Blast (Ye et al. 2012). A TaqMan probe was designed manually, aiming to maximize mismatches with H. sapiens. Final sequences of designed primers and probe were: DogCOI01_fwd: 5′-TTGTGGGAGTAAATATAACTTTCTT-3′, DogCOI01_rev: 5′-GAGGAGACGGTATTTCAGG-3′ for forward and reverse primers, respectively, and DogCOI01_pro: 5′-FAM-TGGGTAGTCAGAGTATCGACGAGGT-BHQ-3′ for TaqMan probe, amplifying a 112 bp amplicon of the COI gene. In vitro testing focused on potential co-amplification of human DNA, as this was expected to be the main source of non-target contamination in the samples. For this, we extracted DNA from buccal swabs of both humans and domestic dogs using the DNeasy Blood & Tissue Kit (Qiagen) using the user-developed saliva protocol (Qiagen 2006). Specificity of the designed primers and probe was conducted on four different human samples and two dog samples (duplicate PCR replicates with every sample). An eight-point standard curve was generated from the dog saliva DNA extracts using 1:10 dilution steps (from 2.14 ng/μL to 2.14 × 10−7 ng/μL), with four PCR replicates per dilution step in order to quantify the assay's efficiency and for conversion of CT-values to absolute target DNA quantities. PCR reaction setup and cycling conditions of the standard curve were identical to conditions of the eDNA samples (see below). For the determination of the assay's limit of quantification (LOQ) and limit of detection (LOD) calculations, we used the approach of Klymus et al. (2019) and applied the LOD/LOQ calculator of Merkes et al. (2019).
For each eDNA sample, eight qPCR replicates were conducted. Each qPCR reaction contained 2 μL template DNA, 300 nM of the forward and reverse primer, respectively, 200 nM of the TaqMan probe, 6.25 μL of TaqMan Environmental Master Mix 2.0 (Thermo Fisher Scientific) and dH2O up to a total reaction volume of 12.5 μL. PCR cycling conditions consisted of an initial stage at 95°C for 10 min, followed by 50 cycles at 95°C for 15 s and 60°C for 1 min and was performed on a Corbett Rotor-Gene 3000 (Qiagen). Filter and extraction blanks were run along with the field samples and with each PCR batch, two PCR negative controls were included, as well as two dilutions of dog saliva DNA extracts as positive controls.
2.3 Statistical Analyses
3 Results
3.1 Development of Dog-Specific TaqMan qPCR
The Primer Blast search resulted in no significant blast matches with non-target species (particularly not humans). Additionally, no amplification was observed in any human saliva sample, whereby the dog saliva samples readily amplified, confirming the assay's specificity. The efficiency of the developed protocol quantified via the generated standard curve was 0.91 and R2 was 0.9972. The limit of quantification (LOQ) was determined to be 2.14 × 10−7 ng/μL, whereby the limit of detection (LOD) could not be calculated from the generated standard curve as it fell below the concentration of the highest dilution of the standard curve. So conservatively, the LOD was assumed to coincide with the LOQ.
3.2 Quantification of Shed Dog DNA in the Experimental Trials
None of the filter blanks, extraction blanks, or PCR blanks showed any amplification in any reaction and were therefore excluded from further analysis. During run 2 of the “passing by” interaction of trial Day 2, the dog briefly touched the water with one paw, resulting in a drastic increase in DNA concentrations in the samples 2–5 of this trial (Figure S3), samples of runs 2–5 of this trial were removed from statistical analyses.
Amplifications were observed for all interaction types, whereby eDNA samples from datasets “drinking”, “crossing through”, “standing still” and “defecating” all amplified in eight out of eight qPCR replicates (with one exception: run 2 of one of the “defecating” interactions, with six out of eight amplifications; Figure S4). With the interaction “passing by”, a steady increase in amplification rate was observed from run 1 (0.8 amplifications on average) to run 5 (3.5 amplifications on average). From the control pool, none of the samples taken at the beginning experiment amplified, whereby 0.6 reactions amplified on average at the end of the experiment. The mean concentrations of all but one (total: 136) samples that amplified fell above the limit of quantification (LOD/LOQ: 2.14 × 10−7 ng/μL DNA extract; concentration of the one sample that fell below: 1.65 × 10−7 ng/μL DNA extract).
