Geographic Genetic Divergence in Tychoplanktonic Taxa Dominating Diatom Communities in Marine Biofilms
Funding: This work was supported by both the Direction Générale de l'Armement, French Government Defense procurement and technology agency (Program DRACONS) and Research French National Agency.
ABSTRACT
Diatoms constitute the main photosynthetic group in marine biofilms throughout the world's oceans, in particular, on plastic debris, which has become a major problem in the marine environment. However, they remain largely unexplored in this context compared to prokaryotes. Here, we aim to understand the dynamics of diatom communities in the plastisphere at different levels: how taxa are selected from the planktonic community and how communities are structured over time and at large spatial scales. Biofilms were collected from PVC (polyvinyl chloride) panels immersed (i) for 1 year in two NW Mediterranean sites, a mesotrophic one (Toulon Bay) and an oligotrophic one (Banyuls Bay), and (ii) for 1 month in Toulon Bay and two other eutrophic sites (Lorient in South Brittany in the Atlantic Ocean and Reunion Island in the Indian Ocean). Plastispheres were analyzed using both microscopic and molecular approaches, focusing on the relationship between diatoms and other microorganisms in biofilms. Light microscopy revealed spatio-temporal differences in cell abundance and biovolume. Metabarcoding, targeting the rbcL gene for diversity and composition, revealed that the richness of diatom species was already maximal in the early stages of biofilm formation, and beta-diversity showed a clear temporal evolution in the Mediterranean Sea. Including prokaryotic and fungal communities, we described microorganism interactions within biofilms throughout the colonization process of the plastisphere. In addition, environmental parameters on a large geographical scale were shown to be stronger drivers in structuring diatom communities, considering both planktonic and biofilm lifestyles. A core biofilm community represented by a few abundant species was observed across sites. The occurrence of tychoplanktonic taxa emphasizes the specificity of diatoms among other microorganisms in biofilms. Finally, the complete absence of common genetic variants among Lorient, Reunion Island, and Toulon suggests that dispersal by marine currents over a large geographical scale has led to adaptation processes.
1 Introduction
Diatoms are unicellular photosynthetic microeukaryotes, which are distributed across marine, freshwater, and terrestrial ecological niches, contributing up to 20% of the global primary production, including roles in carbon fixation and nutrient cycling (Benoiston et al. 2017; Tréguer et al. 2018). Most information is based on their planktonic lifestyle (e.g., Malviya et al. 2016). However, due to their ability to secrete extracellular polymeric substances that notably enable adhesion, as well as bacteria, they are a major component of biofilm communities in marine environments. These include natural and artificial substrates, such as microphytobenthos on sediments, rocks, seagrass, algae, various shells, ship hulls, aquaculture nets, marine renewable facilities, and (micro)plastics (Brandini et al. 2001; Patil and Anil 2005; Molino and Wetherbee 2008; Briand et al. 2017; Amaral-Zettler et al. 2020; Schlundt et al. 2020; Zhao et al. 2021). These primary autotrophic colonizers respond directly to chemical, physical, and biological changes. They have also been shown to be excellent biological indicators in freshwater systems (e.g., Morin et al. 2008; Coste et al. 2009; Tlili et al. 2010, 2011; Wachnicka et al. 2011), although little is known about them in the marine environment (Belando et al. 2017).
Despite their enormous diversity (e.g., Malviya et al. 2016) and non-trivial roles in oceanic and coastal marine environments, few studies have investigated the spatial and temporal dynamics of diatom communities in marine biofilms (Abed et al. 2019; Mitbavkar et al. 2012; Qian et al. 2022). When considering the plastisphere, i.e., microorganisms that form biofilms on plastics, although its scientific output has exploded in recent years, most studies have focused on prokaryotes (e.g., Amaral-Zettler et al. 2020). Only recently have fungal communities begun to be studied (Philippe et al. 2023, 2024; Sérvulo et al. 2023; Lacerda et al. 2020). Although diatoms are reported to be an important part of the plastisphere, e.g., as primo-colonizers (Amaral-Zettler et al. 2020) or involved in the carbon cycle (Conan et al. 2022), surprisingly, studies on their abundance or diversity are rare, probably due to the lack of taxonomic knowledge on marine taxa. If polyethylene (26.9%) and polypropylene (19.3%) are the most commonly collected polymers, polyvinyl chloride (PVC) accounts for 12.9% of global plastic production (Plastics Europe 2022). Probably underestimated (Fernández-González et al. 2022), PVC was among the six dominant polymers found in marine litter (Carney Almroth and Eggert 2019).
Thanks to continuous improvements in DNA-based methods, it is now possible to initiate conclusive studies on the factors that shape microbial communities in biofilms (Briand et al. 2022; Song et al. 2022; Yeh and Fuhrman 2022; Zhao et al. 2021). Global eukaryotic databases still have limited resolution for Bacillariophyceae, mainly limiting taxonomic assignment to the genus level. However, Rimet et al. (2015) developed a specific and open-access database (diat::syst) for freshwater diatoms and biomonitoring, providing a necessary and complementary tool to morphological analysis of diatom communities. This database, now known as Diat.barcode (Rimet et al. 2019), has significantly improved the understanding of diatom community dynamics in river and lake biofilms. Gradually, sequences of marine taxa have also been added to this database, leading to its successful application in marine environments (Rivera et al. 2018; Pérez-Burillo et al. 2022; Védie et al. 2022; Laroche et al. 2023), opening the door to more comprehensive studies of diatom community control in marine biofilms. Such a DNA-based approach appears to be particularly well suited to the study of large datasets covering large geographical areas.
Here we aimed to specifically understand the dynamics of diatom communities in the plastisphere at several levels: (i) how taxa are selected from the planktonic community, (ii) how communities are structured over time, including their potential interactions with prokaryotes and fungi in the biofilm community, and (iii) how communities are structured at large spatial scales. The temporal dynamics were studied over 1 year in the northwestern Mediterranean Sea in the Bay of Toulon, a mesotrophic site. The diatom plastisphere was then compared with a second Mediterranean site in Banyuls sur Mer, considered to be oligotrophic, after 75 and 365 days. In addition, the effect of geographical location and seawater parameters on the diversity of 1-month diatom biofilm diversity was studied in Toulon (with high salinity and temperature) and in two eutrophic sites: one with lower salinity and temperature along the French Atlantic Coast, and the other in a tropical area on Reunion Island in the Indian Ocean. Finally, the specificity of the plastisphere was characterized alongside the planktonic ones (surrounding seawater), in order to compare the two lifestyles for these sites. The biofilms were studied using both light microscopy for cell abundance and metabarcoding targeting the rbcL gene for diversity and composition. By considering prokaryotic and fungal communities from the same biofilm samples, interactions between microorganisms within biofilms have also been discussed.
2 Materials and Methods
2.1 Sampling Strategy and Environmental Variables
Diatom communities were studied at four sampling sites (Figure S1) selected for their contrasting environmental conditions: Toulon Bay (43°06′25″ N;5°55′41″ E) and Banyuls sur Mer (47°27′13″ N;03°32′360″ E) in the Mediterranean Sea (considered as mesotrophic and oligotrophic sites, respectively), Lorient (47°43′8.0178″ N;3°22′7.2048″ W) on the south coast of Brittany in the Atlantic Ocean, and Le Port, Reunion Island (20°56′03″ S;55°17′0.6″ E) in the Indian Ocean (both considered as eutrophic sites, with lower temperature and salinity for Lorient, and in a tropical area for Reunion Island).
Prior to immersion, 5 × 5 cm PVC panels (AbaqPlast) were sandblasted (roughness increased the contact surface area and reduced the biofilm release). The panels were immersed at a depth of 1 to 2 m, with a metal frame fixed to the dock (in Toulon Bay, Lorient, and Reunion Island) or suspended under the SOLA oceanographic buoy (at Banyuls sur Mer). Immersion started on June 18 at 2015, in Toulon and Banyuls sur Mer for 1 to 367 days. Sampling took place at Toulon after 1, 4, 8, 12, 20, 28, 75, and 367 days. In Banyuls sur Mer, sampling was performed only twice, after 75 and 365 days (for details, see (Briand et al. 2022)). One year later (in 2016), new panels were immersed during another immersion campaign as described above, this time for 30 days, starting on July 15 in Toulon and Lorient, and on December 1 in Reunion Island (i.e., all immersions started in summer). Two liters of seawater were sampled each week during the 30 days of immersion (i.e., four samples) for plankton DNA extraction (1 liter) and chemical analysis (1 liter).
For each sampling point, biofilms were scraped as previously described (Védie et al. 2022) directly at the sampling site with sterile scalpel blades from six panels—three panels for microscopic analysis and three panels for metabarcoding. Water temperature and salinity were measured at a depth of 1–2 m using a Hydrolab DS5X probe (Hatch Hydromet, United States). Dissolved organic carbon (DOC) and total nitrogen (TN) were analyzed using a TOC-VCSH analyzer (Shimadzu) (Oursel et al. 2014). Nutrients (NO3−, PO43−, Si(OH)4) were measured using standard colorimetric methods (Coclet et al. 2018). Data from Banyuls sur Mer were obtained directly from the French coastal monitoring network SOMLIT (Service d'Observation en Milieu Littoral, https://www.SOMLIT.fr/). Trace metal concentrations (Cd, Cu, Pb, Zn) were measured by voltammetry using a fully automated Metrohm/Ecochemie system (Cindrić et al. 2015).
