CRISPR-Based Environmental Biosurveillance Assisted via Artificial Intelligence Design of Guide-RNAs
Funding: This work was supported by Ministry of Business, Innovation, and Employment (MBIE) project: A toolbox to underpin and enable tomorrow's marine biosecurity system (MBIE CAWX1904) funded the cost for this study and B.D.-V. PhD scholarship.
ABSTRACT
Environmental biosecurity challenges are intensifying as climate change and human activities accelerate the spread of invasive species, disrupting ecosystem composition, function, and essential services. Environmental DNA (eDNA) has transformed traditional biosurveillance by detecting trace DNA fragments left by organisms in their surroundings, primarily by applying quantitative polymerase chain reaction (qPCR) methods. However, qPCR presents challenges, including limited portability, reliance on precise thermal cycling, and susceptibility to inhibitors. To address these challenges and enable field-deployable monitoring, isothermal amplification techniques such as recombinase polymerase amplification (RPA) paired with clustered regularly interspaced short palindromic repeats and associated proteins (CRISPR-Cas) have been proposed as promising alternatives. CRISPR-Cas technology also presents challenges, including searching and optimizing a guide RNA (gRNA) that is highly sensitive and has no off-target interactions for use as an effective environmental biosurveillance tool. We present here the development of SENTINEL (Smart Environmental Nucleic-acid Tracking using Inference from Neural-networks for Early-warning Localization) that harnesses the programmability, specificity and sensitivity of a one-pot RPA-CRISPR-Cas13a reaction by integrating an accessible and pre-trained neural network to accelerate assay design for rapid deployment. We challenged SENTINEL with waterborne eDNA from two marine sites invaded by species not native to New Zealand as proof-of-concept fluorescence-based tests: Sabella spallanzanii (Mediterranean fanworm) and Undaria pinnatifida (Wakame). Off-target effects were explored by challenging the SENTINEL assays on gDNA from a suite of co-occurring species. SENTINEL presented a robust, streamlined method incorporating the trained neural network, achieving a sensitivity down to 10 attomolar using recombinant DNA and down to ~0.34 copies/μL for eDNA samples with 1 h, costing 3.5 USD per sample. There was a 100% agreement between SENTINEL results and qPCR-based analysis of the eDNA samples. SENTINEL displayed no off-target activity when challenged against 23 gDNA samples from co-occurring species. Thus, our study showcases SENTINEL's potential as a robust platform for eDNA screening applications.
1 Introduction
Environmental biosecurity challenges in ecosystems are evolving under pressures from climate change, invasive species, and human activities that collectively affect ecosystem sustainability, endemic biodiversity, and related economic activities (Britton et al. 2023; Davis et al. 2023; Shim et al. 2023; Tsirintanis et al. 2022). Combatting these pressing issues demands innovative, reliable, and robust solutions to enhance biosurveillance efforts in the management and mitigation of biosecurity risks (Durán-Vinet et al. 2023; Schenekar 2022; Shim et al. 2023). Current biosurveillance efforts are mostly based on traditional biomonitoring methods (e.g., capture and diving surveys) that can be laborious and require specialist knowledge such as morphological taxonomy expertise (Bell et al. 2024; Hawthorne et al. 2024). Recent studies have highlighted environmental DNA (eDNA) as a novel, minimally invasive approach for the precise and sensitive detection of invasive species (Blasko et al. 2024; Kim et al. 2024; Shashank et al. 2024; Zhang et al. 2024) and conservation efforts (Leugger et al. 2024). By targeting specific gene regions, molecular tools such as quantitative polymerase chain reaction (qPCR) or digital droplet PCR (ddPCR) allow ecologists to efficiently detect the presence or absence of species of interest at scale (Jeunen et al. 2022; Wood et al. 2019, 2017).
Although qPCR-based methods now have portable options (Billington et al. 2021; Yolda-Carr et al. 2022), they still require precise temperature cycling and may be susceptible to amplification inhibitors (Durán-Vinet et al. 2021).
A proposed solution is using isothermal amplification technologies for eDNA detection to tackle current qPCR challenges; however, the most used isothermal amplification technologies have critical shortcomings that make initial testing complex. For example, recombinase polymerase amplification (RPA) provides quick DNA amplification, but it lacks specificity and can lead to false positives and high background noise (Munawar 2022), while loop-mediated isothermal amplification (LAMP) approaches display high specificity but have a complex primer design, which can lead to false negatives and undesired poor sensitivity performance (Moehling et al. 2021; Yigci et al. 2023).
Recent studies have harnessed the natural prokaryotic adaptive immune system known as CRISPR-Cas (Clustered regularly interspaced short palindromic repeats and associated CRISPR proteins) (Kaminski et al. 2021; Zuo et al. 2017), coupled with isothermal methods such as RPA and LAMP (Abudayeeh and Gootenberg 2021; Yigci et al. 2023) to address problems when using isothermal techniques alone. Using this approach, the isothermal methods enrich the sample prior to Cas nuclease detection, thereby circumventing the standalone drawbacks and further increasing the specificity and sensitivity of the overall diagnostic platform (Kaminski et al. 2021). This has allowed swift and robust isothermal eDNA detection, including use in point-of-need in-field testing (Osborn et al. 2021; Phelps 2019; de Puig et al. 2024; Yang, Matsushita, et al. 2024). One significant advantage is that CRISPR technology can be applied directly to briefly heated, unextracted saliva or urine samples, showing potential effectiveness even in the presence of residual qPCR inhibitors while remaining suitable for in-field deployment through lateral flow screening (Myhrvold et al. 2018).
CRISPR-Cas-based detection platforms harness RNA molecules known as CRISPR RNAs (crRNAs) or guide RNAs (gRNAs), which direct Cas nucleases to the target through nucleotide complementarity (Jinek et al. 2012). Therefore, a critical portion of success is concentrated on achieving an optimal crRNA configuration for effective detection (Huang et al. 2024; Mantena et al. 2024; Metsky et al. 2022). Specifically, Cas13a, upon recognizing and cleaving its single-stranded RNA target (cis activity), triggers a cis-activated trans-collateral cleavage activity that further targets short RNA molecules (Abudayyeh et al. 2017; Gootenberg et al. 2018). This property has been harnessed to develop rapid diagnostic platforms by coupling short single-stranded RNA probes with a fluorophore (e.g., 6-carboxyfluorescein—6-FAM) and a quencher (e.g., Black hole quencher 1—BHQ1) or biotin for lateral-flow screening (Gootenberg et al. 2018). A positive reaction produces either a fluorescence peak or a band on a lateral flow device (Kellner et al. 2019; Li, Kielich, et al. 2023; Pena et al. 2023). These types of CRISPR-based platforms have already been developed for eDNA-based detections (Leugger et al. 2024; Williams et al. 2022; Yang, Matsushita, et al. 2024). Importantly, to differentiate CRISPR-based diagnostics (CRISPR-Dx) widely used in clinical settings (Arizti-Sanz et al. 2020; Patchsung et al. 2020) from environmental settings (Shashank et al. 2024; Williams et al. 2022; Yang, Matsushita, et al. 2024), we refer to the use of CRISPR for environmental nucleic acids detection as CRISPR-based environmental biosurveillance (CRISPR-eBx).