The total DNA in the water body after run 5 differed by several orders of magnitude among interactions (Figure 2, Table 3). Hereby, “defecating” produced the most DNA in the water bodies, with a mean value of 14,494 ng in the water body after run 5. This was followed by “crossing through” (749 ng), “drinking” (391 ng), “standing still” (215 ng) and “passing by” several orders of magnitude lower (0.062 ng). The control pool contained, on average 0.036 ng at the end of the trial days.

| Interaction type | Run number | Interaction time cumulative (s) | Number of amplifications | Total DNA in water body (ng) |
|---|---|---|---|---|
| A—passing by | ||||
| 1 | 120 (±0) | 0.8 (±0.8) | 0.015 ± (0.02) | |
| 2 | 240 (±0) | 0.5 (±0.6) | 0.08 ± (0.009) | |
| 3 | 360 (±0) | 1.75 (±2.4) | 0.023 ± (0.028) | |
| 4 | 480 (±0) | 2.25 (±2.9) | 0.046 ± (0.057) | |
| 5 | 600 (±0) | 3.5 (±2.9) | 0.062 ± (0.073) | |
| B—drinking | ||||
| 1 | 32.8 ± (11.5) | 8 (±0) | 143.275 ± (55.128) | |
| 2 | 67.2 ± (22.2) | 8 (±0) | 284.464 ± (200.657) | |
| 3 | 106.8 ± (23.2) | 8 (±0) | 315.355 ± (220.748) | |
| 4 | 142.8 ± (23.5) | 8 (±0) | 315.355 ± (200.289) | |
| 5 | 177.2 ± (25.9) | 8 (±0) | 390.725 ± (251.654) | |
| C—crossing through | ||||
| 1 | 14 (±0) | 8 (±0) | 88.650 ± (84.379) | |
| 2 | 28 (±0) | 8 (±0) | 235.667 ± (115.771) | |
| 3 | 42 (±0) | 8 (±0) | 325.038 ± (204.230) | |
| 4 | 56 (±0) | 8 (±0) | 506.136 ± (227.424) | |
| 5 | 70 (±0) | 8 (±0) | 749.381 ± (428.720) | |
| D—standing still | ||||
| 1 | 120 (±0) | 8 (±0) | 66.131 ± (78.143) | |
| 2 | 240 (±0) | 8 (±0) | 111.215 ± (109.399) | |
| 3 | 360 (±0) | 8 (±0) | 120.036 ± (94.458) | |
| 4 | 480 (±0) | 8 (±0) | 188.148 ± (99.451) | |
| 5 | 600 (±0) | 8 (±0) | 214.851 ± (104.274) | |
| E—defecating | ||||
| 1 | 120 (±0) | 8 (±0) | 381.330 ± (426.465) | |
| 2 | 240 (±0) | 7.6 (±0.9) | 1956.934 ± (1729.543) | |
| 3 | 360 (±0) | 8 (±0) | 7920.198 ± (6002.859) | |
| 4 | 480 (±0) | 8 (±0) | 10520.032 ± (9114.899) | |
| 5 | 600 (±0) | 8 (±0) | 14494.228 ± (12865.360) | |
| F—control | ||||
| 0—start | 0 (±0) | 0 (±0) | 0 ± (0) | |
| 6—end | 6180 (±164.3) | 0.6 (±0.9) | 0.018 ± (0.033) |
The glmm revealed significant effects of interaction time and interaction type on the deposited eDNA (Table 4). For the latter, all interactions differed significantly from the reference category “passing by”.
