Quantifying the Temporal Dynamics of Marine Biodiversity Under Anthropogenic Impacts Using eDNA Metabarcoding
Funding: Funding support comes from a 2022 Seed Fund for Basic Research for New Staff (University Research Committee) and Collaborative Research Fund #C7013-1s9GH from the Hong Kong Research Grants Council.
ABSTRACT
Marine biodiversity is rapidly declining due to anthropogenic activities; however, the key causal agents of this decline remain largely elusive. Improving our understanding of anthropogenic impact on biodiversity dynamics requires fine-scale temporal observations, which are logistically challenging using traditional methods. Coral reef ecosystems are key biodiversity hotspots for a wide range of organisms, making them essential for wider marine ecosystem stability and important focal points for marine biodiversity dynamics research. The use of autonomous reef monitoring systems (ARMS) has been effective in assessing reef benthic biodiversity. However, it remains unknown how ARMS marine biodiversity changes over time or in response to anthropogenic activity. Here, we utilized eDNA and a full factorial experiment consisting of 12, 2,042 L mesocosms, seeded with ARMS previously colonized from natural reef habitats. A total of 240 eDNA samples were collected over 20 time points across 232 days to assess: (1) eDNA accumulation dynamics, (2) effects of anthropogenic activity on biodiversity dynamics and (3) eDNA degradation rates. Biological communities changed drastically over time, with 814 ASVs across 617 marine families identified over the course of the experiment. Arthropoda dominated during the accumulation phase, Mollusca during the treatment phase, and Platyhelminthes and Bacillariophyta during the degradation phase. Richness across all mesocosms increased to 110 ± 18 initially, then stabilized at 147 ± 31 during the treatment phase before declining rapidly to 49 ± 17 during the degradation phase. We found that fish feed had a significant negative effect on community composition over time (p < 0.01). Additionally, we identified Lithodesmiaceae and Haminoeidae as potential bioindicator groups. eDNA decay rates across species groups showed faster declines for fish compared to algae and invertebrates. Overall, our findings demonstrate that eDNA metabarcoding offers a detailed assessment of biodiversity, allowing us to differentiate between spatiotemporal and anthropogenic impacts on community dynamics.
1 Introduction
Anthropogenic activities are the strongest drivers in marine biodiversity loss, especially for benthic ecosystems, with negative influences on ecosystem functioning and provision of ecosystem services (Sala and Knowlton 2006; Pawar 2016). The marine benthic ecosystem itself provides a habitat for a multitude of highly diverse and largely unknown species assemblages (Walag 2022). Coral reefs are archetypical benthic ecosystems found in warm, oligotrophic, anshallow depths in tropical waters that serve ecologically vital roles in ecological and ecosystem stability (Vinithkumar et al. 2008). Human activities, however, including overexploitation, habitat destruction, and increasing ocean temperatures have directly contributed to declines in many benthic species populations and subsequent declines in total biodiversity (Sala and Knowlton 2006; Elahi et al. 2015; Prakash and Verma 2022). Specifically, anthropogenic activities, including sedimentation from land reclamation and development, pollution from excess nutrients from aquaculture and fertilizer runoff from agriculture, have been linked to drastic declines in biodiversity and ecosystem functioning (Roberts 2012; Ouyang et al. 2022). Our understanding of the underlying temporal biodiversity dynamics under different anthropogenic stresses is severely limited; however, as studies have predominately focused on endpoint exposure community assessments or on exposure observations of individual species (Moore et al. 2012; Runge et al. 2019). Given the complexity, diversity, and interconnectedness of marine biological communities, it is essential to monitor temporal ecosystem-wide dynamics to gain a comprehensive understanding of their responses to human disturbances and to evaluate the long-term effects (Isbell et al. 2015). Furthermore, understanding temporal responses to anthropogenic stressors is crucial for effectively assessing marine ecosystem resilience and for developing effective conservation strategies to mitigate further biodiversity loss.
Excess nutrients from aquaculture and agricultural runoff, including excess fertilizer and fish-feeding waste, can lead to eutrophication, causing algal blooms that deplete oxygen levels in the water, ultimately resulting in a loss of biological and functional diversity (Price et al. 2015; Isbell et al. 2013; Breitburg et al. 2018). Anthropogenic sedimentation is largely due to activities such as land reclamation and urban development. Studies indicate that annual sediment deposition rates can be as high as 1 m per year, with 78% of major coastal cities contributing to a total of 253,000 ha of sedimentation (Smith et al. 2008; Schratzberger and Somerfield 2020). The accumulation of sedimentation has been linked to increased mortality of coral reefs, with an estimated 50% of coral beds having been lost to sedimentation, to date (Ouyang et al. 2022).