Cas12a nucleases can directly target DNA and also have a trans-collateral activity like Cas13a, but over single-stranded DNA instead. However, Cas13a nucleases do not experimentally require a coupling incubation time that can extend the protocol by 10–20 min (Low et al. 2025; Williams et al. 2022). Although this is not significant in lab settings, it may be significant for in-field deployments. Moreover, by the time this study was designed, no artificial intelligence tool existed to streamline and accelerate Cas12a-based diagnostics, but now it has recently been reported with a good sample training size (~11,000 experimental validations (Huang et al. 2024)) in comparison to the AI tool used in this study that was trained for Cas13a-based diagnostics (~19,000 experimental validations (Metsky et al. 2022)).
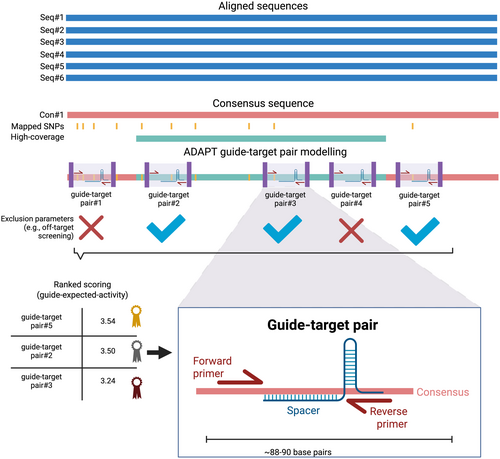
Our framework proposes an integrated approach combining CRISPR, RPA, and AI to enhance environmental biosurveillance efforts using eDNA, namely Smart Environmental Nucleic-acid Tracking using Inference from Neural-networks for Early-warning Localization (SENTINEL, Figure 1). SENTINEL harnesses different cutting-edge technologies, starting with an already-trained neural network modeling for the discovery of highly active guide-target pairs (i.e., RPA primers and crRNA, Step 1, Figure 1). Then, these guide-target pairs are screened, tested on the known samples to verify efficacy, and then used to detect the target of interest (Steps 2–4, Figure 1).

One of the major bottlenecks that CRISPR-Dx and CRISPR-eBx technology share is that different crRNA configurations on the spacer region (complementarity region) directly impact the on-target and off-target activity of Cas nucleases (Liu et al. 2020). This is observed in genome editing and Cas characterization studies (Tambe et al. 2018; Wessels et al. 2024; Yang and Patel 2024), including target inaccessibility (Spangler et al. 2022; Wessels et al. 2019), crRNA mismatch tolerance sensitivity (Molina Vargas et al. 2024), and Cas:crRNA complex dynamics that can lead to target binding but no nuclease activation (Tambe et al. 2018). Altogether, these challenges can significantly increase crRNA screening efforts as design principles are still considered coarse (Kaminski et al. 2021), which can generate unwanted outcomes. These challenges are naturally inherited by CRISPR-eBx as different crRNAs have already displayed different sensitivity across CRISPR-based eDNA detection studies (Baerwald et al. 2020; Yang, Matsushita, et al. 2024). Therefore, we exploited a previously trained neural network, ADAPT (Activity-informed Design with All-inclusive Patrolling of Targets), to tackle these challenges (Metsky et al. 2022). The use of AI, such as neural networks, can discover unusual, intricate data pattern structures from high-dimensional data (Lecun et al. 2015). ADAPT explores existing genomic data to predict maximally fit diagnostic assays for the enzyme Leptotrichia wadeii Cas13a nuclease (LwaCas13a) based on a trained model (Ackerman et al. 2020; Metsky et al. 2022). Using ADAPT, we can maximize on-target specificity and sensitivity and avoid unwanted off-target activity.
We used ADAPT on two invasive species in New Zealand as proof-of-concept, Sabella spallanzanii (Mediterranean fanworm; Soliman and Inglis 2018) and Undaria pinnatifida (Asian seaweed; South et al. 2017) to showcase the SENTINEL platform's utility. We designed species-specific CRISPR-eBx assays targeting the cytochrome c oxidase subunit I (COI) gene. We then evaluated SENTINEL under various controlled conditions to optimize the detection platform. Our screening approach included optimal buffer selection to increase activation speed; selection of reporter probes to optimize final signal output; testing for off-target effects; and making a sensitivity assessment using plasmid DNA and gDNA to explore SENTINEL limits. These tests were followed by screening samples containing S. spallanzanii and U. pinnatifida eDNA, as confirmed by ddPCR and qPCR, respectively. SENTINEL demonstrated 100% concordance with ddPCR/qPCR-based methods and represents an easily accessible, streamlined CRISPR-eBx platform, enabling rapid and efficient target detection for biosurveillance of emerging environmental biosecurity threats using LwaCas13a.
2 Material and Methods
Our primary work can be summarized in four major steps (Figure 2): (1) sequence retrieval and alignment, (2) pre-trained neural network design of guide-target pairs, (3) guide-target pair screening and selection, and (4) environmental DNA screening.

2.1 Construction of the Target Reference
The COI gene was used as the target for our assays. A consensus sequence for each invasive species was built for later use in the ADAPT pipeline when generating guide-target pairs for our SENTINEL platform. We retrieved Sabella spallanzanii (KY472787.1) and Undaria pinnatifida (NC_023354.1) COI genes from GenBank (release 258.0; www.ncbi.nlm.nih.gov/genbank/). Each reference sequence was used in GenBank megaBLAST (version 2.14.0; (Camacho et al. 2009)) to retrieve all sequences with a ≥ 99.6% and ≥ 99.5% pairwise identity to the target sequence of the respective species, obtaining 18 and 14 sequences, respectively. Accession numbers are available in Table S1.
Retrieved sequences for each species were aligned using the Clustal Omega alignment algorithm (version 1.2.2; Sievers et al. 2011). High-coverage regions and polymorphic sites were identified with a 50% minimum sequence frequency threshold. This means that for either potential polymorphic sites or a high coverage region, the said region was required to be present on ≥ 50% of the retrieved sequences. Then, we generated consensus sequences for S. spallanzanii and U. pinnatifida with a 0% majority call threshold (most common bases are called in each site to build a consensus to avoid ambiguities in plasmid inserts (Table S2)). We used these consensus sequences in the next step of the ADAPT pipeline. These steps are crucial to correctly assess and understand COI gene polymorphic sites and the high coverage region landscape for both target species. All in silico analyses were performed in Geneious Prime (2024.0.5; https://www.geneious.com).
We also used these consensus sequences for gene synthesis and custom plasmid construction (GenScript, US, custom product) for optimization of SENTINEL later. Plasmids with known inserts containing the target regions were used as the first proof-of-concept prior to further assay development.
2.2 ADAPT Local Deployment for Environmental DNA Target Discovery
ADAPT (version 1.6.0; https://adapt.run/) is an end-to-end deep-learning neural network that predicts highly active CRISPR RNAs (crRNAs) for viral diagnostic deployment, trained with 19,209 guide-target pair outcomes from Leptotrichia wadeii Cas13a (LwaCas13a) to enhance trans collateral cleavage activity (Metsky et al. 2022). Guide-target pairs refer to the spacer region of the crRNA and flanking primers for RPA (Metsky et al. 2022).