2.2 Determination of Diatom Abundance by Microscopy
Collected biofilms were fixed in 10 mL of 0.3% Lugol's sterile ASW solution (with a sea salt concentration of 36 g L−1) and stored at 4°C in the dark until analysis. Cell counts were performed under an inverted light microscope (×1000 magnification, minimum 30 fields or 100 cells per sample). Biovolumes were estimated from cell sizes (Hillebrand et al. 1999). Sampling times with missing values were excluded from statistical analysis.
2.3 DNA Extraction, Amplification, and Sequencing
Scraped biofilms were immediately immersed in liquid nitrogen. In parallel, seawater samples were filtered through 0.2-μm filters. Scraped biofilms and filters were stored at −80°C until DNA extraction using the Power Biofilm Kit (Qiagen). PCR amplification of diatom communities was performed using the Ribulose Bisphosphate Carboxylase Large subunit (rbcL) gene, a genetic marker commonly used for diatom metabarcoding (e.g., Bailet et al. 2020; Vasselon et al. 2018). Equimolar mixtures of forward primers (Diat_rbcL_708F_1 (AGGTGAAGTAAAAGGTTCWTACTTAAA), Diat_rbcL_708F_2 (AGGTGAAGTTAAAGGTTCWTAYTTAAA) and Diat_rbcL_708F_3 (AGGTGAAACTAAAGGTTCWTACTTAAA)) and reverse primers (R3_1 (CCTTCTAATTTACCWACWACTG) and R3_2 (CCTTCTAATTTACCWACAACAG)) were used as described by Vasselon et al. (2017). The PCR program was as follows: 95°C-15′, 95°C-45″, 55°C-45″, 75°C-45″, 75°C-5′. Steps 2–5 were performed in 40 cycles. Amplicons were then paired-end sequenced (2 × 300 bp, Illumina platform at Eurofins Genomics (Konstanz, Germany)).
2.4 Sequencing Data Processing
The DADA2 pipeline adapted for diatom metabarcoding data was applied as described by Tapolczai et al. (2019) (available on Github: https://github.com/fkeck/DADA2_diatoms_pipeline). The data are available in the NCBI BioProject database (accession number PRJNA1166302).
The global ASV table was taxonomically assigned using the FROGS pipeline with the rbcL Rsyst Diat.barcode 10.1 database (Rimet et al. 2019) and the RDP assignment. Multi-affiliations were manually selected for the most abundant ASVs (> 1000 reads for the entire table), prioritizing species previously recorded in marine habitats. The final taxonomic table was filtered based on several criteria: sequences with at least 95% blast identity were retained (as for the chosen multi-affiliations), while taxonomic ranks, from species to classes, with an RDP value < 0.6 were considered uncertain and labeled as “unknown” (e.g., “unknown genus”, “unknown species,” etc.). Species listed as “sp.” in the Diat.barcode database (e.g., “Stricosus sp.”) were linked to their original environmental sequence numbers to maintain traceability and to distinguish them from uncertain assignments (due to an RDP value < 0.6). ASVs classified as uncertain Bacillariophyta (RDP value ≠ 1) were discarded. The corresponding biom table and phylogenetic tree were exported from FROGS for subsequent statistical analyses. Reads were normalized to the minimum number of reads among the entire dataset (17,563 reads). The 365-day sampling point at Banyuls sur Mer was removed as it did not meet the rarefaction threshold.
2.5 Bioinformatics and Statistical Analyses
All statistical analyses were performed using Microsoft Excel and R (version 4.1.1) with the phyloseq, vegan, microbiome, stats, agricolae, and pairwiseAdonis packages. Shannon and Chao1 indices were calculated at the species level. Non-metric multidimensional scaling (NMDS) was performed using the “ordinate” function of the phyloseq package, with the Bray–Curtis dissimilarity method, again at the species taxonomic level. Analysis of variance (ANOVA) and permutational multivariate analysis of variance (PERMANOVA) were performed on the distance matrix, considering site, lifestyle (in biofilm or planktonic), and immersion time. Interactions between diatom species from biofilms (co-occurrence network) were assessed using the CoNet plugin (v 1.1.1 beta) (Faust and Raes 2016). Data were processed using Pearson and Spearman correlations, with Bray–Curtis and Kullback–Leibler distances, and a mutual information score, applying a threshold of 150 edges. Randomization was calculated according to Brown's method, with the Bonferroni correction for multiple testing (p < 0.05).
Interactions between microorganisms in biofilms were processed using raw sequencing data obtained from the same DNA extracts from previous studies focusing on prokaryotic communities (Catão et al. 2021, 2019) (NCBI accession n°. PRJNA608237, BioSamples SAMN14167926 to 28 and under PRJNA608293, BioSamples SAMN14169847 to 52, and under PRJNA504753). A similar processing workflow in R (cutadapt, dada2) and ASV assignment to the 16S rDNA Silva 138.1 database was performed with FROGS. For the fungal communities, the ASV table provided by G. Burgaud (Philippe et al. 2023) was used. A multi-block analysis DIABLO from the MixOmics R package (v 6.26.0) was also performed to obtain a network showing positive correlations between diatoms, prokaryotes, and fungal species. The networks were then imported and analyzed using Cytoscape software (v 3.9.1).
Distance-based Redundancy analysis (dbRDA) was performed on a reduced subsampled dataset (biofilms immerged 30 days in 2016 in Lorient, Toulon and La Reunion) against environmental variables using the Bray–Curtis distance matrix at the species taxonomic level. The “anova.cca” function was used to test the statistical significance of global analyses, as well as axes and variables. LEfSe analysis (Segata et al. 2011) with a linear discriminant analysis (LDA) set at 4 or 4.5 allowed us to identify specific taxa according to conditions, using lifestyles used as classes to identify relevant taxa for each site. Finally, ASVs and species were exported from R using the “core_members” function to generate Venn diagrams for each site or between sites, distinguishing exclusive and core (common to all samples) communities, with results visualized using the online program Venny 2.1 (Oliveros 2007).
3 Results
3.1 Sequencing Data
Processing of the sequencing data with DADA2 resulted in 6,311,870 reads (from an initial 7,112,973) yielding 3105 ASVs. Of these, 34 ASVs (84,118 reads), mainly assigned to the brown algae Feldmannia irregularis and Hecatonema terminale, were unclassified, while 99 ASVs (151,474 reads) were identified outside the Bacillariophyta phylum. Our decision to filter Bacillariophyta with an RDP value ≠ 1 removed a further 520 ASVs (1,678,022 reads), including 425 ASVs with an “unknown class” (RDP < 0.6). Of these, six ASVs, representing 888,816 reads, were among the 10 most abundant ASVs in the entire set. After normalization, all further analyses were performed on the resampled ASV table containing 2295 ASVs (843,024 reads) in 48 samples.
3.2 Temporal Dynamics in the Northwestern Mediterranean Sea
3.2.1 Diatom Density and Surface Coverage in Biofilms
In Toulon Bay, diatoms were undetectable after 1 day of immersion, and the second sampling time after 4 days showed 68 cells/cm2 ± 44 cells/cm2 (Figure S2A). Diatom abundance increased steadily, showing a significant increase after 28 days (p-value = 0.001, 56,930 cells/cm2) and reaching a plateau after 365 days (p-value < 0.0001, 125,678 cells/cm2). Although no statistical differences in biovolumes were observed between sampling points from 1 to 365 days due to large standard deviations, a general increase in mean biovolume was observed (Figure S2B). Furthermore, diatom numbers and biovolumes remained stable from 2015 (Tln_T28) to 2016 (Tln_T30_N + 1), under similar conditions after 1 month of immersion in summer (p-value = 0.579 and p-value = 1000, respectively). Comparing the two Mediterranean sites, Banyuls sur Mer showed a decrease in biovolume after 1 year compared to 75 days of immersion (p-value < 0.0001), while their density remained unchanged (p-value = 1.000, Figure S2A).
3.2.2 Alpha- and Beta-Diversity of Diatom Communities
Alpha-diversity was analyzed at the species level (Figure 1). Similar values were found for different immersion times in Toulon and Banyuls sur Mer, all in the Mediterranean Sea.