Understanding the temporal dynamics of marine biodiversity is crucial for assessing the full impact of anthropogenic stressors, including sedimentation and nutrient enrichment (Mieszkowska et al. 2014). Temporal observations of biodiversity (e.g., richness, beta-diversity) are critical as they enable researchers to capture dynamic trends and biodiversity shifts over time (Seymour et al. 2024; Bista et al. 2017). In contrast, studies that rely on end-point observations, where changes are assessed between two points in time, are often unable to detect nuanced changes needed to understand biodiversity dynamics (Borja et al. 2016; Ouyang et al. 2022). Many studies are limited in their ability to capture temporal biodiversity trends due to their labor-intensive nature and restricted scope (Lotze 2021). However, end-point observations, while highly valuable, often provide only a static view of the ecosystem, which may not account for seasonal variations, annual fluctuations, or long-term trends that influence biodiversity composition and ecosystem health (Meyer et al. 2011; Hallam et al. 2021). Long-term data are essential for uncovering delayed or cumulative effects of environmental stressors, such as the gradual decline of coral reefs or the progressive eutrophication of coastal waters (Djurhuus et al. 2020). By incorporating temporal dynamics, researchers can better understand the resilience and adaptability of marine ecosystems to human-induced changes, leading to more informed and effective conservation strategies. Additionally, temporal data is invaluable for predicting future impacts under varying scenarios of anthropogenic pressure, providing the basis for proactive marine management frameworks (Watts et al. 2020). Whereas traditional methods are logistically limited in providing the necessary fine-scale temporal data, the advent of environmental DNA (eDNA) methods offers a promising solution to these limitations, enabling more efficient and comprehensive monitoring of marine biodiversity over time (Miya 2022; Jensen et al. 2022).
Recent advancements in molecular-based biodiversity assessment provide an innovative and indirect method for sampling and evaluating biological diversity across temporal scales (Seymour et al. 2021; Bista et al. 2017). Environmental DNA (eDNA) is the genetic material that originates from biological sources that are indirectly isolated from environmental samples, such as water, soil, sediment, or air (Seymour 2019; Deiner et al. 2017). The use of eDNA has proven to be a powerful and innovative approach in ecological research and conservation biology, presenting numerous advantages over conventional methodologies through improved detection of cryptic species (Baudry et al. 2023; Banerjee et al. 2022) and increased spatial and temporal scope (Seymour et al. 2021; Rivera et al. 2023). Consequently, there is an increasing number of studies on the application of eDNA sampling strategies to monitor temporal variations within aquatic ecosystems (Salter 2018; Jensen et al. 2022; Djurhuus et al. 2020). Several studies have also highlighted that the composition of eDNA is subject to diverse influences, including large-scale seasonality and localized degradation processes (Seymour et al. 2018; Bista et al. 2017). By leveraging eDNA with high-throughput sequencing, fine-scale temporal dynamics can be assessed, providing valuable insights into how anthropogenic pressures affect complex and diverse marine biological communities.
Hong Kong is located on the southern coast of China and experiences a monsoonal climate, characterized by distinct dry and wet seasons; The dry season spans from November to March, featuring lower water temperatures (17°C–21°C) and low mean monthly precipitation (67 mm), while the wet season occurs from May to October, with higher water temperatures (25°C–29°C) and high mean monthly precipitation (330 mm) (Lai et al. 2016). This seasonal climatic pattern shapes Hong Kong as a marginal environment for coral community development (Cybulski et al. 2020). The Pearl River, located to the west of Hong Kong, discharges substantial volumes of freshwater during the wet season, creating a pronounced east–west gradient in salinity, turbidity, and nutrient concentrations across Hong Kong's marine waters (Xie et al. 2020). From west to east, Hong Kong's coastal waters transition from estuarine zones to oceanic regions, resulting in significant differences in coral ecosystems due to the unique geographical and environmental conditions. In the western region, influenced heavily by the Pearl River's freshwater input, corals fail to form true reefs and instead develop shallow, marginal coral communities (Xie et al. 2020). In contrast, the eastern region, largely unaffected by the Pearl River Delta, supports coral communities with relatively higher seabed coverage and species diversity (Goodkin et al. 2011; Cybulski et al. 2020). Despite these marginal environmental conditions, Hong Kong boasts exceptionally rich marine biodiversity, with an estimated 6,000 marine species, including over 90 species of hard corals (Duprey et al. 2017).
In this study, we used eDNA and conducted a full factorial mesocosm experiment, where mesocosms were seeded using ARMS previously colonized from natural sites, with different levels of biodiversity, in Hong Kong. The aim was to dynamically assess the changes in complex marine benthic communities and their response to anthropogenic influences. The primary objectives were: (i) to monitor the establishment of benthic communities over time using eDNA to assess shifts in large and complex benthic communities, (ii) to investigate the temporal effect of anthropogenic stressors on benthic biodiversity, and (iii) to examine the variation in eDNA decay rates across species groups and treatment effects.
2 Methods
2.1 Experimental Design
The experiment was carried out at The Hong Kong University's Swire Institute for Marine Science (SWIMS), Hong Kong, using a set of 12 mesocosms (2,042 L volume). The mesocosms were evenly positioned with approximately 1 m spacing between each unit and were subjected to outdoor conditions over the course of the 232-day experiment. During inclement weather, such as rainy days, the mesocosms were covered to mitigate abrupt fluctuations in salinity. To facilitate water circulation from the external seawater source, each mesocosm was equipped with a Hailea HX-8670a submersible pump (Hailea, China), with the valve set at 75% open and a 90° elbow pipe configuration. The water flow rate was maintained at 1,048 L per hour, resulting in a water turnover rate of approximately 12 times per day.