We used ADAPT with the COI consensus sequences (S. spallanzanii and U. pinnatifida) as “on-target sequences” in FASTA format while incorporating identified potential off-target sequences as “excluded sequences” in FASTA format. For each species, the COI consensus sequence was run and optimized independently. Off-target sequences were identified as 100% hits (full complementarity—100% pairwise identity) using GenBank MegaBLAST on obtained spacer sequences after a first exploratory ADAPT run without the “--specific-against-fastas” command. Identified off-target accession numbers were added as a single FASTA file in a second final run (Table S1). Additional parameters, including primer length, primer GC content, spacer length, spacer specificity, and other customizations, were applied to optimize LwaCas13a crRNA modeling and diagnostic performance prediction for SENTINEL. An important command to ensure high specificity in ADAPT is “--id-m 4,” which enforces ADAPT specificity by considering off-target sequences with four mismatches as on-target activity (default configuration), discarding that design as a potential off-target guide. Therefore, all our designed crRNAs have highly enforced in silico specificity against known off-target sequences. The pipeline used for S. spallanzanii and U. pinnatifida is provided in Table S3. ADAPT command features are fully explained in the original study (Metsky et al. 2022) and at the online repository: https://github.com/broadinstitute/adapt. The output of ADAPT was a TSV file with the predicted activity, crRNA spacer sequence (28 nucleotides) and RPA primer sequences (30 nucleotides each). Only the spacer portion of the crRNA was checked with megaBLAST, and full specificity was enforced (no mismatches allowed). This is an important detail, as RPA in the SENTINEL assay is used to enrich target DNA to increase sensitivity while LwaCas13a provides specificity and detection.
Forward primers were appended with a T7 promoter sequence at the 5′ end (5′-gaaatTAATACGACTCACTATAGGG-3′) (Baerwald et al. 2023; Gootenberg et al. 2018; Thakku et al. 2022), while reverse primers and spacer sequences were reverse complemented. The spacer sequence was then converted to RNA and appended with the corresponding direct repeat for LwaCas13a at its 5′ end site (5′-GAUUUAGACUACCCCAAAAACGAAGGGGACUAAAAC-3′). All primers, crRNAs, and probes used in this study were synthesized as custom products (GenScript, US; Table S4). A summary of the overall workflow is illustrated in Figure 3.
2.3 Genomic DNA Extraction
Genomic DNA (gDNA) of S. spallanzanii was extracted using the DNeasy Qiagen Blood and Tissue (Qiagen, Germany #69504) following the manufacturer's instructions and performed in a PCR-free laboratory for all gDNA extractions. All gDNA samples were used as a higher complexity performance test for SENTINEL after screening plasmid DNA to explore sensitivity and specificity further.
Extracted gDNA samples of S. spallanzanii were stored at −20°C until further processing. As previously reported, we used an optimized qPCR assay to check the gDNA signal (Wood et al. 2017). The qPCR configuration was as follows: 95°C for 3 min, followed by 30 cycles at 95°C for 15 s and 60°C for 30 s. The primers were as follows: forward primer: GCT CTT ATT AGG CTC TGT GTT TG; reverse primer: CCT CTA TGT CCA ACT CCT CTT G; probe: AAA TAG TTC ATC CCG TCC CTG CCC.
Undaria pinnatifida gDNA extractions followed the PDQeX extraction method as previously reported (Stanton et al. 2019). Fresh U. pinnatifida tissue samples were collected with a 3 mm biopsy punch (~10 mg), the tissue rinsed with Milli-Q water, and manually homogenized with 80 μL 1X Green Buffer (MicroGEM PLC, New Zealand) using a pestle. Forty microliters of the homogenate was added to the extraction mastermix (48 μL of RNAse-free water, 40 μL homogenate, 10 μL 10X Green Buffer, 2 μL PrepGem enzyme). The extraction mastermix was dispensed into PDQeX extractor cartridges and run through the following protocol in the PDQeX 2400 device: 37°C for 5 min, 75°C for 5 min, and 95°C for 2 min. At the end of the program, extracts containing purified DNA were collected in 0.2-mL PCR tubes. Purified DNA samples were stored in 0.2-mL PCR tubes at −20°C until further processing. U. pinnatifida gDNA and eDNA samples were screened with qPCR (95°C for 3 min, then cycled 45 times: 95°C for 15 s, 61°C for 15 s and 72°C) as previously described (Bott and Giblot-Ducray 2011), using the following primers: forward primer: TACAGCAATGTCTGTTTTTATCC; reverse primer: ACATTATACAACTGATGATTTCCC; probe: ATTGCAATTAGCTAGCCCTG.
Blank extractions were performed as internal controls for both species but were not used in SENTINEL assays. DNAse and RNAse-free distilled water (Invitrogen, #10977015) were used as a negative control instead for SENTINEL assays. Both gDNA extractions were quantified with a Qubit 4 Fluorometer (ThermoFisher Scientific, US) following the manufacturer's instructions. The initial concentration for S. spallanzanii gDNA was 18 ng/μL, and U. pinnatifida gDNA was 3.4 ng/μL. Consecutive tenfold dilutions were set up and used immediately for gDNA sensitivity assays.
To create an off-target library of co-occurring marine species to challenge SENTINEL, we obtained gDNA from 23 co-occurring algae species (Table S5). Cystophora torulosa, Chondria macrocarpa, Unidentified red algae, Scytothamnus australis, Xiphophora gladiata, Codium fragile, Cystophora retroflexa, Macrocystis pyrifera, Durvillaea poha, Caulerpa brachypus, and Caulerpa parvifolia gDNA were extracted with the same PDQeX method for U. pinnatifida. Bryopsis vestita, Caulerpa taxifolia, Caulerpa proliferia, and Caulerpa brownii gDNA were extracted as previously reported (Pearman et al. 2024).
Further gDNA samples were from University of Milan Bicocca (Italy): Caulerpa cylindracea, Caulerpa lentillifera, Caulerpa selago, Caulerpa serrulata, Caulerpa sertularioides, Caulerpa racemosa, Caulerpa chemnitzia, and Caulerpa nummularia.
2.4 Environmental DNA Samples
Processing of environmental DNA samples was conducted in a dedicated PCR-free laboratory. Benches and equipment were decontaminated using a 10-min exposure to 10% bleach solution and wiped with ultrapure water (Invitrogen, #10977015) before laboratory work commenced. As SENTINEL also works with RNA, RNaseZAP (Invitrogen, #AM9780) was used to remove RNAses from benches and equipment.