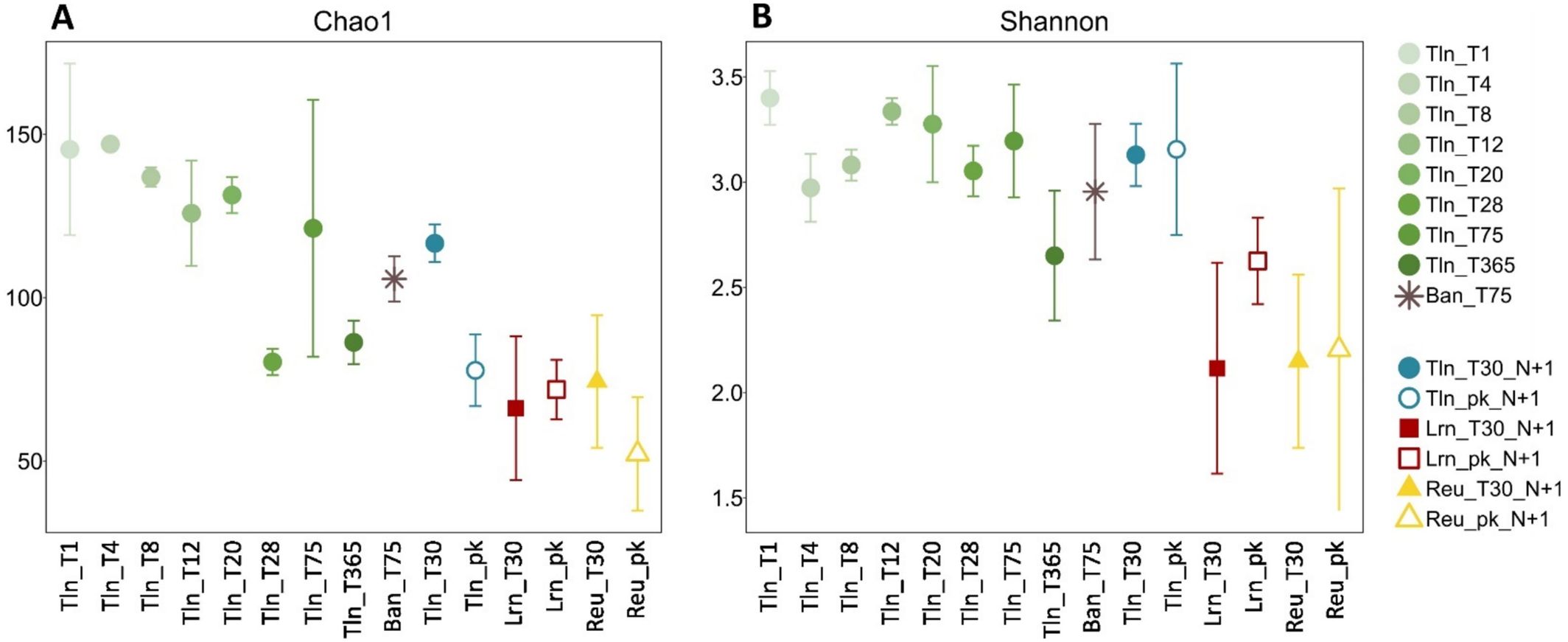
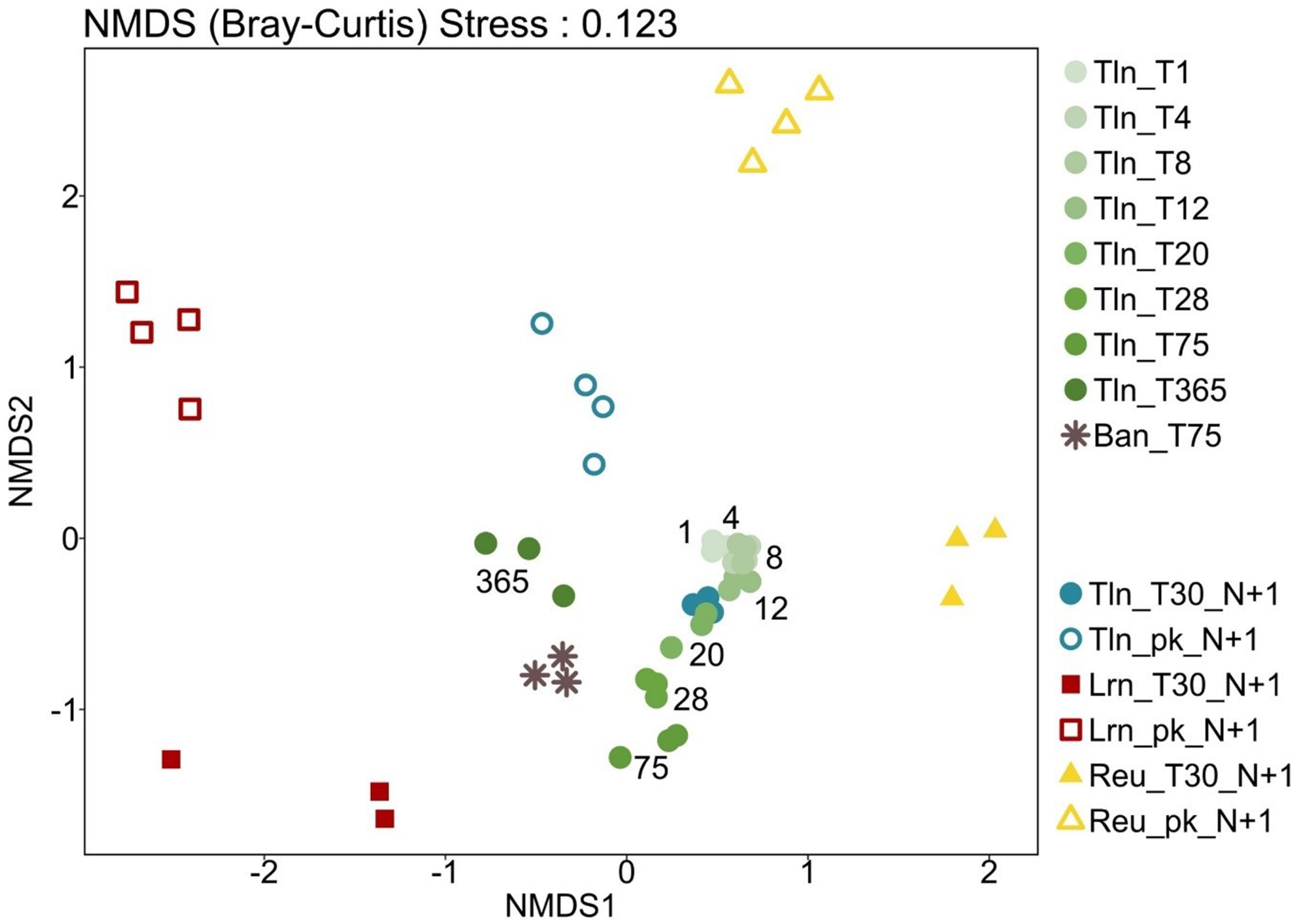
Beta-diversity was analyzed using NMDS ordination based on the Bray–Curtis dissimilarity (Figure 2). In Toulon, a significant temporal dissimilarity in diatom communities was observed between T1 and T365 in 2015 (p-value = 0.0001), and PERMANOVA showed no significant difference between diatom communities in biofilms immersed for 1 month in 2015 and 2016.
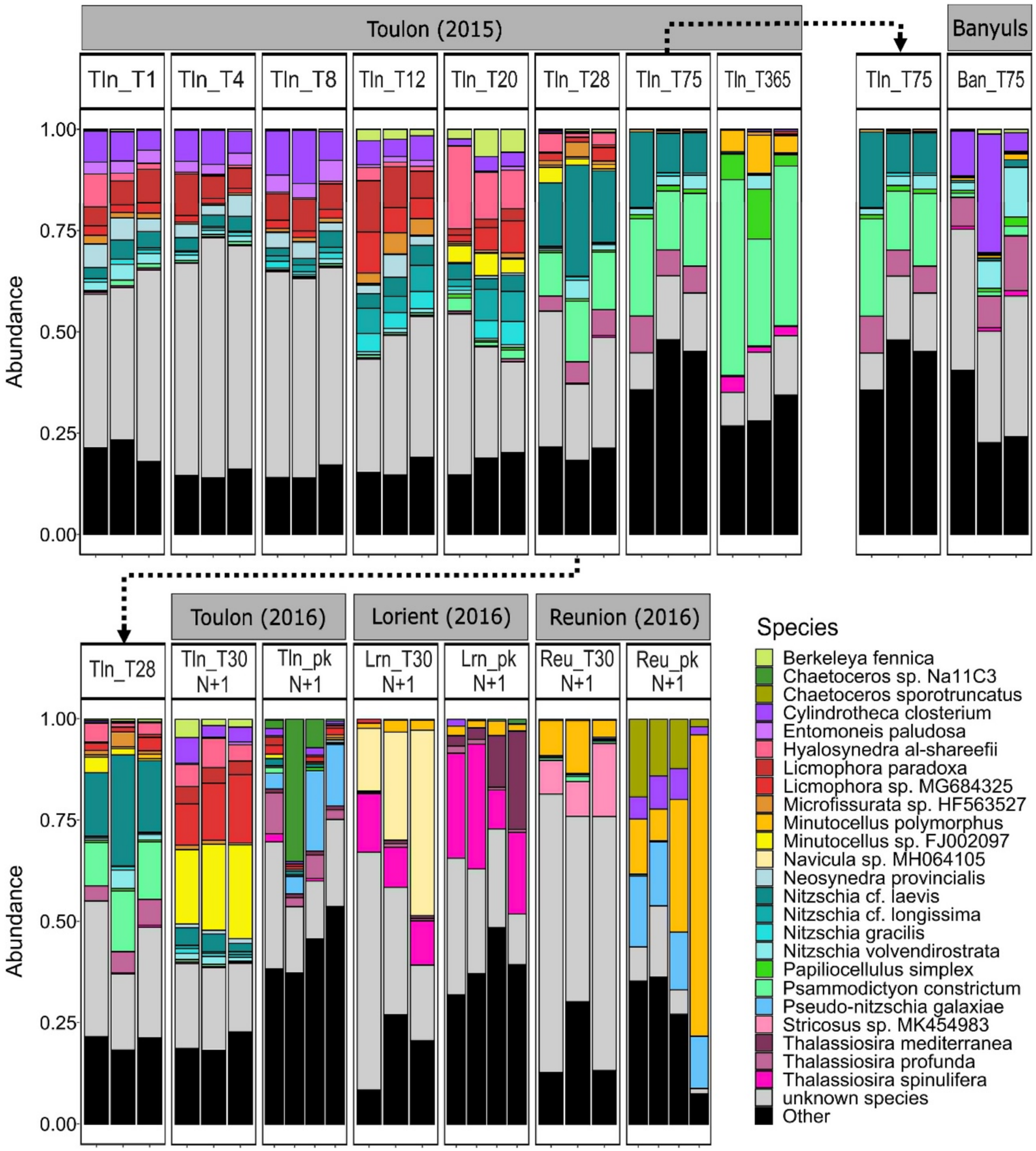
Considering the taxonomic composition of the diatom communities in the whole dataset (Figure 3), the proportion of unknown species (49 species out of 489 total species) ranged from 1% (in seawater from La Reunion) to 69% (in biofilm from La Reunion) of species depending on the sample. Of the 25 most abundant species, 14 have been previously observed in marine habitats, while 2 species were only known from freshwater (Minutocellus polymorphus and Nitzschia gracilis), and 8 species had a habitat not yet defined (Table S1). Hyalosynedra al-shareefii, Microfissurata sp. HF563527, Entomoneis Paludosa, Licmophora paradoxa, Licmophora sp. MG684325, Neosynedra provincialis, Nitzschia gracilis, Nitzschia cf. longissima were prevalent in Toulon in the early stages but decreased with time and became rare after 28 days. Cylindrotheca closterium followed a similar trend but remained abundant at Banyuls after 75 days. Conversely, Psammodictyon constrictum, Papiliocellulus simplex, and Thalassiosira profunda (which was also present in Banyuls) became abundant after 28 days and persisted. Interestingly, the latter was still present the following year but with reduced relative abundances. Taxa identified as less abundant at T28 in 2015, such as Berkeleya fennica or Minutocellus sp. FJ002097, became dominant in 2016, highlighting the variation in species dominance in mature biofilms at Toulon between consecutive years.