Marine communities were introduced into mesocosms using autonomous reef monitoring systems (ARMS), which are standardized and non-invasive devices that simulate coral reef complexity through their nine-layer PVC plate design (Zimmerman and Martin 2004; Yang et al. 2023). Each vertically stacked unit, separated by spacers, creates sheltered habitats for marine organisms (Figure 1a). ARMS are passive samplers and effectively attract both mobile (moving) and sessile (non-moving) taxa, including many cryptic species often missed by conventional methods (David et al. 2019). The ARMS multilayered structure creates distinct microhabitats, with the uppermost plate serving as a substrate for larger sessile organisms, while lower layers capture smaller fauna (Yang et al. 2023). ARMS are recognized as an international standard for hard benthic monitoring and have been successfully deployed across diverse marine environments (Villarino et al. 2025; Palomino-Alvarez et al. 2021).
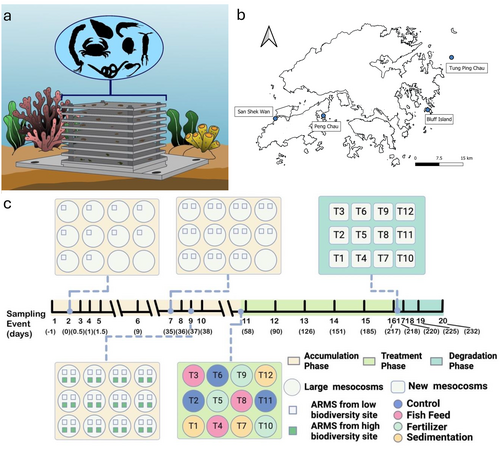
Two years prior to the initiation of this experiment, 48 ARMS were deployed across four distinct sites in Hong Kong: San Shek Wan (Airport), Peng Chau, Bluff Island, and Tung Ping Chau (Figure 1b). The preliminary deployment was carried out to facilitate the recruitment of marine benthic communities. The four selected sites were categorized into two groups based on biodiversity: low biodiversity (San Shek Wan, and Peng Chau) and high biodiversity (Bluff Island, and Tung Ping Chau) (Ng et al. 2017). Subsequently, each of the 12 mesocosms used in the experiment received one ARMS from each of the 4 sites.
To study the effects of anthropogenic stressors on temporal changes to biological communities, three treatments were used across the 12 mesocosms, including sedimentation, fertilizer leakage, and aquaculture nutrification. Each treatment included four mesocosms to serve as replication (Figure 1c). For the sedimentation treatment, we applied a uniform distribution of 3 kg of sand per day for a week; thus, a total of 21 kg of sand was added to each mesocosm in the sedimentation treatment and then resuspended twice per week (Xie et al. 2016). To stimulate the leakage of fertilizer and excess aquaculture, we referred to the maximum value of nitrogen concentration in Hong Kong from the Environmental Protection Department of Hong Kong (EPD 2024). To keep the nitrogen concentration around 14 mg/L, we filled four mesh bags with a total of 205 g of fertilizer, Nutricote 14-13-13 TYPE 140, which contains 8.5% NH4, 5.5% NO3, 13% P2O5, 13% K2O, and 4% S. These mesh bags were placed in the mesocosm and replaced monthly to ensure a constant concentration of nitrogen in the water. Similarly, for the fish feed, we used Grouper Feed #1 (Everlasting A-One Trading Co, Kaohsiung, Taiwan), which has a crude protein content of 45% and a crude fat content of 4%. Initially, mesh bags were filled with 359 g of fish feed with the intent to refill the bags on a weekly basis. However, we observed after the first week that the mesh bags floated on the water surface and failed to release the fish feed effectively. To address this issue, we removed the mesh bags and instead directly added 179.5 g of fish feed twice per week, ensuring even distribution across each mesocosm.
2.2 eDNA Sampling
Twenty eDNA sampling events (hereafter, individually indicated as M1–M20) were carried out over 7 months (Figure 1c) with a total of 251 eDNA samples collected (240 mesocosm samples and 11 experimental blank samples). For each mesocosm, eDNA samples consisted of 1 L of water collected from a single mesocosm using a sterilized 1 L Nalgene bottle and immediately filtered through a 0.45 μm enclosed Sterivex filter membrane (Merk, Darmstadt, Germany) using a Geopump II field peristaltic pump (GeoTech, Boulder, USA). Concurrently, a liter of sterile Mili-Q water, which served as the negative control, was filtered at each sampling time point. To account for the eDNA signal from the water intake, we also collected and filtered experimental blanks consisting of an empty mesocosm with no ARMS for sample events M1 to M8. When ARMS were added to the rest of the empty mesocosms (Figure 1c), we took experimental blanks directly from the inlet valve. The filters with eDNA samples were then preserved on the ice during transportation to the eDNA lab at The University of Hong Kong and subsequently stored in a freezer at −20°C until DNA extraction.