Environmental samples from three sites within Otago Harbor where U. pinnatifida is occurring (Otago Harbor—OH#1–3) were obtained following the protocol previously described (Jeunen et al. 2023). OH2 and OH3 eDNA samples were obtained by filtering 1 L seawater while OH1 was obtained by filtering 2 L seawater. We used a 1.2 μm ~30 mm cellulose acetate filter (Whatman) for OH1 and OH3 eDNA samples, while OH2 used a 0.22 μm ~30 mm cellulose acetate filter (Whatman). It has been reported that either 1 L or 2 L works for eDNA capture, purification and detection (Capo et al. 2020). A different filter size was used in OH2. It has been reported that large and small pore sizes can capture eDNA (Miya et al. 2016; Turner et al. 2014). This methodology difference did not impact the results of this study as the main scope was to assess the consistency of detection of target species with the “gold standard” qPCR/ddPCR versus SENTINEL assays. Environmental samples from Marsden Cove, where S. spallanzanii is present, were obtained from a previous study (von Ammon et al. 2025).
Lastly, environmental samples free of S. spallanzanii and U. pinnatifida were obtained from Doubtful Sound, Fiordland, New Zealand, and were collected as part of a previous study (Jeunen et al. 2020). All samples were aliquoted in the PCR-free clean room and always stored at −20°C. Aliquots were only taken out of the PCR-free clean room for qPCR benchmarking.
In summary, for this study, we used a total of 12 environmental samples (Table S5), three Doubtful Sound environmental samples (Doubtful Sound 1–3), three Otago Harbor environmental samples (OH1–3) benchmarked with qPCR, and six Marsden Cove environmental samples benchmarked with ddPCR. All environmental samples were benchmarked with either qPCR or ddPCR.
2.5 Cas13 Fluorescence Assays
The Cas13 mastermix for fluorescence assays (Table S6) was standardized and modified from previously reported Cas13-based fluorescence assays (Ackerman et al. 2020; Baerwald et al. 2023; Gootenberg et al. 2018, 2017; Leugger et al. 2024; Li, Shang, et al. 2023; Thakku et al. 2022). For all SENTINEL reactions, we used a 1:1 (LwaCas13a:crRNA) ratio. Moreover, some modifications were tested, including reaction buffer and reporter probe, to explore different configurations that may work better for SENTINEL assays. The mastermix composition is as follows: 5.18 μL of RNase-free water (Invitrogen, US, #10977015), 4 μL of 5X optimized Cas13a reaction buffer (see Table S7) or 2 μL 10X Cas13a reaction buffer (GenScript, US, #Z03486), 0.5 μL of Murine RNAse inhibitor (New England Biolabs—NEB, US, #M0314S), 0.8 μL of ribonucleotide mix (NEB, US, #N0466S), 1 μL of 10 μM reporter (poly-U5 or poly-U15, GenScript, US, custom), 0.96 μL of 10 μM forward primer (GenScript, US, custom), 0.96 μL of 10 μM reverse primer (GenScript, US, custom), 0.6 μL of T7 RNA polymerase (NEB, US, #M0251S), 2 μL of 500 nM LwaCas13a (GenScript, US, #Z03486; diluted in RNAse-free water and 1X RNAse-free PBS pH 7.4, Invitrogen, US, #AM9624), 1 μL of 1 μM crRNA (GenScript, US, custom) and 1 μL of 280 mM MgOAc (TwistDx, US). All components were added in the described order. We considered a 5% pipetting error when scaling up the reactions. All mastermixes were freshly produced and used immediately. Cas13a optimized reaction buffer (Table S7) composition was used as previously reported (Arizti-Sanz et al. 2020).
After setting up the mastermix, it was slowly mixed with one RPA pellet (TwistDx, US). Eighteen microliters of mastermix was aliquoted in a white 8-strip tube using a 96-well adaptor (Roche Diagnostics, US, LightCycler 8-Tube Strip Adapter Plate, #06612598001), and then 2 μL of sample was added. Then, strips were spun down for 15 s at full speed in a minifuge and immediately run in LC480 II LightCycler qPCR systems (Roche Diagnostics, US) at 39°C as previously reported (Higgins et al. 2022), for 1 h with data acquisitions every 60 s in the FAM channel (excitation at λ465 nm and detection at λ510 nm). RNAse-free water was used as a negative control in all assays unless otherwise specified. All assays were run in triplicate plus a negative non-target control per RPA pellet. A list of the samples used in this study is provided in Table S5. A cost breakdown is provided in Table S8.
Additionally, to comprehend LwaCas13a activity under different buffer conditions, we performed a simple fluorescence experiment to screen test the manufacturer buffer (GenScript, US, #Z03486, buffer composition not disclosed) and a previously reported optimized buffer for LwaCas13a (Table S6; Arizti-Sanz et al. 2020) to determine which buffer would be best for SENTINEL. Both buffer configurations were only tested with U-cr180, at 10 pM plasmid DNA and using poly-U5 reporter.
To test novel reporters for enhancing the fluorescence signal of LwaCas13a (Yang et al. 2023), we screened two poly-U reporters for LwaCas13a: the novel poly-U15 (5′-6-FAM/UUU UUU UUU UUU UUU/BHQ1-3′; (Yang et al. 2023)) versus the traditional reporter poly-U5 (5′-6-FAM/UUU UU/BHQ1-3′; (Gootenberg et al. 2018; Kellner et al. 2019)). We explored whether a shorter or longer poly-uracil reporter influences the diagnostic performance of SENTINEL. We tested SENTINEL under the same reporter concentration (500 nM final), adding a fixed plasmid final concentration (10 pM or ~0.3 ng/μL) containing the corresponding target sequence.
2.6 Statistical Analysis and Calculations
The t-test and correlation estimates were performed with Prism 10 (Version 10.4.0 for Windows, GraphPad Software, Boston, Massachusetts USA, www.graphpad.com). The t-test and p-values are given in parentheses when corresponding. All data plots (heatmap, column plots, and curves) were created with Prism 10. All illustrations were created with BioRender (www.biorender.com/). Fold-change calculations were calculated with end-point raw intensity data, dividing the control-subtracted end-point raw intensity for the target from the average end-point raw intensity of controls. To standardize the intensity fold-change calculations, we used the standard deviation of end-point raw intensities from 11 no-target control replicates across different assays (average = 0.302, standard deviation = 0.435). The average was used as the end-point baseline for intensity fold change calculations for all the samples in this study (sample end-point fluorescence—average end-point baseline/average end-point baseline). The standard deviation was used to set the arbitrary detection threshold equal to five times the standard deviation (~7-fold change units or ~2.17 raw fluorescence intensity).
3 Results
3.1 CRISPR RNA Design With ADAPT
ADAPT provided six and four highly active guide-target pairs (i.e., forward primer, crRNA and reverse primers, Figure 3) for S. spallanzanii and U. pinnatifida, respectively, with no off-target hits (using 100% hit search) on the spacer region when checked using megaBLAST. We selected the best and worst guide-target pairs for each species to test further (Table S4). A full list of guide-target pairs obtained from ADAPT with their scoring for S. spallanzanii and U. pinnatifida is provided in Table S9.