3.2.3 Interactions Among Diatom Species in the Marine Environment
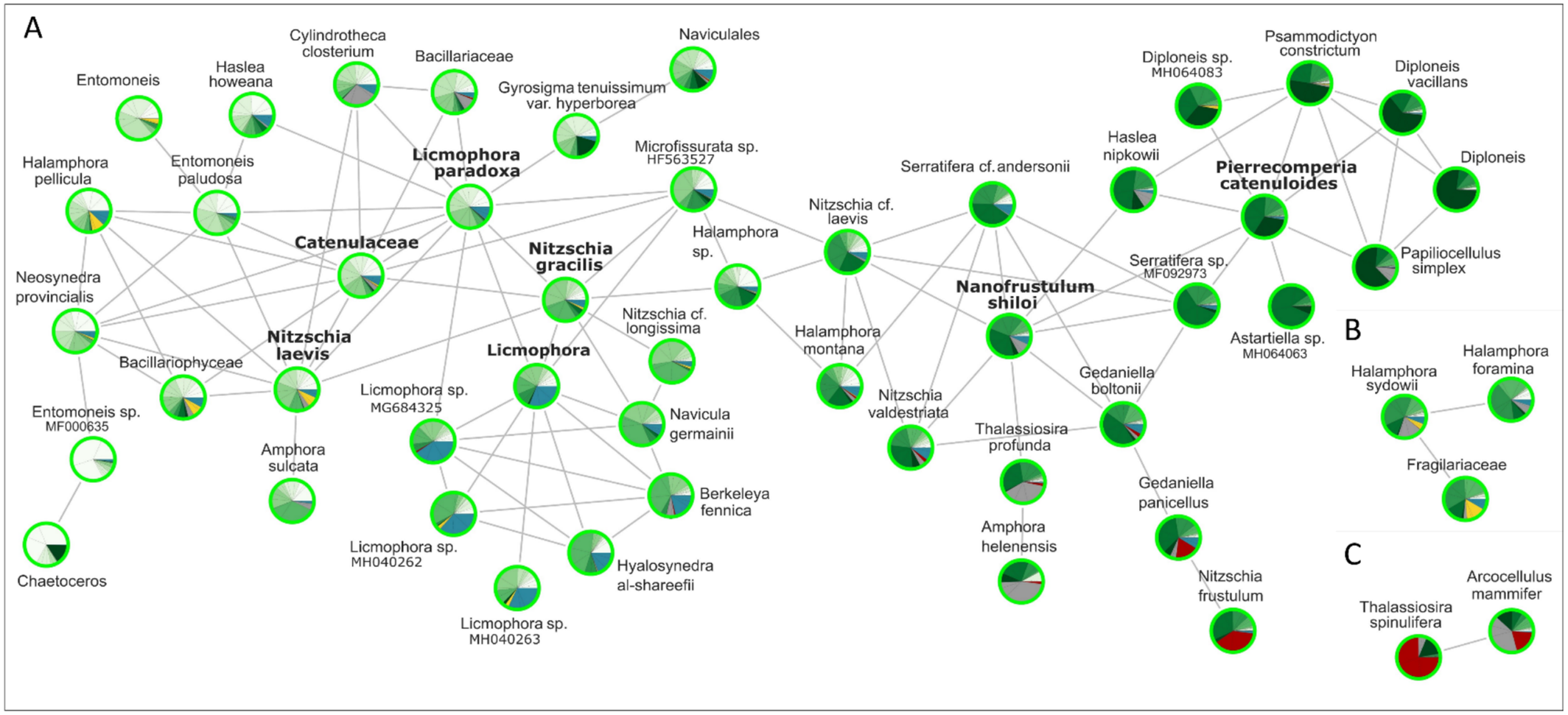
The diatom network (Figure 4) showed 103 positive interactions (edges or degrees) between 51 species (nodes) across the entire dataset. A main cluster of 46 nodes (Figure 4A) was identified, together with two smaller clusters of three and two nodes (Figure 4B,C, respectively). Cluster A consisted of species mainly from Toulon and Banyuls sur Mer, which were mostly found in biofilms. An unknown Catenulaceae, Licmophora paradoxa, Nitzschia gracilis, N. laevis (associated with species found during the first month of immersion), Nanofrustulum shiloi, and Pierrecomperia catenuloides (mostly associated with to species observed after long-term immersion) had the highest number of putative interactions (edges> = 8), each co-occurring with 4–108 other genera. They can therefore be considered as keystones in the network. Some of these genera (e.g., Nitszchia, Licmophora, Navicula, Halamphora, Diploneis, Serratifera, Gedaniella and Entomoneis) seemed to be more represented and clustered in groups ranging from two to five species. Cluster B contained three species mostly from 2015 samples in Toulon and Banyuls sur Mer, belonging to the Catenulaceae (Halamphora species) and Fragilariaceae classes. Cluster C contained two co-occurring species (Arcocellulus mammifer and Thalassiosira spinulifera) mainly from Lorient or Banyuls sur Mer, belonging to the Mediophyceae.
3.2.4 Effect of Geographical Location on Biofilm and Planktonic Communities of Diatoms After 1 Month
3.2.4.1 Diatom Density in Biofilms Depending on Sites
PVC substrates immersed in Reunion Island (Reu_T30_N + 1) tended to be more colonized (76,741 cells/cm2) and more covered (891 μm3/cm2) than in Toulon (17 μm3/cm2) (Figure S2), although statistical analyses could not be performed due to missing values for Reunion Island. After 30 days, significant differences were observed for the Chao1 (Figure 1A) and Shannon (Figure 1B) indices between Toulon and Lorient (p-value = 0.00012 and p-value = 0.00061, respectively) or Reunion Island (p-value = 0.9 × 10−6 and p-value = 0.6 × 10−4, respectively). Notably, the only difference between planktonic and biofilm samples was observed in Toulon, with a lower richness (Chao1, p-value = 0.037) in planktonic communities, as observed in Lorient and in Reunion Island.
The diatom communities appeared to be structured by two main factors (Figure 2): immersion site (with Toulon differed from Lorient and Reunion (p-value = 0.006), and oceanic sites differed from each other (p-value = 0.012)), and lifestyle (biofilm vs. surrounding seawater, p-value = 0.001). Regarding the taxonomic composition (Figure 3), biofilms from Reunion Island and Lorient were dominated by two species pairs, Stricosus sp. MK454983 and Minutocellus polymorphus in the Indian Ocean, Navicula sp. MH064105 and Thalassiosira spinulifera in the Atlantic Ocean. In addition, Chaetoceros sporotuncatus and Minutocellus polymorphus (reaching 74%) were prevalent in the planktonic mode in Reunion Island, while Thalassiosira mediterranea dominated in Lorient, Chaetoceros sp. Na11C3 in Toulon, and Pseudo-nitzschia galaxiae in both the Indian Ocean and the Mediterranean Sea.
3.2.4.2 Core Community Between Sites and Lifestyles
Venn diagrams allowed us to detect common taxa (core community) among the three sites of Toulon, Lorient, and Reunion Island, for each lifestyle (Figure 5A,B), and for both lifestyles at each site (Figure 5C). The core community of planktonic samples (Figure 5A) contained fewer species or ASVs than the communities of the individual sites. Nevertheless, the 22 species involved (8%) accounted for 52% of the reads, indicating a core community composed of abundant taxa such as Minutocellus polymorphus, Pseudo-nitzschia galaxiae, or an unknown Bacillariophyceae (with 12%, 9%, and 9% of the reads, respectively). The number of common genetic variants (ASVs) was much lower (1% of ASVs for 14% of the reads), reflecting a greater site specificity in terms of genetic variants. Toulon and Lorient shared more species (11%, corresponding to 20% of the reads) and genetic variants (4%, corresponding to 16% of the reads) than Reunion.