eDNA samples from the mesocosms were collected across three phases: the accumulation phase (M1–M10), the treatment phase (M11–M16), and the degradation phase (M17–M20). In the accumulation phase, ARMS were introduced into the mesocosms serially to simulate the process of community establishment. Due to logistics constraints in retrieving the ARMS, the introduction of the ARMS to the mesocosms was done across multiple stages (Figure 1c). Initially, 10 mesocosms received one ARMS from the low biodiversity site (Airport), with the other 2 mesocosms serving as experimental controls. After 35 days, 10 mesocosms received another ARMS from the low biodiversity site (Peng Chau), and one of the previous experimental control mesocosms received two ARMS from the low biodiversity sites (Airport and Peng Chau), with the remaining empty mesocosm continuing to serve as an experimental control. On day 37 (prior to M9), two ARMS from high biodiversity sites (Bluff Island and Tung Ping Chau) were added to the currently occupied mesocosms, and 4 ARMS (from each of our sites) were introduced into the previously empty mesocosm (Figure 1c). During the accumulation phase, the eDNA sampling occurred 45 min before the first addition (M1), 45 min after the first addition (M2), 12 h (M3), 24 h (M4), 36 h (M5), 9 days (M6), 35 days (addition of ARMS from second low biodiversity sites, M7), 36 days (M8), 37 days (addition of ARMS from the high biodiversity sites, M9), and 38 days (M10) (Figure 1c). Treatments were initiated 55 days after the start of the experiment, which initiated the treatment phase of the experiment. During the treatment phase, eDNA sampling was carried out at 72 h after adding the stressors (M11) and then monthly for 5 months (M12–M16, Figure 1c).
For the degradation phase, 20 L of seawater from each mesocosm was filtered through a sterile 20 μm cloth mesh to remove the (visible) fauna, as previous studies have shown that ARMS can collect microorganisms of approximately 1 mm (Yang et al. 2023), and transferred into new sterile mesocosms to simulate the extinction of the community, and therefore to assess the degradation of the eDNA signal (Figure 1c). These mesocosms were placed in an aquarium room with a controlled temperature (24°C), a diurnal light cycle (6:30 a.m. to 5:45 p.m.), and covered to avoid evaporation. Air was provided using clean air stones. For the decay phase, each of the 20 L mesocosms was filled with 20 L directly from the corresponding large mesocosm, with no water from the external environment added. No other water source was provided, and the water was not circulated during the degradation phase (M17–M20), thus the mesocosms were operated as closed systems. Water samples were collected 45 min after transferring into the tanks (M17), and then at 48 h (M18), 7 days (M19), and 14 days (M20, Figure 1c). Seawater (20 L) from the large mesocosm inlet was also kept in a new sterile mesocosm and served as the experimental control for M17 to M20.
2.3 DNA Extraction and Library Preparation
The eDNA samples were extracted from the Sterivex filter membrane following the protocol described by Spens et al. (2017), and then quantified using a NanoDrop (Thermo Scientific, USA) to measure the absorbance of each DNA sample. Extracted eDNA was cleaned using the PowerClean Pro Cleanup Kit (Qiagen) to remove PCR inhibitors. Amplicon libraries, consisting of a ~313 bp fragment of the cytochrome c oxidase subunit I (COI) gene (mlCOIintF: 5′-GGWACWGGWTGAACWGTWTAYCCYCC-3′) and (jgHCO2198: 5′-TAIACYTCIGGRTGICCRAARAAYCA-3′), were generated for high throughput sequencing (Leray et al. 2013; Geller et al. 2013). A two-step PCR approach was used for library preparation using unique dual index tags (Illumina, USA) (Bohmann et al. 2022). Round 1 amplification (PCR1) was generated using a reaction mix of 12.5 μL master mix, 2 μL DNA template, 1 μL of each primer, and 8 μL nuclease-free water. The thermal protocol for PCR1 consisted of an initial denaturation step at 95°C for 5 min, followed by 25 cycles of denaturation (95°C, 30 s), annealing (54°C, 30 s), and DNA extension (72°C, 60 s), and a final extension step at 72°C for 10 min. PCR1 was performed in triplicate, which was pooled prior to PCR2. The PCR1 amplicon libraries were then submitted to the Centre for PanorOmic Sciences, Hong Kong, for subsequent amplicon cleaning, PCR2, normalization, and high throughput sequencing on an Illumina MiSeq using V3 2 × 300 bp chemistry.
2.4 Bioinformatic Processing
Bioinformatics processing was carried out using a series of Linux-based scripts. First, primer sequences were removed using Cutadapt (Martin 2011). Next, quality filtering was conducted using the fastq_filter command in VSEARCH (Rognes et al. 2016). Reads were then combined and dereplicated using the derep_fulllength function in VSEARCH, followed by denoising using the unoise3 algorithm implemented in VSEARCH. Lastly, chimera removal was performed using the uchime3_denovo function in VSEARCH. The resulting amplicon sequence variant (ASV) frequency table was then used for subsequent taxonomy assignments and statistical analyses.