| Fixed effect | Level | Estimate | Z | Sign. |
|---|---|---|---|---|
| Intercept | −4.989 (±0.358) | −13.94 | < 0.001 | |
| Interaction type | Drinking | 10.225 (±0.360) | 28.41 | < 0.001 |
| Interaction type | Crossing through | 10.731 (±0.377) | 28.44 | < 0.001 |
| Interaction type | Standing still | 8.486 (±0.329) | 25.81 | < 0.001 |
| Interaction type | Defecating | 11.360 (±0.320) | 35.54 | < 0.001 |
| Interaction time | 3.84 × 10−3 (±6.48 × 10−4) | 5.92 | < 0.001 |
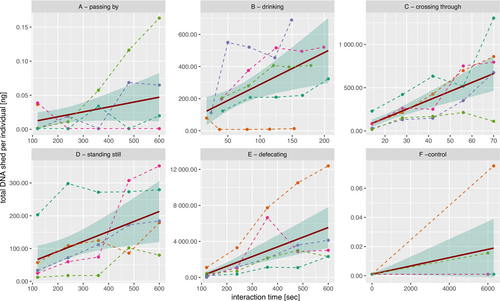
Generalized linear regressions revealed a significant effect of interaction time on the amount of DNA shed per individual for the interactions “drinking” “crossing through” “standing still” and “defecating” but not for “passing by” and “control” (Table 5, Figure 3). For the significant interactions, shedding rates varied between 18.3 ng/min/ind (standing still) and 645 ng/min/ind (defecating), whereas the non-significant interactions (passing by and control) had much lower shedding rate estimations (0.004 and 0.0001 ng/min/ind, respectively).
| Interaction type | Model coefficients | Model fit | DNA shedding rates | ||
|---|---|---|---|---|---|
| Intercept | Interaction time | Adjusted R-squared | (ng/min/ind) | (pg/h/ind) | |
| A—passing by | 3.99 × 10−3 (±1.03 × 10−2) | 7.18 × 10−5 (±4.29 × 10−5) | 0.062 | 4.31−3 (±2.58 × 10−3) | 2.59 × 102 (±1.55 × 102) |
| B—drinking | 8.88 × 101 (±5.38 × 101) | 1.99 × 100 (±6.87 × 10−1)** | 0.071 | 1.20 × 102 (±4.12 × 101) | 7.17 × 106 (±2.47 × 106) |
| C—crossing through | -5.63 × 101 (±4.21 × 101) | 1.03 × 101 (±1.95 × 100)*** | 0.509 | 6.17 × 102 (±1.17 × 102) | 3.70 × 107 (±7.02 × 106) |
| D—standing still | 3.02 × 101 (±3.21 × 101) | 3.04 × 10−1 (±1.20 × 10−1)* | 0.183 | 1.83 × 101 (±7.18 × 100) | 1.10 × 106 (±4.31 × 105) |
| E—defecating | −9.15 × 102 (±3.59 × 102)* | 1.08 × 101 (±2.46 × 100)*** | 0.445 | 6.45 × 102 (±1.48 × 102) | 3.87 × 107 (±8.87 × 106) |
| F—control | 9.99 × 10−4 (±5.4 × 10−4) | 2.85 × 10−6 (±1.62 × 10−6) | 0.464 | 1.71 × 10−4 (±9.73 × 10−5) | 1.03 × 101 (±5.84 × 100) |
4 Discussion
This study represents the first effort to quantify eDNA shedding rates of a terrestrial mammal into surface water bodies while interacting with them. Every interaction resulted in detectable DNA, with all direct interactions having virtually 100% detection probability. Although the indirect interaction (“passing by”) resulted in detectable DNA in the water body as well, the detection probability never reached 100%. The control pool resulted in amplifications from two out of five replicates at the end of the trial days (with one and two out of eight amplifications respectively), revealing a very unreliable detection probability even if the target organism has been in the ultimate vicinity during the last 1–2 h.