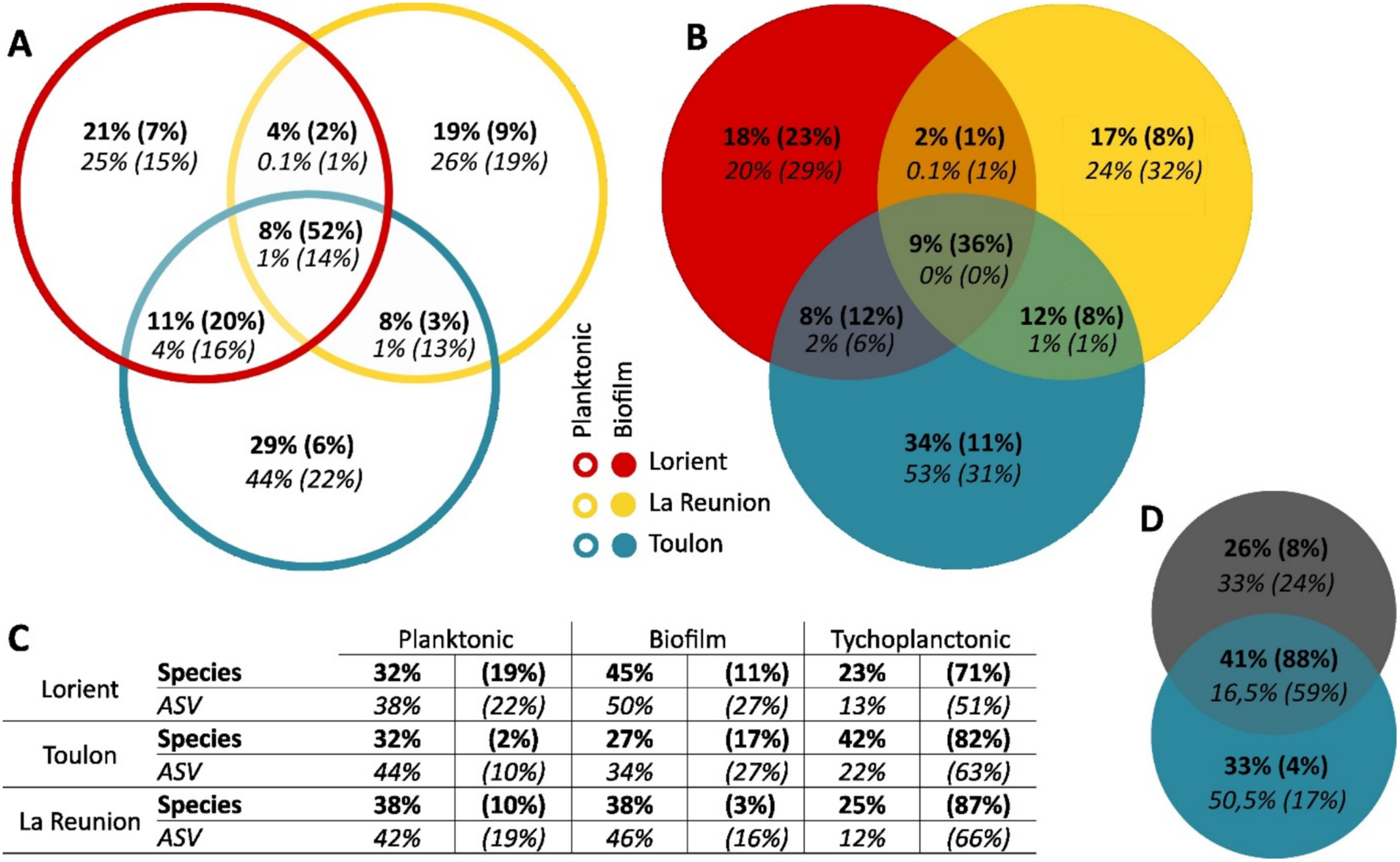
Analyses of the biofilm communities (Figure 5B) showed a similar pattern. Although the core community also contained 22 species (9%), they represented a lower proportion of reads (36%) compared to those from planktonic samples collected from surrounding waters. Common species included Nitzschia sp. (17%), Minutocellus polymorphus (4%), and two unknown species (6% and 3% of the reads). Notably, the three biofilm communities did not share any ASV. For comparison, looking specifically at Mediterranean biofilms (Figure 5D), Toulon and Banyuls sur Mer () shared 41% of the species and 16.5% of the ASVs (corresponding to 88% and 59% of the reads, respectively). Furthermore, similar analyses after 1 month (Table S2) of immersion at Toulon (Tln_T28 and Tln_T30_N + 1), 1 year apart (2015 and 2016), revealed 42% of shared species and 26% of shared ASVs (corresponding to 97% and 86% of the reads, respectively).
3.2.4.3 Effects of Environmental Parameters on Diatom Community Structure and Composition
Reunion Island had the highest temperatures and phosphate concentrations, while Lorient had the highest nitrate concentrations (Table S1). Salinity was lower at Lorient compared to Toulon, with intermediate values in Reunion Island. Toulon had the lowest silicate concentrations, which were significantly different from Reunion Island and Lorient. Bray–Curtis distance-based redundancy analysis (dbRDA, Figure S3) including both biofilm and planktonic samples, significantly (p-value = 0.001) explained 44% (dbRDA1) and 43% (dbRDA2) of the variance accounted for by the constrained variables (43%). Statistical analysis showed that temperature (anova.cca, p-value = 0.001), salinity (p = 0.001), and nitrates (p-value = 0.05) influenced the distribution of diatom communities in both planktonic and biofilm lifestyles. Phosphates and silicates were significantly correlated with temperature (r = 0.89) and salinity (r = −0.89), respectively.
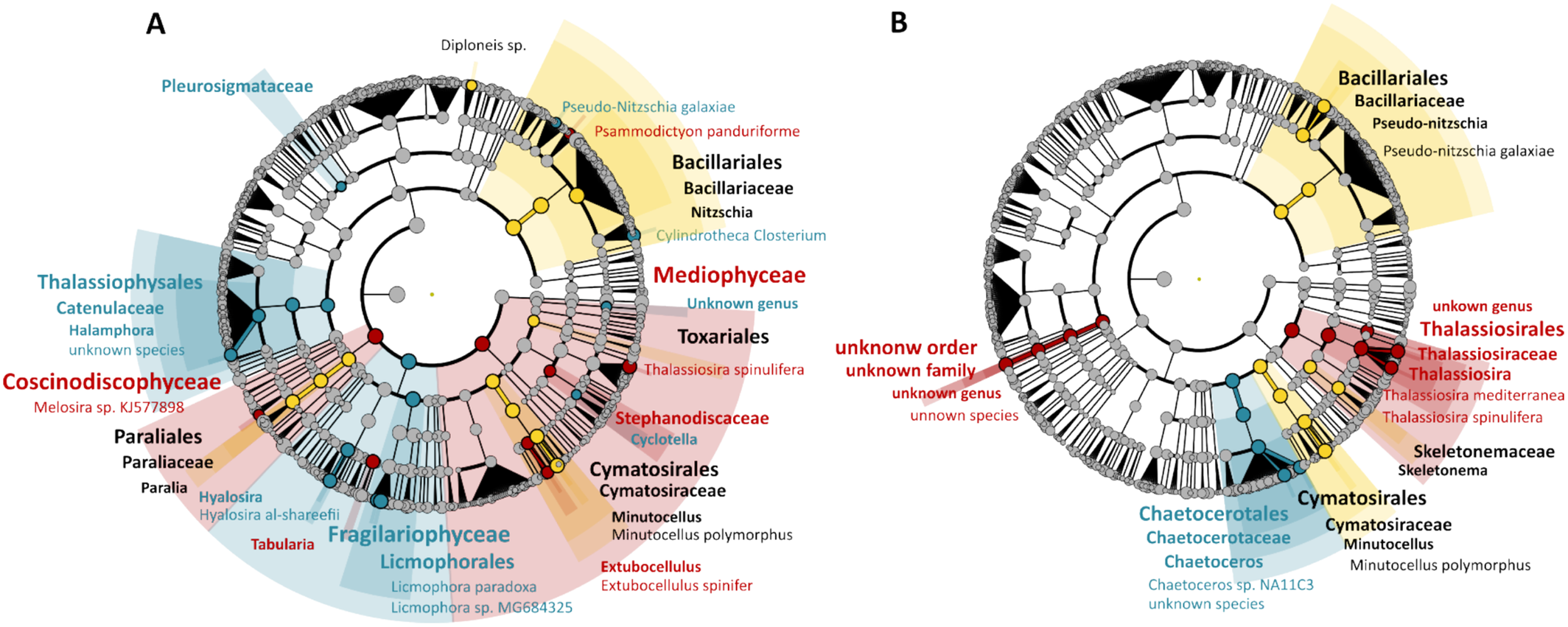
LEfSe analysis (Figure 6) identified the Cymatosirales, together with the family and genus of Minutocellus polymorphus, as discriminant taxa in Reunion Island, with no distinction between lifestyles. This was also the case for Bacillariales and Bacillariaceae. Nitszchia spp. and Pseudo-nitzschia spp. (and especially the species Pseudo-nitzschia galaxiae) were specific for biofilms (Figure 6A) and planktonic lifestyles (Figure 6B), respectively. Pseudo-nitzschia galaxiae was also found as a discriminant species in Toulon Bay biofilms, together with Cylindrotheca closterium and Psammodictyon panduriforme. Thalassiophysales, Pleurosigmataceae, and Catenulaceae (together with Halamphora spp., and an unknown species of Halamphora) were also specific taxa of Toulon biofilms. Fragilariophyceae appeared specific to Toulon biofilms as well (except for the genus Tabularia, which was discriminant in Lorient), with the genus Hyalosira and the species Hyalosira al-shareefii, or with Licmophorales represented by two species (Licmophora sp. MG684325 and L. paradoxa). Diploneis sp. MH064083 was discriminant in biofilms from Reunion Island. This was also the case for Toxariales, Paraliales, Paraliaceae, and Paralia spp. Mediophyceae and Coscinodiscophyceae were two discriminant classes in Lorient biofilms, as were the family Stephanodiscaceae (except for the genus Cyclotella, which was specific to Toulon), the genus Extubocellulus, and E. spinifer species. In addition, two Chaetoceros species (and their respective family and order) were discriminant species in the planktonic community the surrounding waters of Toulon. Apart from an unknown Coscinodiscophyceae species, the surrounding waters of Lorient were characterized by Thalassiosirales, Thalassiosiraceae, Thalassiosira, and in particular T. mediterranea and T. spinulifera, which were also discriminant in the biofilms.