BLAST+ was used to assign taxonomic information to each of the unique ASVs created in the prior bioinformatics step checked against a locally curated database of NCBI sequence data. The local database was generated using the CRABS workflow (Jeunen et al. 2023). The BLAST+ command was carried out using a Linux-based script. The output file was then closely examined for each ASV taxonomic identification. All ASVs that did not have taxonomic identification to at least the domain level or were not identified as marine species were excluded. The dataset was screened with a threshold of 97% percentage identification for species, 90% for genera, and 80% for family. To eliminate possible contamination, we removed all ASVs that were detected in the negative controls. Additionally, we applied a criterion to filter ASVs based on their read counts in the seawater control. Specifically, an ASV was retained only if its read count was at least twice the read count of the same ASV in the seawater control. If the read count of an ASV in the sample was less than twice its read count in the seawater control, that ASV was excluded from further analysis. Then we removed non-target species based on the habitat information provided by the World Register of Marine Species. Only those species that are known to live in marine or brackish environments were retained for further analysis (Costello et al. 2013). To minimize the influence of fish feed and potential exogenous DNA sources, we screened and identified two fish species, Engraulis ringens and Engraulis encrasicolus, exclusively in the fish feed treatment groups. Since these species are not native to the Hong Kong marine environment, we classified their detection as likely contamination from the fish food and excluded them from subsequent analyses. The resulting family-, genus-, and species-level species table was converted from read counts to relative reads using Hellinger transformation for subsequent statistical analyses (Borcard et al. 2018).
2.5 Statistical Analysis
The statistical analyses and data visualization in this study were carried out using R version 4.4.1 (R Core Team 2024), and figures were generated through ggplot2 (Wickham 2011).
Generalized Additive Models (GAMs) were used to assess the relationship between species richness in response to treatment and time (explanatory variable). The GAMs were fitted using the gam function in the mgcv package, with a smooth term for time using cubic regression splines to allow for nonlinear patterns in the data. We evaluated the effect of different taxonomic levels, including species, genus, and family, with the best model selected based on the lowest Akaike information criterion (AIC) using the AIC function. Principal coordinates analysis (PCoA) was carried out to analyze community dynamics with the function pco in the r-package ecodist (Goslee and Urban 2007), and the principal coordinate that explains the largest data change (PCoA 1) was then virtualized to illustrate the consistency in community dynamics.
ANOVA and Tukey's HSD test were used to test the difference in richness and abundance between different treatments. The metaMDS function in the r-package vegan was used to perform Non-metric Multidimensional Scaling (NMDS) under the Bray-Curtis method, 999 random starts in search of a stable solution, and three dimensions. The ANOSIM test was used to test the significance of the difference between fish feed and other groups. In order to detect which species or group of species caused the shift of community under fish feed treatment, we calculated the species contribution to beta-diversity (SCBD) for each species, taking into account their dominance and occurrence within the community (Borcard et al. 2018).
In the degradation phase, the marine communities were categorized into three distinct groups—aquatic invertebrates, eukaryotic algae, and fish to facilitate a more precise analysis of eDNA signal decay. Linear regressions of natural log-transformed eDNA read against time for each group under different treatments were built to elucidate the decay pattern of eDNA signals at both community and species level using the r-package ggpmisc (Aphalo 2024).
3 Results
3.1 Composition of Marine Community in Mesocosms
A total of 240 samples, 11 experimental controls, and 20 negative controls were collected across 20 sampling events (M1–M20), divided into three phases—the accumulation phase (M1–M10), the treatment phase (M11–M16), and the degradation phase (M17–M20) (Figure 1c). All 271 samples were successfully sequenced across 3 Illumina MiSeq runs, yielding 63,395,168 single-end reads. After quality filtering and denoising, sequences were clustered into 12,958 ASVs, of which 10,586 were taxonomically assigned to at least the family level. After screening with a threshold and removing non-target ASVs, the final analyzed dataset contained 829 unique “putative species” including 22 phyla and 617 families. The most abundant phylum across all time points was Arthropoda (30.3%), followed by Mollusca (15.1%), Cnidaria (13.7%), and Porifera (9.5%). The variation in community composition was high between mesocosms over time; however, the overall temporal composition dynamics were similar. In short, mesocosm communities in the accumulation phase were dominated by species belonging to members of the phylum Arthropoda, by Mollusca and Arthropoda in the treatment phase, and by Platyhelminthes and Bacillariophyta in the degradation phase (Figure 2a).

3.2 Temporal Dynamics in the Community
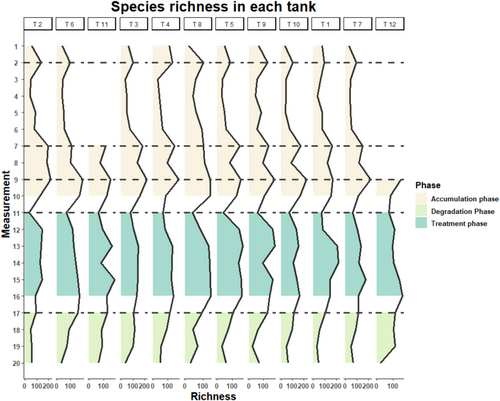
Species-level richness increased rapidly from an average of 55 ± 26 to 110 ± 18 during the first 12 h after the first ARMS introduction on day 0 (M2). Richness noticeably increased as the second and third ARMS were introduced, with average richness changes from 69.4 ± 14 to 147 ± 31 (M6–M7) and from 111 ± 31 to 182 ± 32 (M8–M9) with some minor declines noted before saturating around day 38 (M10) (Figure 3). The addition of two ARMS from high biodiversity sites at day 37 (M9) resulted in a smaller increase in species richness compared to the increase following the second introduction of ARMS from lower biodiversity sites at day 35 (M7). During the treatment phase, richness was generally stable before a large drop in richness was observed on day 58 (M11) followed by a period of restabilization with some oscillation throughout the treatment phase. The results of ANOVA and Tukey HSD tests showed that there is no significant difference in either species richness or abundance among the treatment groups (p = 0.548 and p = 0.911, respectively). Most of the species became undetectable after 48 h into the degradation phase (M17–M20) with average richness declining from 115 ± 16 to 80 ± 23 (M17–M18) and further declining to 49 ± 17 after 14 days (M20). A consistency in richness dynamics across 12 mesocosms throughout time was also suggested in Figure 3.