3.2 Optimized Cas13a Buffer Displays Better Diagnostic Activity Than the Manufacturer's Buffer
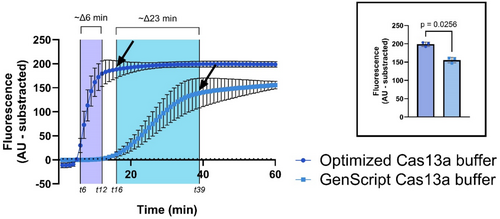
We observed that the U-cr180 guide-target pair had a faster activation speed with the optimized Cas13a buffer than the manufacturer's buffer (t-test, t = 6.1340 p = 0.0256 when using p < 0.05, Figure 4). The optimized reaction buffer was 6 min faster than the manufacturer's buffer in getting beyond the arbitrary detection threshold (set at sevenfold change units or ~2.17 raw intensity). Additionally, the Cas13a optimized reaction buffer reached the saturation point at ~16 min (i.e., faster activation speed) while the manufacturer's buffer reached saturation at ~39 min, making the Cas13a optimized reaction buffer ~2.4 times faster. This is consistent with results from the original study that described the optimized buffer (Arizti-Sanz et al. 2020). We understand activation speed as the elapsed time of the LwaCas13a reaction to reach fluorescence saturation when providing a positive result. Accordingly, we used the optimized Cas13a reaction buffer for all following SENTINEL assays as it reached the saturation point faster.
3.3 A Shorter Poly-U5 Reporter Displays Better Diagnostic Activity Than a Longer Poly-U15 Reporter
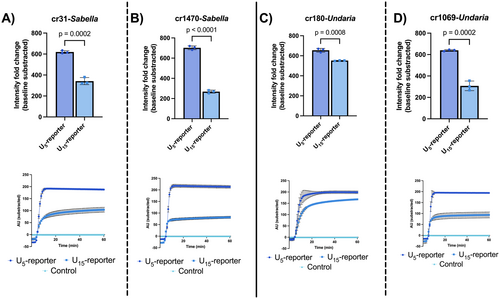
The U5-reporter displayed significantly better end-point intensity fold change after 1 h of reaction time (t-test, tS-cr31 = 13.3500; tS-cr1470 = 30.8200; tU-cr180 = 9.0790; tU-cr1069 = 12.6400, using p < 0.01) across all crRNAs (Figure 5). Moreover, we observed that all crRNAs displayed similar activation speeds in the 10 pM range when using the Poly-U5 or Poly-U15 reporter (Figure 5). Thus, poly-U5 was selected for all further assays.
3.4 ADAPT Predicted Scores Showed no Difference Between the Best and Worst Guide-Target Pair When Using gDNA
The predicted score versus the experimental end-point fold change output was only significant for S. spallanzanii guide-target pairs S-cr31 vs. S-cr1470 when targeting plasmid DNA (t-test, t = 6.1725 p = 0.0035, using p < 0.05), but the same guide-target pairs were not significant when tested against gDNA (t-test, t = 0.6618 p = 0.5443, using p < 0.05). For U. pinnatifida guide-target pairs, U-cr180 versus U-cr1069 assays on plasmid DNA and gDNA were both not significant (t-test, t = 1.2800 p = 0.2699 and t = 1.1130 p = 0.3281, respectively, using p < 0.05; Figure S1).
3.5 Off-Target Assays With Co-Occurring Marine Species Showcase SENTINEL as a Specific Assay for Environmental DNA Biosurveillance
We first tested internal off-target activity on plasmids containing the region of interest to assess whether the SENTINEL assays were working as intended. Tests were performed on gDNA from S. spallanzanii and U. pinnatifida and human DNA (extracted from induced pluripotent stem cells provided by Dr. Indranil Basik from the Department of Biochemistry, University of Otago) to assess detection potential and limitations. No visible off-target activity was observed across species after 1 h (Figure 6A), in concordance with previous in silico results that showed no hits in the NCBI public database. Guide-target pairs S-cr31 and U-cr180 for S. spallanzanii and U. pinnatifida, respectively, were selected for further experiments to reduce the number of assays to screen.
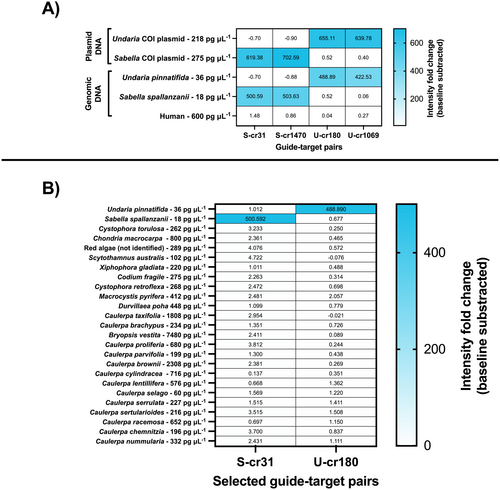
Next, we tested SENTINEL's potential off-target activity in a more realistic off-target assay setup, using gDNA from 23 other marine species that could co-occur with our target species (Table S8), ranging in tested concentration from 18 to 7480 pg/μL (~4.3 nM to ~10 pM) (Figure 6B). Additionally, we set an arbitrary positivity threshold of sevenfold-change units as maximum background noise, similar to other CRISPR studies (Leugger et al. 2024; Nagarajan et al. 2024; Williams et al. 2021). The fold-change arbitrary detection threshold was obtained by multiplying five times the standard deviation of the average end-point raw intensity from no-target controls (average = 0.302; standard deviation = 0.435, raw intensity arbitrary detection threshold ~2.17, fold change arbitrary detection threshold ~7 fold-change units).
3.6 SENTINEL Is a Highly Sensitive Biosurveillance Tool With a Semi-Quantitative Window
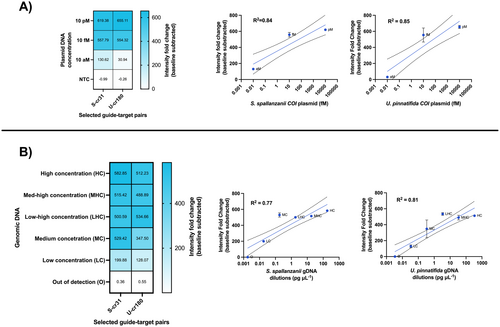
We challenged SENTINEL assay sensitivity in plasmid DNA and gDNA, obtaining attomolar sensitivity with a significant endpoint fold-change (t-test, tS-cr31 = 31.5000 p < 0.0001; tU-cr180 = 4.3070 p = 0.0126, using p < 0.05) when compared with the control (Figure S2). The tested guide-target pairs obtained a theoretically semi-quantitative measurement for plasmid DNA (R2 = 0.84 for S. spallanzanii and R2 = 0.85 for U. pinnatifida, Figure 7A). For gDNA, both crRNAs (S-cr31 and U-cr180), when tested at high, medium, and low DNA concentrations, returned R2 values = 0.77 and 0.81, respectively (Figure 7B). S-cr31 and U-cr180 showcased a sensitivity down to 10 aM in plasmid DNA (copy ranges ~5 copies/μL). Moreover, S-cr31 and U-cr180 achieved gDNA detection down to 0.018 and 0.036 pg/μL, respectively.