3.2.4.4 Interactions Among Prokaryotes, Fungi, and Diatoms in Marine Biofilms
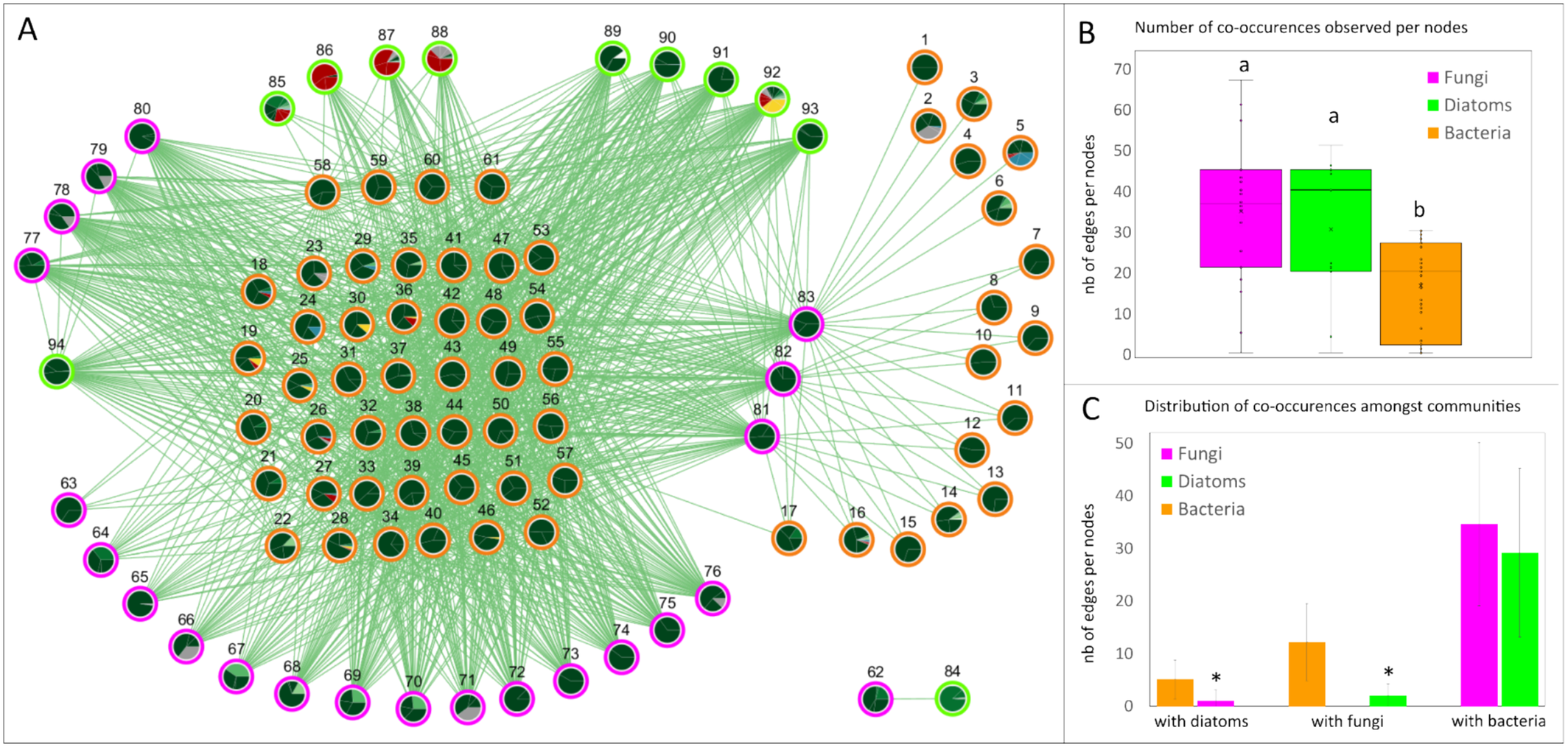
Within biofilms, we also focused on co-occurrences between diatoms, fungi, and bacterial taxa represented in a MixOmics network (Figure 7A). This network contained 1076 edges and 94 nodes (Table S5), including 61 bacteria (nodes 1–61), 22 fungi (nodes 62–83), and 11 diatoms (nodes 84–94), distributed in two clusters. Bacteria had fewer edges (1–31) than fungi (1–68) and diatoms (1–52) (Figure 7B). Co-occurrences between bacteria and fungi or bacteria and diatoms were more frequent than co-occurrences between fungi and diatoms (Figure 7C). However, node 84 (an unknown diatom species from the order Achnanthales) did not co-occur with any bacteria but with a Coniothyrium fungus (node 62). Some nodes had a minimal number of edges, such as bacteria from nodes 1 to 17 (see Table S5), which co-occurred with only one to four fungi (nodes 77, 81, 82, and 83). The diatom Extubocellulus spinifer (node 85) was related to five bacteria (nodes 58–61) belonging to the genera Methyloceanibacter, Flagellimonas, Pelagibius, and Ahrensia. Two species of diatoms, Chaetoceros socialis and a Thalassiosirales species (nodes 86–88), mainly present in Lorient, were only associated with bacterial species. This was also the case for fungi from nodes 63 to 76 (see Table S5). In the large and central cluster of bacteria (nodes 18–61), co-occurrences were distributed with fungi (5–21 edges) and diatoms (1–10 edges). Finally, the nodes 89–93 and node 91 (Chaetoceros diversus, C. danicus, Skeletonema costatum, Minutocellus polymorphus, Rhizosolenia imbricate, and R. fallax) also co-occurred with fungi from nodes 81 to 83 (Nectriopsis sp., Parathyridaria rosae and Buckleyzyma aurantiaca) and nodes 77 to 80 (Exophiala xenobiotica, Lycoperdon pretense, unknown species of the class Agaricomycetes, and Flavoplaca sp.).
4 Discussion
4.1 Diatom Richness Is Nearly Established in the Early Stages of Biofilm Formation
Microbial adhesion and colonization of plastic substrates in the marine environment occurs rapidly, typically within minutes to hours (Latva et al. 2022). Our experiment started in summer, corresponding to maximum irradiance and temperature, when the maximum diversity of diatom species is generally observed (Munda 2005). The colonization trend of diatoms in Toulon Bay is consistent with previous studies (Molino and Wetherbee 2008; Molino et al. 2009; Patil and Anil 2005). The evolution of diatom density determined by microscopy (Figure S2) indicated that initial colonization rates for diatoms are lower than for bacteria. The maximum richness and diversity observed by metabarcoding within the first few days (Figure 1) also confirmed that after an initial settlement phase, most diatoms on surfaces proliferate primarily by asexual reproduction of existing biofilm cells, rather than direct recruitment from planktonic cells, resulting in “monospecific” cloning patches (Cooksey et al. 1984; Mitbavkar et al. 2012). Mitbavkar and Anil (2008) also found that diatom recruitment and invasive reproduction increased cell numbers on surfaces. This process likely explains the lack of variation in both richness and diversity of such r-strategists over time. This is also consistent with recent studies using long-term deployments (up to 52 weeks) where diversity was highest after 1 week of immersion (Amaral-Zettler et al. 2020; Laroche et al. 2023). However, this pattern, however, contrasts with observations for prokaryotes and fungi in the same samples. Prokaryotic communities in Toulon displayed rapid temporal dynamics with a rapid increase in diversity during the first few days. For fungi, the increase in richness and diversity seems to come later (based on ITS, Philippe et al. 2023). As no clear trend was observed for diatom alpha-diversity, this indicates a distinct dynamic. However, the specificity of biofilm communities associated with geographical locations, including the similarity of Mediterranean sites and their higher diversity (H′), seems to be shared by all microbial components studied and linked to environmental parameters.