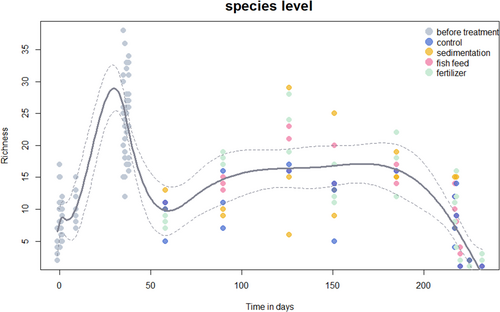
GAM models were consistent across different taxonomic levels with family, species, genus, and species levels (Figure 4, Figure S1) and indicated a significant effect of time (day) on family richness (p < 0.01) with a non-significant effect of treatment on richness (p = 0.935 at the family level, p = 0.906 at the genus level, and p = 0.849 at the species level). The species-level GAM model was the most parsimonious (lowest AIC).
3.3 Dynamics of Community Composition Under Anthropogenic Stressor
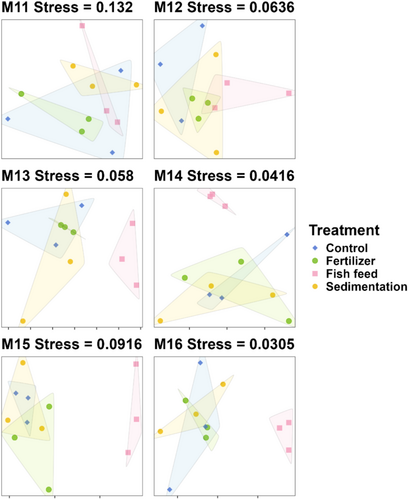
The results of NMDS showed clear community differences progressing through the treatment phase of the experiment (day 58–217; M11–M16). At the start of the treatment phase (day 58; M11), treatment group communities were relatively similar. Differences between treatment communities became increasingly dissimilar over the 6 months of the treatment phase, particularly when comparing communities in the control and other treatments to the fish-feed treatment group (Figure 5). The results of the ANOSIM showed a significant difference between communities in the fish-feed treatment and other treatment groups at day 185 (M15) and day 217 (M16) (p < 0.01, Table 1). The species contribution to beta-diversity (SCBD) analysis identified two unique families associated with the difference in fish feed versus other treatment communities, including Lithodesmiaceae (class Bacillariophyta) and Haminoeidae (class Gastropoda) (SCBD 0.079 and 0.057, respectively). The relative abundance of Gastropoda remains relatively constant with slight fluctuations in the control, fertilizer, and sedimentation groups, while it shows a notable increase in the fish-feed group. The relative abundance of Bacillariophyta increased in the control, sedimentation, and fertilizer groups, with the most rapid growth observed in the fertilizer group, while only a slight fluctuation was noted in the fish-feed group.
| Sampling event | p-value of comparisons between all treatments | p-value of comparisons between fish feed and other treatment groups |
|---|---|---|
| M11 | 0.443 | 0.561 |
| M12 | 0.257 | 0.039 |
| M13 | 0.110 | 0.017 |
| M14 | 0.126 | 0.135 |
| M15 | 0.020 | 0.005 |
| M16 | 0.021 | 0.005 |
3.4 eDNA Decay
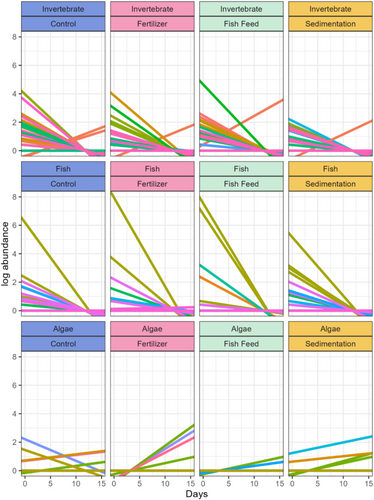
Individual decay rates were significantly different between groups (p < 0.01), but did not show differences between treatments (p = 0.869) or for the group × treatment interaction (p = 0.821). eDNA decay was observed for 56 individual species across three main taxonomic groups (aquatic invertebrates, algae, and fish) (Figure 6). Aquatic invertebrates were the most diverse signal group with 31 species with decay rates ranging from −0.379 log reads/day to 0.201/day (mean −0.022/day) with Ascidia ahodori providing the only positive signal. There were 16 species of fish decay observed, which ranged from −0.623 to 0.007 log reads/day (mean = −0.033). Eukaryotic algae included nine species with decay rates ranging from −0.152 to 0.257 log reads per day (mean = 0.001). Species with positive eDNA rates were predominately Takayama acrotrocha (7 out of 12 tanks) and Fibrocapsa japonica (2 out of 12 tanks).