eDNA shedding rates differed by several orders of magnitude among interactions, generally following the trend of more intense interactions leading to more eDNA shed. The control pool contained on average only a small fraction (1/25th) of the final concentration of the “passing by” pool, which was the tested interaction having the lowest DNA concentrations measured. Therefore, we deem that airborne eDNA deposition during the experimental trials was negligible. “Crossing through” and “defecating” had the highest eDNA shedding rates, both with approximately 4 × 107 pg/h/ind, whereas the other two direct interactions were one order of magnitude less. Note that per our unit definition for the “defecating” interaction, “per individual” is equal to “per scat”. In the previous studies on eDNA shedding rates, where rates were also in mass DNA per time per individual (Pilliod et al. 2014; Sassoubre et al. 2016; Allan et al. 2020, see Table 1), the reported overall shedding rates fell several orders of magnitude below the shedding rates of “crossing through” and “defecating”, but none of these studies focused on terrestrial mammals. However, we would like to highlight that while our study design allowed us to assess the shedding rates of specific interaction types individually, the aforementioned studies did not differentiate between these interaction types. As a result, their calculated shedding rates likely represent a combination of multiple behaviors. This makes direct comparisons of the absolute numbers difficult.
Previous studies also showed drastically differing shedding rates among species with the same environmental conditions (Allan et al. 2020), which has been attributed to different body plans of animal forms. The most relevant distinctive feature of the body plan of terrestrial mammals is clearly the possession of hair or fur on their skin, which is frequently lost and replaced by the body (Ling 1970). These lost hairs, when shed with parts of the follicle cells they grow from, are presumably a major contribution to eDNA shed by terrestrial mammals. Hair and skin cells are presumably also the major source of eDNA in our studies for the interactions “passing by”, “crossing through” and “standing still”. The eDNA of the “drinking” interactions presumably stems from a mixture of epithelial cells from the buccal cavity and tongue as well as skin and hair follicle cells from the outside of the dog snout, which was partially submerged while drinking. eDNA from the “defecating” interactions presumably stems largely from epithelial cells of the digestive tract. Physical forces and abrasion through active movement through water seem to drastically increase eDNA shedding, as shown by the higher shedding rates from the interaction “crossing through” (4 × 107 pg/h/ind) than “standing still” (1.10 × 106 pg/h/ind). Increased eDNA shedding through abrasion could therefore also contribute to increased eDNA shedding observed at higher animal activities (i.e., increased movement of individuals; Thalinger et al. 2021) or higher individual densities or biomass (Klymus et al. 2015; Jo et al. 2019).
Given the comparably high eDNA shedding rates from scat and from active animal movement through water, we conclude that direct water interactions are an important factor for eDNA deposition of terrestrial mammals into surface water bodies. For this, the water body needs to be accessible for the animal so that physical movement into or through it is possible, and/or defecation into the water body is likely. The latter also depends on the biology and behavior of the target species and is particularly likely for semi-aquatic mammals, such as beavers or hippopotamuses. However, if defecation into the water occurs, feces typically remain in the water body and may release eDNA over an extended period. In our experiment, scat proved to be a highly productive source of eDNA showing constant eDNA emission over the entire sampling period. Feces has also been suggested as an important source of eDNA for aquatic organisms (Klymus et al. 2015; Stewart 2019; Wilder et al. 2023) and the pivotal role of scat as a source of eDNA also highlights the importance of how defecation is handled in eDNA shedding experiments of aquatic organisms. Although several studies did not report on scat removal during the experiments, Jo et al. (2019, 2020) removed feces 1 h after feeding and starved the fish on the sampling day, and Maruyama et al. (2014) excluded data from one trial because of observed feces in the tank.