Beta-diversity analysis (Figure 2) revealed a gradual but notable evolution of diatom communities over 1 year, with biofilm assemblages generally clustering, particularly during the first 20 days. Laroche et al. (2023) recently confirmed that exposure time significantly affects diatom community structure on conventional plastics, with significant differences observed after 12 weeks of immersion. Furthermore, our experiment was conducted in a nearly closed, highly contaminated area (Misson et al. 2016; Pollet et al. 2018), which may explain the slow recruitment of new species (Mitbavkar and Anil 2008; Breznak et al. 1984) and minimal changes in biofilm composition due to selection pressure. However, the diatom communities after 365 days of immersion appeared highly different, with an increase in the number of abundant taxa. This shift may be due to the presence of multicellular colonizers competing with diatoms for space or providing additional substrates through epibiosis. In addition, recent work supports our findings, suggesting that the cell size distribution shifts towards smaller cells with longer exposure times, as observed with conventional polymers over periods extending up to 12 weeks (Zhao et al. 2021).
The colonization dynamics of prokaryotes are known to be influenced by the characteristics of the substrate (natural or artificial), mostly during the first hours to days (e.g., Pinto et al. 2019). Among polymers, only the biodegradable ones will select different bacterial communities compared to conventional ones (Dussud et al. 2018; Derippe et al. 2024; Odobel et al. 2021; Jacquin et al. 2021), as well as for fungi (Kettner et al. 2019; Philippe et al. 2023). Although the effect of substrates on diatoms has not been extensively studied, existing evidence suggests that conventional polymers do not significantly affect the composition of mature diatom biofilm communities. This is consistent with observations from both Toulon and a Swedish site where diatom communities on different conventional polymers showed similar characteristics (Védie et al. 2022).
The analyses carried out in Toulon over two consecutive years and in the bay of Banyuls sur Mer, which experiences stronger hydrodynamic effects, gave us the opportunity to document the relatively little studied diatom biofilm communities in the Mediterranean Sea. Considering the cell size observed with microscopy, larger diatom species seemed to be favored on sandy substrates with strong hydrodynamic conditions (Andriana et al. 2021), which may explain why diatoms in Toulon were generally smaller compared to those in Banyuls sur Mer. In any case, these results should be interpreted with caution considering the limit of the numeration of individual cells from diversified and complex biofilms, resulting in high variation between replicates. Nevertheless, the Banyuls sur Mer biofilm communities analyzed by metabarcoding were more similar to those in Toulon, regardless of the immersion time, than to those in Lorient or in Reunion Island, both at the species (Figure 2) and ASV (data not shown) level. Mediterranean samples (Toulon and Banyuls sur Mer) shared almost 40% of the abundant species, representing 88% of the reads. This suggests that environmental parameters common to Mediterranean regions (Table S4), mainly temperature and salinity, are more important drivers than nutrients or contaminants such as trace metals. In particular, biofilms sampled after 1 month of immersion at the same site in the Toulon Bay in 2015 and 2016 with similar seawater parameters at the beginning of summer showed very similar communities with 26% of ASVs and 86% of the reads shared between the 2 years, suggesting a relative seasonal stability of the communities.
The co-occurrence network captured well the temporal variability of these and other keystone diatom species. Licmophora paradoxa and other species of the same genus are known for their weak attachment strength, which explains their absence from the most turbulent areas (Woods and Fletcher 1991; Hunsucker et al. 2014). This trait may explain why L. paradoxa became a dominant (Figure 3) and discriminant (Figure 6) species in the Bay of Toulon, which is protected from strong hydrodynamics by a dam. Moreover, their dominance during the first month of immersion indicates that they are r-strategists. Not surprisingly, L. paradoxa and Nitzschia gracilis (among the most abundant species in Toulon Bay) stood out in the co-occurrence network (Figure 4) with the highest number of interactions with other diatom species mainly occurring during the early stages of our study. In contrast, Pierrecomperia catenuloides, which was present in all Mediterranean biofilms, was not abundant but emerged as a keystone species with potential interactions with several other diatom species in mature biofilms. This seems quite surprising for a centric diatom in biofilms where mostly pennates have been reported. Unfortunately, this taxon which is phylogenetically close to Extubocellulus spp. (Sabbe et al. 2010; Dąbek et al. 2017, 2019), has hardly been described so far. The abundance of Cylindrotheca closterium in all Mediterranean samples was expected. As an important cosmopolitan benthic diatom species (Djaoudi et al. 2022), it thrives in high salinity conditions (Rao and Ragothaman 1988; Chiu et al. 2005) and has already been observed as a dominant pioneer microalga in French sites (Briand et al. 2012). Interestingly, its dominance after 75 days of immersion in Banyuls sur Mer, whereas it became rare after the same immersion time in Toulon, may indicate that nutrient limitation in Banyuls sur Mer slowed down the temporal dynamics of diatom colonization. Furthermore, Berkeleya fennica and Neosynedra provincialis, known for their metal and nutrient tolerance, may reduce the colonization success of more sensitive species (Belando et al. 2017). Their higher abundance in Toulon compared to Banyuls sur Mer may highlight their preference for Cu and Zn concentrations, which differed notably between these two sites (Table S4).
A progressive shift in the abundance of species was observed in Toulon Bay, starting after 20 days of immersion. Predation, interactions between plastisphere microorganisms, recruitment of new taxa during the time of the immersion, completion and diversification of functional traits within the biofilms are inevitably involved in these temporal dynamics (Tobias-Hünefeldt et al. 2021; Bech et al. 2024). However, environmental parameters may also offer some explanation as temperature increased from 23.4°C (T0) to 25.8°C (T20), along with Cu and Zn (2-fold increase and 2.6-fold decrease, respectively) between T0 and T28, while other parameters remained relatively stable. This phenomenon is complex, and several variables not investigated here (e.g., the influence of light (Doose and Hubas 2024)) may also play a role. Psammodictyon constrictum became remarkably dominant (reaching up to 48%) after 1 year of immersion. This widely distributed species has recently been detected in biofilms from sea turtles and floating debris in the Persian Gulf (Azari et al. 2020) and described as a dominant species in red tides along the Egyptian coast (El-Hadary et al. 2022). It would be worthwhile to study the possible reappearance of these species in relation to biotic and abiotic factors. The increase in relative abundance of the centric tychoplanktonic diatoms Papilliocellus simplex, Thalassiosira spp., and Minutocellus sp. could be due to blooms that displace the benthic diatom community and temporarily alter the pennate/centric ratio, as previously shown for Melosira nummuloides by Patil and Anil (2005). Furthermore, Pollet et al. (2018) showed that after 75 days of immersion, the bacterial community in biofilms showed 65% of dissimilarity compared to those in the first month of immersion. Thus, the variable abundance of bacterial taxa within biofilms should be considered as a factor that may influence the composition of diatom communities (Tang et al. 2011). Cruz and Neuer (2022) showed that the bacterial community influenced the aggregation capacity and consequently the settlement of Minutocellus polymorphus, one of the planktonic discriminant taxa in Reunion Island.
4.2 Environmental Parameters Shape Diatom Communities in Marine Biofilms More Than Lifestyle
All three sites, although exposed to harbor activities, differed in terms of temperature, salinity, nutrients, and trace metal contamination, which largely explain the diatom dissimilarities in both biofilm and planktonic communities (dbRDA, Figure S3). Our results are consistent with findings that biofilm diatom communities differ significantly from surrounding seawater communities (Chiu et al. 2005; Patil and Anil 2005), as do bacteria or fungi. Thus, our data are in agreement with Patil and Anil (2005), showing that physico-chemical factors of the surrounding environment influence both biofilm diatom and planktonic communities. Furthermore, it was observed that site remained the primary clustering factor even in the case of biofilms on biocidal antifouling coatings (Zargiel et al. 2011; Briand et al. 2017). Here, environmental parameters accounted for up to 40% of the variation in community clustering between biofilm and planktonic samples recovered in 2016 (Figure S3). Recent studies have highlighted the structuring role of seawater parameters on biofilm diatom communities using metabarcoding, a finding consistent with previous microscopy-based observations of marine plastispheres (Belando et al. 2017; Azari et al. 2020; Munda 2005; Mitbavkar and Anil 2008; Chiu et al. 2005; Nodine and Gaiser 2014; Hillebrand and Sommer 2000).
For prokaryotes and fungi, biofilm communities showed higher diversity than planktonic communities (Yeh and Fuhrman 2022; Catão et al. 2021; Philippe et al. 2023). However, diatom diversity did not show similar differences in our samples. To date, studies on the alpha-diversity of diatom communities from biofilms and surrounding waters are scarce and have shown contrasting results depending on the site (Patil and Anil 2005; Laroche et al. 2023). The lower richness and diversity observed in biofilms from both oceanic sites (Lorient and Reunion Island) compared to Mediterranean biofilms echo recent results on fungal communities (Philippe et al. 2023). This trend can be attributed to nutrient loading (Desrosiers et al. 2013), which is the only common measured factor between the sites, although Lorient has higher nitrate concentrations and Reunion is characterized by higher phosphate levels. Hydrodynamics is also a notable factor structuring diatom assemblages in biofilms (Wachnicka et al. 2011; Hunsucker et al. 2014; Nodine and Gaiser 2014), with diatom species possessing stronger attachment capabilities (morphological and adhesive properties) being favored (Zargiel and Swain 2014). Although lower diatom biomass is typically observed on sheltered shores compared to moderately exposed ones, regardless of site and season (Thompson et al. 2005), the generally sheltered nature of our sites (all within harbors, except for Banyuls sur Mer) suggests that hydrodynamics may not be a primary driver of community composition when comparing abundances in Toulon Bay, Lorient, and Reunion. As there is still a gap in the literature regarding lifestyle-specific diversity comparisons for marine diatoms, it was not possible to investigate this parameter further.