3.7 Environmental DNA Assays Showcase SENTINEL as a Specific and Highly Sensitive Biosurveillance Tool
To finalize the trial of our newly developed SENTINEL assays, we tested different eDNA samples that were known to contain S. spallanzanii (Marsden Cove), U. pinnatifida (Otago Harbor), as well as a control site where neither species was present (Doubtful Sound). All eDNA samples from Marsden Cove and Otago Harbor were benchmarked using species-specific ddPCR (von Ammon et al. 2025) or qPCR method (this study), respectively. The results obtained from SENTINEL were highly sensitive and species-specific (Figure 8). Briefly, comparing the qPCR results from Otago Harbor eDNA samples (n = 3), we found that all were positive for qPCR and SENTINEL for U. pinnatifida (100% positive agreement), achieving detection down to 59.2 copies/μL. A similar result was obtained from the Marsden Cove eDNA samples (n = 6) benchmarked using ddPCR for S. spallanzanii and U. pinnatifida. All these eDNA samples were ddPCR and SENTINEL positive for S. spallanzanii (100% positive detection) and all negative for U. pinnatifida (100% negative detection). Detection sensitivity was down to 0.34 copies/μL when detecting S. spallanzanii eDNA. All positives exceeded the arbitrary threshold set previously in this study.

Linear regressions with ddPCR and qPCR copies/μL for Sabella eDNA samples gave an R2 = 0.84, while Undaria eDNA samples had an R2 = 0.54 (Figure S3). We further explored off-target detection for both SENTINEL assays by testing eDNA samples from Doubtful Sound taken at different depths (0 m—D1, 4 m—D2, and 15 m—D3). No known invasion of S. spallanzanii or U. pinnatifida has been reported in Doubtful Sound. All Doubtful Sound samples were negative for SENTINEL. The raw fluorescence intensity plot of all eDNA samples used in this study is provided in Figure S4.
4 Discussion
Amid the ongoing global biodiversity crisis in aquatic habitats, driven primarily by climate change and bioinvasions (Britton et al. 2023), eDNA monitoring holds transformative potential for environmental biosurveillance. Its ability to provide highly accurate species identification, combined with the capacity to survey extensive areas with minimal resource demands, makes it a powerful tool for conservation efforts (Schenekar 2022). To address these needs, novel molecular tools are being developed to enhance portability and ease of use without sacrificing specificity and sensitivity in eDNA detection (Cordier et al. 2019; Makiola et al. 2020; Schenekar 2022). In this context, our study introduces SENTINEL, a new CRISPR-Cas-based strategy designed to detect S. spallanzanii and U. pinnatifida from eDNA samples, leveraging a trained neural network to facilitate and streamline target in-field discovery and assay design to minimize screening efforts and deploy in-lab environmental biosurveillance assays faster.
We first deployed ADAPT locally to design crRNAs targeting the COI genes of S. spallanzanii and U. pinnatifida, separately (Tables S1 and S2) (von Ammon et al. 2019; Razeghi et al. 2021). ADAPT was locally run (Table S3) and identified the shortest possible target fragments (~88–90 nucleotides per assay) containing highly active guide-target pairs for S. spallanzanii and U. pinnatifida (Tables S4 and S9), as illustrated in Figure 3. This feature is particularly advantageous, as RPA performs optimally with smaller amplicons (~100–200 nucleotides) (Lobato and O'Sullivan 2018). Additionally, eDNA in natural environments is often fragmented (Jo 2023), making short targets more suitable for detection. By swiftly generating these compact gene targets, ADAPT enhances the efficiency and reliability of eDNA-based biosurveillance.
For the first screening of the SENTINEL platform, we used the guide-target pair U-cr180, where we observed that ADAPT-provided constructs were working as intended, preliminarily highlighting its advantages to streamline LwaCas13a designs for CRISPR-eBx. Moreover, we also observed that the optimized LwaCas13a reaction buffer yielded the best results, providing an activation speed ~2.4 times faster than the Cas13a manufacturer's buffer (Figure 4). The observed difference in activation speed might be due to the addition of PEG-8000, which has been reported to enhance enzymatic reaction performance via enzymatic stabilization and preventing aggregation (Akabayov et al. 2013; Yang, Li, et al. 2024). Interestingly, if PEG-8000 increases Cas13a reaction performance and therefore increases activation speed, it should only lead to a quicker signal plateau but similar final fluorescence. This is not what we observed, as the difference between each buffer's end-point fluorescence is significant (Figure 4). Therefore, the signal difference likely has another source. Accordingly, the variation in raw intensity suggests that inactivation of LwaCas13a activity may be occurring because of potential depletion of the cofactor (Mg2+) rather than a potential variation of poly-U5 reporter concentration, as they were prepared under the same conditions and screened in parallel (Table S6). However, the lack of detailed information on the LwaCas13a GenScript reaction buffer limits further analysis. Nevertheless, buffer selection was pivotal for the SENTINEL platform, as it could detect low eDNA abundances in less than 40 min (Figures 8 and S4), a frequent scenario when using eDNA (Lee et al. 2024). Therefore, we used the LwaCas13a optimized buffer (Table S7; Arizti-Sanz et al. 2020) for all subsequent SENTINEL assays.
We tested all obtained crRNAs with two different poly-Ux reporters (poly-U5 and polyU15), as longer Uracil chains have been shown to enhance LwaCas13a trans activation (Yang et al. 2023). However, we found that the smaller reporter, poly-U5, performed better under the same conditions (target concentration: 10 pM final, reporter concentration: 500 nM final), yielding the highest intensity fold change (Figure 5). This discrepancy may be explained by two factors: (1) we employed a novel optimized buffer that accelerates the LwaCas13a reaction (Arizti-Sanz et al. 2020), whereas other studies used different reaction buffers (Gootenberg et al. 2018; Kellner et al. 2019; Yang et al. 2023) that influence reaction kinetics (Arizti-Sanz et al. 2020); (2) LwaCas13a trans-collateral activity exhibits a dinucleotide preference for uracil pairs (UU) (Gootenberg et al. 2018), which, as described below, has been utilized in previous studies using the poly-U5 reporter (Baerwald et al. 2023; Gootenberg et al. 2018; Patchsung et al. 2020).
Assuming that each UU motif results in a single cut, a poly-U5 reporter could produce up to two trans cuts per reporter molecule. We hypothesize then that longer UU chains in a poly-U reporter could lead to more trans cuts, resulting in a similar activation speed (Figure 5) but a lower raw intensity. This may occur because, though the enzyme cuts the reporter at more sites, the fluorescence is only reported once, thus depleting LwaCas13a activity faster without contributing to the signal. This is potentially due to a finite number of substrate cis cuts available per active Cas13a (East-Seletsky et al. 2017, 2016). Hence, cis-activated trans-collateral activity is limited by the cis activity of Cas13a (Bot et al. 2022; East-Seletsky et al. 2016). To put this in perspective, the same molarity was used when comparing poly-U5 and poly-U15 (i.e., the same number of molecules), indicating that in terms of raw intensity release (i.e., required LwaCas13a trans cleavage cuts that effectively release the fluorophore), shorter poly-U reporters, such as poly-U5, may be better suited than longer poly-U reporter chains, as they have more UU motifs available that deplete LwaCas13a activity before maximum intensity can be provided. Therefore, from an application perspective, under the conditions of this study, a shorter reporter provides a better diagnostic efficiency in terms of activation speed towards saturation. Consequently, we used poly-U5 for all subsequent SENTINEL assays.