We would like to emphasize that the ultimate amount of target eDNA in a water body is influenced by additional factors that were beyond the scope of this study. These include: (1) The frequency of individual interactions: For instance, in a water body where animal crossings occur frequently but defecation is relatively rare in both frequency and duration, the dominant source of eDNA might not be defecation. This would be especially true given the comparable eDNA shedding rates observed for these two interaction types. (2) The degradation rate of target eDNA: Degradation rates can vary substantially among water bodies due to a variety of abiotic and biotic factors. These include water chemistry, temperature, microbial activity, and UV exposure. Ideally, these rates should be assessed for each specific natural setting to make robust inferences about species presence or abundance (Barnes et al. 2014; Allan et al. 2020). Furthermore, degradation can be affected by the molecular state of the target DNA, as highlighted by Sedlmayr and Schenekar (2024). (3) The time span since the interaction: The duration between the eDNA-releasing interaction and sample collection can also significantly impact the quantity of eDNA detected, as older eDNA is more prone to degradation.
The possibility of scat as eDNA origin further complicates interpretations of waterborne eDNA in these systems, as scat can enter the water independently of the organism it originated from, for example, by adhering to the paws or hooves of other animals. These remnants can lead to species detections even when the target species was never directly near the water body.
Finally, consistent with previous studies (Allan et al. 2020), we observed high variability in eDNA concentrations across replicates, as indicated by the low R-squared values in our generalized linear regressions used to estimate shedding rates. We exerted maximum effort to mix the water bodies in order to homogenize the water bodies before sampling; however, more intense mixing would have affected the integrity of scat samples in the water body, reducing the comparability to natural systems. The high variability among replicates could be due to the high stochasticity of eDNA deposition during shedding processes, leading to considerably varying eDNA concentrations among experimental runs using the same interaction. Alternatively, this variability could originate from the patchiness of eDNA concentrations in water bodies, which is particularly known from stagnant water bodies, where mixing of dissolved particles is less likely (Brys et al. 2021; Bruce et al. 2021; Schenekar et al. 2024). However, to confirm either, additional experiments using multiple independently analyzed eDNA sampling replicates per experimental run are needed. In any case, this phenomenon underscores the importance of thorough sampling replication.
This study was the first one to quantify eDNA shedding rates from a terrestrial mammal. However, future work is needed to expand this work to other species and interactions, as shedding rates have been shown to vary greatly among species and among interactions. Furthermore, as mentioned in the introduction, the total amount of DNA in a given system results from a combination of eDNA, shedding rate, interaction duration/frequency, and eDNA degradation. In our tested system, different types of interactions are highly likely to shed different types of cells into the water (e.g., epithelial cells of the oral cavity via drinking, hair follicle and skin cells via passing through and epithelial cells of the intestinal tract via defecation). These cells vary in their physiological structure (e.g., keratinization) which is likely to affect also eDNA degradation rates (Mercer 1965; Born et al. 1992; Sedlmayr and Schenekar 2024). Therefore, assessing tissue-specific degradation rates of eDNA is highly needed to gain a better understanding of the factors affecting the eDNA amount of a target organism at a given time. Finally, the frequency of specific interactions additionally determines their contribution to the total eDNA deposited by a species in a given system. These are expected to be highly variable across systems, and additional research is required to quantify these for a specific study system. However, our study reveals the importance of direct interaction of terrestrial mammals with the waterbody for eDNA deposition. Taken together, a better knowledge of eDNA shedding rates and degradation rates, also from terrestrial animals, is crucial in our understanding of how to interpret eDNA-based results and is therefore essential for the further development of this tool for biodiversity assessments.
Author Contributions
G.S. and T.S. conceived the study design and carried out the field experiments. T.S. conducted laboratory work and statistical analyses. T.S. wrote the first draft of this manuscript, and G.S. made contributions to the final version of this manuscript.
Acknowledgments
First and foremost, we would like to thank Quora, Ulma, Camou, Pompeia, and Iuma for their enthusiastic participation in the experimental trials. Furthermore, we thank D. Hummel and H. Schenekar for their support in conducting the experimental trials, water filtering, and cleaning equipment, and S. Weiss for feedback on the initial version of this manuscript. This research was funded in whole or in part by the Austrian Science Fund (FWF) [10.55776/P35059]. For the purpose of open access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Raw qPCR data and R scripts of statistical analyses are included as Data S2 in this publication.