Prokaryotes and fungi exhibited distinctly different clear dissimilar communities when, as mentioned above, the diatom lifestyle appeared to be less specific as evidenced by the definition of tychoplanktonic taxa. The tychoplanktonic lifestyle (Kuhn and Waser 1981), corresponding to taxa able to switch from planktonic to biofilm and vice versa, was dominant at all sites with about a third of the species but only a quarter of the ASVs, representing about 80% of the reads. Our analysis revealed that some planktonic biomarkers from centric genera (Chaetoceros, Thalassiosira, Minutocellus, Skeletonema) were represented by specific species in biofilm communities (e.g., Minutocellus polymorphus in Reunion Island, Thalassiosira spinulifera in Lorient). Conversely, few diatoms were specific to the planktonic lifestyle (2% of species and 10% of reads in Toulon), suggesting that the biofilm lifestyle may be more common for diatoms, at least for certain life stages Notably, while bacteria mostly exhibit a specific lifestyle (Catão et al. 2021; Zhang et al. 2019), fungi tend to adapt to both lifestyles (Philippe et al. 2023).
Diatom communities are characterized by few dominant taxa complemented by a high number of rare taxa, a pattern also observed in planktonic diatom communities worldwide (Malviya et al. 2016). Rare species exhibited a specific lifestyle and a higher genetic diversity. As these comparisons were limited to summer (T30 samples), further studies are needed to understand seasonal variability in diatom biofilm diversity.
Among the dominant (Figure 3) and site-specific (Figure 6) species, Navicula and Nitzschia (motile diatoms) are known to be high competitors under nutrient-enriched conditions, which can explain why the genus Nitzschia was found as a biomarker in Reunion Island and why the community was largely dominated by Navicula sp. in Lorient. However, this selection may vary between species (Belando et al. 2017) as we were able to observe several species of Nitzschia among the abundant ones in Toulon. Similarly, Tabularia spp. are highly nutrient tolerant (Hillebrand and Sommer 2000), which is consistent with the specificity of this genus in Lorient (Figure 6). Thalassiosira spp. are centric and cosmopolitan marine diatoms (195 species referenced), already described in both the Mediterranean Sea and the French Atlantic coasts (Percopo et al. 2011). This seems to be in line with the fact that, depending on the species, Thalassiosira has been found either as abundant in Mediterranean biofilms and waters (in Toulon and Banyuls) or as a biomarker of Lorient. Finally, Pseudo-nitzschia, which includes at least 12 toxic species potentially producing domoic acid (Trainer et al. 2012), has been reported to be widespread in the Mediterranean Sea, with a high abundance of cells attached to plastic matrices (Casabianca et al. 2019). In our study, P. galaxiae appeared as a major species in the surrounding waters of Toulon and Reunion and, interestingly, serves as a biomarker either in both biofilms in Toulon and planktonic species in Reunion.
A limited biofilm core community, consisting of a few dominant species (9% with 36% of reads), showed no shared genetic variants between sites. This suggests that genetic variants from the same species, transported by marine currents, were able to adapt to different local conditions. It also seems to indicate that a genetic variant from Lorient reaching the Mediterranean Sea may not thrive in this new habitat. The importance of intraspecific variation for adaptive success, which could also be related to the ecotype concept, has already been reported for planktonic diatoms (Godhe and Rynearson 2017), but this study provides the first evidence for marine biofilms. Such genetic divergence, mainly observed over large geographical distances, seems to be in line with our data showing intraspecific variability between oceans but high similarity between Mediterranean sites. Recent studies in French and Hungarian rivers (Pérez-Burillo et al. 2021; Tapolczai et al. 2021) based on ASVs have highlighted their utility in analyzing intraspecific and cryptic diversity among diatom species based on dispersal patterns and ecological preferences. Similar results have been shown for diatoms in alpine lakes from France and Georgia, with remarkable intraspecific differentiation (Rimet et al. 2023). Considering heterotrophic protists, a less clear trend has been observed, with some species showing restricted distribution patterns and others being widely distributed. It would be worthwhile to look at the functional potential of diatom communities to better unravel how diatom communities adapt to different environments (Pearson et al. 2015). To our knowledge, intraspecific genetic variability has not yet been explored for fungi. Finally, this adaptation process at the subspecies level remains difficult to test for prokaryotes as metabarcoding does not allow for a sufficient level of taxonomic assignment at the species level.
4.3 Microbial Potential Interactions Between Prokaryotes and Microeukaryotes in Marine Biofilm Communities
The prokaryotic and fungal communities of the same samples used here for diatom diversity have already been published independently (Catão et al. 2021, 2019; Pollet et al. 2018; Philippe et al. 2023). This study advances our understanding of biofilm dynamics and interactions between the three main taxonomic groups using for the first time a co-occurrence approach with a MixOmics network. Although these interactions appear primordial, they remain poorly studied, particularly in marine biofilms.
In the network, most of the nodes were abundant in mature biofilms in Toulon, which could be related to the largest number of samples in Toulon and the increasing cell density over time. Prokaryotic nodes were mainly composed of low abundant but core biofilm taxa (Verrucomicrobiae and Planctomycetes, Catão et al. 2021), rare taxa in biofilms but involved in photosynthesis and nitrogen fixation (filamentous cyanobacteria and an ammonia-oxidizing Thaumarchaeota), a marine pathogen (Vibrio splendidus), and three abundant and diverse groups in biofilms (Flavobacteriaceae, Rhodobacteraceae, and Rhizobiales). Many of these taxa are known to be associated with diatoms in natural habitats or cultures (e.g., Amin et al. 2012; Abed et al. 2019). Recently, a Rhizobiales was identified as the first non-cyanobacterial nitrogen-fixing symbiont of a marine diatom belonging to the genus Haslea (Tschitschko et al. 2024). Rhizosolenia and Chaetoceros spp. constituted half of the diatom nodes, although, as centric diatoms, they represent low abundant taxa in biofilms. Interestingly, these genera are involved in N2-fixing symbioses with cyanobacteria (Foster et al. 2011), which may indicate that this lifestyle is also reflected in the network. We also noticed Minutocellus polymorphus, a diatom biomarker of La Reunion biofilms, but also abundant in mature biofilms in Toulon, highly associated with several bacteria and three fungi, yet little described in the marine environment, possibly including Nectriopsis sp., known as a mycoparasite and also a lichen parasite (Lawrey and Diederich 2003). The diatom biomarkers of Lorient (Extubocellulus spinifer and Thalassiosira spinulifera) show associations only with bacteria. Among the fungi, Exophiala xenobiotica co-occurred with several bacteria and the Coscinodiscophyceae diatom Rhizosolenia fallax. This fungus is an ubiquitous taxon frequently detected in soil and water contaminated by monoaromatic hydrocarbons and alkanes (De Hoog et al. 2006), with a closely related species, E. jeanselmei, described as a styrene degrader (Cox et al. 1997). Other fungi associated with a variety of bacteria, are known as parasites, such as Ochroconis, known to cause diseases of marine fish (Pang et al. 2016) or Rozellomycota, known as the basal fungal lineage, which includes species that are all parasites, whether of water molds and algae, crustaceans, or amoebae (Corsaro et al. 2020), epibionts (Ochrocladosporium frigidarii, isolated from brown algae (Crous et al. 2007)), or algal endophytes (Acrostalagmus luteoalbus (Cao et al. 2019)).
Interactions between microorganisms are critical to the functioning of ecosystems. For example, using isolated strains, Koedooder et al. (2019) suggested that interactions between bacteria and diatoms in mudflats are a key factor in the structuring of the benthic microbial community and its overall functioning, as reported for specific bacteria-diatom associations (Amin et al. 2012; Seymour et al. 2017; Filek et al. 2022). While our results are largely consistent with the literature, the complexity of natural, diverse communities makes it difficult to draw firm conclusions. Understanding the interactions and dynamics between prokaryotes, fungi, and diatoms in marine biofilms is still in its early stages.
5 Conclusions
This study provided significant insights into diatom communities, as major players in marine biofilms living on plastics, by combining classical microscopic analysis with advanced molecular approaches. The results highlighted the specificity of diatoms within biofilms, with a strong influence of environmental parameters on the structuring of their communities, beyond lifestyle aspects. The presence of a core of dominant species on a large geographical scale, but with local genetic differentiation, underlines the importance of adaptive processes in the diversity of diatoms within marine biofilms. These findings contribute to a better understanding of the functioning and dynamics of such microbial ecosystems in coastal environments.
Author Contributions
R.B.M. performed P.C.R., the bioinformatic and statistical analyses, and wrote the article. T.P. was responsible for experimental design and performed DNA extraction. C.G. was in charge of trace metal quantification as well as water quality analysis. F.F. was responsible for immersion and sample recovery in Lorient. A.T.L. and J.T. were responsible for immersion and sample recovery in Reunion Island. A.P. assisted with the statistical analysis of the MixOmic network. G.B. supplied the fungi ASV table and reviewed the article. F.R. helped with the diatbarcode use and reviewed the article. J.F.B. led the project and wrote the article.
Acknowledgments
We thank Marine Maintenay for her help in biofilm samplings at Toulon. We also thank the scuba diving service of the Oceanographic Observatory of Banyuls sur Mer (OOB) for the immersion at the SOLA Buoy at Banyuls.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets generated during the current study are available in the SRA (BioProject PRJNA1166302) using the MIxS package.