Next, we challenged SETINEL with a proof of concept using potentially co-occurring off-target species, employing plasmids containing COI inserts from S. spallanzanii and U. pinnatifida, as well as gDNA from S. spallanzanii, U. pinnatifida, and humans. Firstly, all crRNAs performed as expected, with no detectable off-target activity, even in the presence of potential human gDNA contamination and the constructed plasmid for this study (Figure 6A). Additionally, ADAPT score ranking yielded similar results when comparing crRNAs targeting the same species (e.g., S-cr31 vs. S-cr1470) using either plasmid or gDNA (Figure S1). Based on these findings, we selected S-cr31 and U-cr180 for all subsequent assays.
In an environmental biosurveillance context, any species of interest will co-exist with others, which will also be co-captured and co-isolated during eDNA sampling. To account for this, we expanded our off-target assays by creating a panel of 23 potential co-occurring species as potential off-target organisms for the SENTINEL assay targeting S. spallanzanii and U. pinnatifida (Figure 6B; Table S5). No significant intensity fold change was observed above the arbitrary detection threshold (set at sevenfold-change units, representing the maximum arbitrary background noise) when gDNA from off-target organisms was present.
We then assessed the sensitivity of SENTINEL using plasmids with COI inserts and gDNA from our target species. Our results showed that SENTINEL detected plasmid DNA as low as 10 aM (10−18 mol/L or ~5–6 copies/μL or ~0.024 fg/μL; Figure 7A), with a significant endpoint intensity fold change compared to the control (Figure S2A,B). For gDNA, we detected S. spallanzanii at 0.018 pg/μL and U. pinnatifida at 0.036 pg/μL, corresponding to approximately ~10 fM range (10−15 mol/L; Figure 7B), consistent with previous studies using extracted gDNA and LwaCas13a (Yang, Matsushita, et al. 2024). The SENTINEL assay also detected plasmid DNA at 10 aM within 30 min (Figure S2A), a speed similar to other CRISPR-based assays that outperformed qPCR in terms of result turnaround time (Baerwald et al. 2023); however, due to the potential risk of false negatives when shortening assay screening time, we chose to maintain a 1-h endpoint to minimize this risk as eDNA samples can be in low abundance (ref). Furthermore, both species displayed a semi-quantitative response with plasmid DNA and gDNA, showing an R2 of 0.77 and 0.81 for S. spallanzanii and U. pinnatifida gDNA samples (Figure 7B), respectively. Similar results were obtained with plasmid DNA (Figure 7A).
This semi-quantitative feature of SENTINEL was more pronounced at lower concentrations, as high concentrations quickly saturated the reaction due to faster activation, resulting in the same end-point fluorescence. In contrast, lower-concentration samples did not reach saturation, leading to varying end-point fluorescence at the 1-h mark based on concentration, enabling semi-quantification. While this reflects the relative abundance of the eDNA target, it does not directly correlate with species abundance (Rath et al. 2024) but rather with the genomic region used for detection. Overall, this provided a solid foundation for our SENTINEL assays and gave us the confidence to apply them to eDNA samples.
Finally, we tested eDNA samples from locations with known invasions of S. spallanzanii (Marsden Cove, New Zealand) and U. pinnatifida (Otago Harbor, New Zealand), as well as from a site with no reported invasions of S. spallanzanii and U. pinnatifida (Doubtful Sound, New Zealand; Table S5). Our results showed 100% agreement between qPCR/ddPCR versus SENTINEL in all tested environmental samples: 6/6 for S. spallanzanii and 3/3 for U. pinnatifida. As expected, no SENTINEL assay exceeded the arbitrary positivity threshold in negative eDNA samples from Doubtful Sound (Figure 8). (Miya et al. 2016; Turner et al. 2014).
When comparing qPCR/ddPCR versus SENTINEL results on eDNA samples, we observed that the quantification window for S. spallanzanii (R2 = 0.84) and U. pinnatifida (R2 = 0.54) (Figure S3) aligned with previous results in controlled experiments using plasmid DNA and gDNA (Figure 7). However, we must mention that these quantification windows may serve as semi-quantitative for qualitative inference, i.e., high or low abundance of the target rather than a fixed number of copies/μL. We believe this can be extremely useful, especially in invasive species contexts, as their signal should not be in the environment at any given moment if there is no prior known incursion in a given ecosystem. A full display of all SENTINEL environmental samples run in this study can be found in Figure S4.
When comparing the sensitivity of our SENTINEL assay to that of other recent studies using LwaCas13a (Leugger et al. 2024; Nagarajan et al. 2024; Yang, Matsushita, et al. 2024), similar detection limits were achieved. The best-performing SENTINEL assay detected S. spallanzanii COI region at concentrations ranging from 2.33 to 0.34 copies/μL, as determined by ddPCR quantification, corresponding to approximately ~10 aM range by the fold-change intensity obtained by SENTINEL. This is consistent with other CRISPR-eBx studies that have achieved single copy detection (Baerwald et al. 2023; Nagarajan et al. 2024; Yang, Matsushita, et al. 2024). For U. pinnatifida eDNA samples, the detection range was from 364 to 73.2 copies/μL, according to qPCR quantification, which would be within the fM–aM ranges by the fold change intensity obtained by SENTINEL. However, as both SENTINEL assays targeted genomic regions different from qPCR/ddPCR, these values are valid references but not absolute ones.
During this study, a new invasion of S. spallanzanii was reported near Otago Harbor (Port Chalmers) between August 26 and 30, 2024, with a full specimen retrieved (transect coordinates: −45.81192312, 170.6268429; end of transect: −45.81142622, 170.6271924). However, our assay did not detect this incursion in the Otago Harbor eDNA samples, likely because the invasion occurred after our samples were collected on July 17, 2024. Additionally, the sampling site for U. pinnatifida was approximately 10.5 km from Port Chalmers. These findings highlight the importance of targeted and timely CRISPR-eBx sampling at sites of interest to ensure the accurate detection of biosecurity threats.
Our results support SENTINEL as a reliable proof-of-concept for the rapid design of CRISPR-based diagnostic technologies using AI tools like ADAPT. Remarkably, the designs in this study were generated within minutes, significantly reducing screening efforts while delivering high-quality, cost-efficient results at approximately 3.5 USD per sample (Table S8). However, this study also identified three key areas requiring further development: (1) further specificity validation, including co-occurring species closely related species for S. spallanzanii and U. pinnatifida to fully comprehend ADAPT capabilities to discover and detect unique diagnostic sequence regions for environmental biosurveillance deployments; (2) further tests on eDNA samples from different locations and abundance of target populations to estimate detection probabilities using SENTINEL assays in situ; (3) further train ADAPT with high-quality SENTINEL eDNA assays to maximize and tailor ADAPT guide-target pairs specifically for CRISPR-eBx context to account for complexities of eDNA-based applications.