4 Discussion
Our study is the first to utilize eDNA to quantify temporal change to ARMS biodiversity using a mesocosm setup across three distinct phases: accumulation, stabilization, and degradation. The observed increase in species richness during accumulation, followed by shifts in community composition due to fish feed treatment effects, and variations in eDNA degradation across taxonomic groups underscores the sensitivity of eDNA as a monitoring tool in detecting community dynamics and environmental stressors. Overall, our study contributed to a growing body of evidence that eDNA metabarcoding is a promising method for detecting marine biodiversity (Alexander et al. 2020; Czachur et al. 2022) and can be used as a robust tool for monitoring temporal dynamics of marine biodiversity, in terms of community composition, offering critical insights into temporal and compositional changes influenced by human activities (Cybulski et al. 2020; Borja et al. 2024).
Our findings demonstrate a consistent pattern of richness changes over time within each mesocosm, indicating that the dynamics of the species-area relationship play a significant role in driving biodiversity dynamics in this study (Li et al. 2024; Hart et al. 2017). However, we also found several instances, through eDNA monitoring, during the experiment where changes in richness and biological community composition were attributed to the introduction of species (Young et al. 2017) and anthropogenic stress (Isbell et al. 2013). These findings support numerous studies that have demonstrated the potential of eDNA with metabarcoding for tracking the recruitment of populations and invasive species (West et al. 2020; Jensen et al. 2022). The results presented here are unique in holistically highlighting support for fine-scale eDNA-based biomonitoring to capture subtle changes in biodiversity with direct implications for determining recent species introductions (Ganguly and Umapathy 2024), species–area relationship dynamics (Mathon et al. 2023) and indications of ecosystem collapse and localized extinction (Martel et al. 2021). These findings also support recent research demonstrating the robustness and reproducibility of eDNA metabarcoding as an environmental biomonitoring tool for benthic bacterial communities (Dully et al. 2021).
Previous research has predominantly focused on the eDNA shedding rate of individual species, such as fish, freshwater mussels, and crown-of-thorns sea stars (Sassoubre et al. 2016; Sansom and Sassoubre 2017; Wilder et al. 2023; Kwong et al. 2021). Consequently, there has been a notable gap in the literature concerning the dynamics of eDNA signal accumulation within entire marine communities. Our study provides valuable insights into this area. During the accumulation phase, 20 ARMS were deployed across 10 mesocosms in the first ARMS introductions, resulting in an average richness increase of 55. This demonstrates that detectable eDNA accumulation occurs within a relatively short period. Interestingly, the second ARMS introduction (M7) yielded the highest species diversity increase, even though the ARMS units originated from low biodiversity sites. This suggests that the magnitude of the richness increase may be independent of the initial community diversity, potentially due to species-area relationships within mesocosms (Hart et al. 2017). During the treatment phase, species richness stabilized after initial declines across all treatments. This pattern likely reflects the loss of species maladapted to the mesocosm environment rather than treatment-specific effects. In the degradation phase, species richness exhibited a continuous decline over 14 days, decreasing from an average of 140 to 49 species after ARMS were removed from the mesocosms. The dominance and persistence of autotrophic organisms, such as algae, indicates their ability to endure in the mesocosm even after ARMS removal, while other species experienced local extinction.
Anthropogenic treatment effects did not significantly impact overall species richness, as we found high similarity in biodiversity under the control, fertilizer, and sedimentation treatment groups during the treatment phase of the experiment. However, we did find a distinct shift in community composition under the fish feed treatment group, which highlights the profound effects of aquaculture wastes on biodiversity composition. The lack of more general and widespread anthropogenic effects on biodiversity for this study was contrary to previous studies, which suggested anthropogenic disturbance significantly influences eukaryotic richness at the family level (DiBattista et al. 2020). This contrast might be due to experimental design and study objectives. Our study utilized a relatively closed system and assessed the anthropogenic impacts over a relatively short time frame, whereas DiBattista et al. (2020) utilized marine samples under longer-term human disturbance. Our results do align with research suggesting that sedimentation may impact community physiology rather than abundance (Magris and Ban 2019). Specifically, our observation of a significant increase in Gastropoda within the fish feed treatment supports previous observations of increased occurrence and abundance of deposit feeders in fish feed-influenced environments (Sanz-Lázaro and Marín 2011). Recent research has primarily focused on the effects of elevated nitrogen, phosphorus, and potassium levels from fertilizer runoff on microbial, seagrass, and algal communities in marine environments, with limited exploration of fertilizers' impact on macrofaunal or meiofaunal abundance (Murase et al. 2015; Zhou et al. 2019; Ferdie and Fourqurean 2004). Our study observed a more rapid increase in algal group abundance under fertilizer treatment, along with enduring effects demonstrated by the slower decay rate of the algal group under fertilizer treatment. While the changes induced by fish feed were noticeable, the subtle increase in the algal group under fertilizer treatment may have been less distinct. These results align with previous findings on anthropogenic-induced shifts in species dominance, emphasizing potential long-term implications for ecosystem health and resilience (Isbell et al. 2013; Ouyang et al. 2022).