There are some limitations to ADAPT that could be further investigated. For example, the differences in the predicted activity between S-cr31 and S-cr1470 are small (objective score of 6.04 vs. 6.34, respectively; Table S9). This small difference was evidenced by the fact that there was no significant difference when using gDNA for S. spallanzanii detection with our guide-target pairs, but there was a significant difference in plasmid DNA (Figure S1), showcasing contradictory results. However, this might show that different sample sources may affect the efficiency of guide-target pairs or that ADAPT was trained with highly pure samples and, therefore, the importance of screening different types of targets during optimization to evidence potential experimental variations. Moreover, we consider gDNA samples more complex than plasmid DNA; thus, gDNA performance is of higher importance in screening weight than plasmid DNA. In this regard, gDNA performance between S-cr31 and S-cr1470 was not significant. For U. pinnatifida, this contradictory observation was not evidenced, as their predicted efficiency difference was marginal in their objectives scores given by the ADAPT pipeline (~0.02 of difference on the overall), providing no significant differences either in plasmid DNA or gDNA. An interesting observation regarding guide-target activity is that in both species, plasmid DNA yielded higher intensity than gDNA, but this might be due to target concentration or sample complexity.
To fully understand the implications of the predicted efficiency provided by ADAPT on the final fluorescence output for eDNA studies, it is necessary to explore a greater difference between guide-target pairs expected activity. This could also be done using a window exploration command within ADAPT (sliding-window search type) rather than looking for the best assay options as it was done in this study (complete-target search type (Metsky et al. 2022)). The sliding-window option will provide a full landscape of all possible guide-target pair combinations in a given sequence, where highly different values could be tested to screen for differences (e.g., objective scores of 3 vs. 6), ultimately exploring any significant differences and correlations with observed signal and predicted objective value. A similar screening was done by Metsky et al. (2022) when they proposed ADAPT; however, this was done for viral detection instead of plasmid DNA, gDNA, or eDNA. Nevertheless, we can assume that the ADAPT guide-target pairs used in this study were highly active as predicted (by using complete-targets search type in ADAPT, see Table S3; Metsky et al. 2022) since all screened crRNAs provided quick fluorescence saturation under the same conditions (Figure 5) without significant computational or bioinformatics efforts, as ADAPT can also be run locally on a low-tier laptop.
Overall, these findings highlight two key take-home messages for SENTINEL application: (1) the semi-quantitative performance is maintained with eDNA samples and (2) SENTINEL results align closely with qPCR/ddPCR calling, underscoring the potential of CRISPR-eBx application for biosurveillance. Most S. spallanzanii and U. pinnatifida eDNA samples could also be called positive within 30 min, except for sample S3.3a, which required 40 min (Figure S4). Moreover, the cost per SENTINEL reaction was approximately 3.5 USD (Table S8), comparable to reported costs for similar CRISPR-LwaCas13a assays (Nagarajan et al. 2024), which may most likely keep decreasing as CRISPR-eBx technologies advance in their uptake by the research and end-user communities.
5 Concluding Remarks
Leveraging ADAPT, a pre-trained end-to-end AI model, we swiftly designed and deployed in the lab highly active guide-target pairs detecting the COI gene region for S. spallanzanii and U. pinnatifida separately. We achieved high sensitivity and high specificity with no off-target activity across 23 co-occurring species. Our findings indicate that SENTINEL can detect DNA concentrations as low as 10 aM in plasmid DNA, within the fM range in gDNA, and down to 0.32 copies/μL in eDNA samples. Additionally, we observed a semi-quantitative detection window, allowing relative assessment of eDNA abundance based on endpoint intensity at low concentrations. This feature provides higher confidence in environmental biosurveillance for future applications where target eDNA is often present in trace amounts. When testing SENTINEL on eDNA samples from two known invasion sites (Marsden Cove and Otago Harbor), it yielded results consistent with ddPCR and qPCR benchmarks for S. spallanzanii and U. pinnatifida, showcasing a 100% agreement and affirming the robustness and reliability of SENTINEL for environmental biosurveillance with an average cost of ~3.5 USD per reaction.
However, while SENTINEL shows promise, two key limitations require attention for further research: expanding off-target activity testing on closely related species to ensure specificity, validating across diverse sampling sites, and tailoring ADAPT to optimize guide-target pairs specifically for eDNA contexts, as eDNA samples can have a complex DNA composition from different sources and inhibitors. Addressing these areas will enhance SENTINEL application in real-world environmental biosurveillance efforts worldwide, supporting global biodiversity efforts through rapid and precise CRISPR-eBx.
Our study successfully demonstrates the use of a novel blend, using RPA-CRISPR-LwaCas13a integrated with an accessible trained AI model—ADAPT, for detecting environmental nucleic acids. We named this system SENTINEL, which can separately detect two invasive species, S. spallanzanii and U. pinnatifida, using eDNA from aquatic habitats as proof of concept for the CRISPR-eBx toolbox. This approach provides a streamlined pipeline for future environmental biosurveillance applications while closing a gap in potentially tedious, time-consuming, and costly crRNA screening for eDNA biosecurity applications, with benefits spanning from invasive species detection like this study to viral environmental biosurveillance and endangered species management.
Author Contributions
Benjamín Durán-Vinet: conceptualization, methodology, investigation, software, illustration, resources, data curation, formal analysis, writing – original draft and writing – review and editing. Jo-Ann L. Stanton: conceptualization (supporting), methodology, investigation, resources, formal analysis, writing – original draft and writing – review and editing. Gert-Jan Jeunen: methodology (supporting), investigation, resources, formal analysis (supporting), writing – review and editing, and supervision. Ulla von Ammon: investigation (supporting), resources, writing – original draft and writing – review and editing. Jackson Treece: methodology (supporting), investigation (supporting) and resources. Xavier Pochon: investigation (supporting), resources, writing – review and editing, and supervision. Anastasija Zaiko: investigation (supporting), resources, writing – review and editing, supervision, project administration, and funding acquisition. Neil J. Gemmell: conceptualization (supporting), methodology (supporting), investigation (supporting), resources, formal analysis, writing – original draft, writing – review and editing, supervision, project administration, and funding acquisition.
Acknowledgments
All authors thank the Cawthron Institute (Nelson, New Zealand), National Institute of Water and Atmospheric Research (NIWA), Prof. Joe Zuccarello (Victoria University of Wellington, New Zealand), Prof. Sarah Caronni (University of Milan, Italy) and Michelle Liddy (Department of Anatomy, University of Otago, New Zealand) for kelp genomic DNA samples. All authors also thank Dr. Indranil Basik (Department of Biochemistry, University of Otago, New Zealand) for extracted human genomic DNA samples. Benjamín Durán-Vinet thanks Dr. Susie Wood for the early support of the CRISPR-based environmental biosurveillance concept in 2019. Open access publishing facilitated by University of Otago, as part of the Wiley - University of Otago agreement via the Council of Australian University Librarians.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
ADAPT public, open-source repository is available at https://github.com/broadinstitute/adapt or also as a web server at https://adapt.run/. Supporting Information (qPCR report and alignments) is available online at https://github.com/bduranvinet/SENTINEL-article. Other raw data will be available upon reasonable request.