Our findings indicate that the dynamics of Gastropoda and Bacillariophyta (i.e., algae) contributed most to shifts in changes in community structure in response to the fish feed treatment. Previous research has suggested that Gastropoda and algae interactions have strong potential to serve as biological indicators for marine environmental monitoring (Salo and Salovius-Laurén 2022; Dobrescu et al. 2023; Fitria et al. 2023). Given Gastropoda predominantly feed on algae (Brown and Lydeard 2010), the slower relative increase in the fish feed group, which showed reduced algae abundance, compared to others is consistent with ecological expectations. These results further underscore that changes in the proportions of Gastropoda and algae within communities could serve as sensitive indicators of fish feed-related stressors. While recent research emphasizes the importance of interaction dynamics within communities under anthropogenic impacts (Gilarranz et al. 2016), a significant knowledge gap persists regarding the use of community composition as a bioindicator. Current research focuses on the presence or absence of specific organisms as bioindicators, which may overlook the complexity of community dynamics and varied responses of different taxa to environmental stressors (Kitaka et al. 2024).
Our study found that the decay of eDNA reads over time is consistent across treatment groups but varied for different species and noticeably among taxonomic groups. These observations align with previous research (Holman et al. 2022), suggesting species-specific decay rates of eDNA persist in marine environments. In general, the detectability of species from eDNA is a reflection of the persistence time of eDNA (Barnes et al. 2014) and its association with the concentration of eDNA influenced by active shedding from living or deceased organisms and decay from environmental and biological factors (Wood et al. 2020). Compared to other environments, the eDNA degradation rate is expected to be relatively high due to increased microbial activity (Lamb et al. 2022; Zulkefli et al. 2019), which would match the general observations in this study, whereby most species detections were nonexistent after 48 h into the decay phase. It should be noted that many studies of eDNA decay focus on the persistence of eDNA in aqueous systems. For instance, amphibians have been reported to remain detectable after 25 days in fresh water, while fish species remain detectable for 7 days in marine aquariums (Barnes et al. 2014). However, these studies often overlook the influence of initial eDNA concentrations and environmental variability, which may account for the wide range of reported persistence times. It is also important to note that while decay rates derived from mesocosm experiments provide insights into decay rate variation among species (Seymour et al. 2018; Andruszkiewicz Allan et al. 2021), decay rates in natural environments are likely faster due to increased effects of dilution, dispersion, or biotic effects (Perry et al. 2024; Barnes et al. 2014). Future research on eDNA detectability and decay should aim to develop refined oceanographic or ecological models that incorporate initial eDNA concentrations alongside species- and environment-specific decay rates to enhance predictive accuracy (Andruszkiewicz et al. 2019).
Long-term marine monitoring has proven to be highly valuable in making informed management decisions aimed at reducing the effects of human activities and environmental constraints (Adade et al. 2021). Based on our results, we would suggest that future conservation efforts prioritize the use of fine-scale eDNA monitoring to capture short-term biodiversity dynamics, particularly in response to aquaculture waste. Employing eDNA with metabarcoding could provide snapshots of community composition with relatively short time intervals, thus allowing conservationists to track rapid changes in biodiversity more effectively, identify early signs of ecosystem stress, and monitor the efficacy of management interventions (Deiner et al. 2017). Additionally, our findings highlight the importance of integrating eDNA metabarcoding with ecological modeling to predict species-specific decay rates and persistence times, thereby enhancing the accuracy and applicability of eDNA data in marine conservation.
Overall, our findings highlight the effectiveness and robustness of eDNA metabarcoding in capturing the temporal dynamics of marine communities (Sevellec et al. 2021; Sales et al. 2021). As human-induced biodiversity loss accelerates, there is an urgent need to develop and implement rapid biodiversity assessment frameworks to safeguard ecosystems, their diversity, and the services they provide. Our study demonstrates that modern eDNA methods can rapidly and effectively detect community-level changes (Seymour et al. 2021), while also disentangling the underlying drivers of these shifts in marine environments (Czachur et al. 2022; Oosthuizen et al. 2023; Adams et al. 2023; Miya 2022). Additionally, we underscore the potential of eDNA for identifying biological indicators to complement traditional approaches (Seymour et al. 2020). By advancing our understanding of eDNA dynamics, we can enhance the reliability of eDNA as a tool for biodiversity monitoring and conservation efforts.
Author Contributions
Z.S. led the writing. M.S. and D.B. designed the study. M.S., Z.S., T.Y.C., C.Q., I.G., and C.F.W. conducted the sampling. M.S., Z.S., and T.Y.C. carried out the molecular work. G.-J.J. and M.S. performed the bioinformatics. Z.S., W.H. and M.S. analyzed the data and structured the manuscript. All authors provided comments on the final version.
Acknowledgments
Funding support comes from a 2022 Seed Fund for Basic Research for New Staff (University Research Committee—URC) and Collaborative Research Fund #C7013-1s9GH from the Hong Kong Research Grants Council. We also thank R. Wong for logistical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study, including the species table, metadata, and R-script, are available via GitHub at https://github.com/MatSeymour/Zhewei_etal.git.




