A Time- and Cost-Effective eDNA Protocol to Survey Freshwater Mussels (Bivalvia: Unionida) in Tropical Rivers
Funding: This project was funded by Technology Grant NGS-61128T-19, granted to A.Z. and K.A.R. by the National Geographical Society.
ABSTRACT
Tropical freshwater mussels (Bivalvia: Unionida) are one of the most endangered groups of animals globally, but conservation is hindered by a lack of species distribution data. Traditional hand-sampling is time- and cost-intensive and not always possible, for example, because of the presence of crocodiles. Surveying freshwater mussel populations by environmental DNA (eDNA) could potentially rapidly increase data availability, but no published study and protocols targeted towards tropical freshwater mussels are available to date. We aimed to develop a reliable and cost-efficient eDNA protocol for surveying tropical freshwater mussels. We first developed and validated a qPCR primer-probe assay within the cytochrome c oxidase subunit 1 (COI) gene for Rectidens sumatrensis. We applied this assay in a controlled laboratory setting on eDNA collected from lake and river water, respectively, at two different R. sumatrensis densities in order to test a set of 18 different protocols for capturing, preserving, and extracting freshwater mussel eDNA. All protocols use equipment that is readily available and reusable. Our results revealed that samples stored in Longmire's buffer (at 4°C) yielded more mussel DNA than when stored in absolute ethanol (at −20°C), with < 1% of ethanol- and 78% of buffer-preserved samples fulfilling the criteria for positive R. sumatrensis eDNA detection (i.e., amplifying above the limit of detection in at least four out of five qPCR replicates). Across buffer-preserved samples, eDNA detection and amplification success rates were higher and quantification cycle values were lower for eDNA captured without pre-filtration and with filter membrane pore sizes > 0.45 μm, and eDNA extracted with the Qiagen DNeasy Blood & Tissue Kit rather than the PowerSoil ProKit (albeit latter exhibiting fewer instances of amplification in negative controls). The assay detailed here was capable of detecting down to R. sumatrensis eDNA concentrations of 6.38 × 10−7 ng/μL and reflected the difference in stocking density.
1 Introduction
Biodiversity is being lost faster in tropical freshwater habitats than anywhere else (Dudgeon et al. 2006; Dudgeon 2019). Prioritizing and developing effective conservation actions requires knowledge of the distribution of species, but these data are largely missing for tropical freshwater taxa. For freshwater mussels (Bivalvia: Unionida), data availability is particularly poor. Freshwater mussels are one of the most endangered animal groups and are rapidly declining, especially in Southeast Asia (Lydeard et al. 2004; Zieritz, Bogan, Froufe, et al. 2018). At the same time, these filter-feeding animals provide crucial ecosystem services, from increasing water quality and promoting benthic biodiversity to providing a source of food (Zieritz, Azam-Ali, et al. 2018; Zieritz et al. 2022). The freshwater mussel fauna of Borneo is poorly studied, highly endemic, and highly threatened. Most of the 21 species currently recognized as native to Borneo—15 of which are endemic to the island—have not been recorded in the past 50–100 years (Zieritz, Bogan, Froufe, et al. 2018; Zieritz, Bogan, Rahim, et al. 2018; Zieritz et al. 2020, 2021, 2024; Graf and Cummings 2023). Four out of five species native to northern Borneo may have already been lost due to large-scale habitat loss and degradation (Zieritz, Bogan, Rahim, et al. 2018). The recent discovery of a new genus and five new species endemic to Borneo illustrates our poor understanding of this fauna (Zieritz et al. 2021, 2024). However, many rivers/sites currently cannot be surveyed for freshwater bivalves, as traditional hand-sampling is too dangerous due to the presence of Estuarine Crocodiles (Crocodylus porosus Schneider, 1801). For example, in 2021, 45 crocodile attacks—26 of them fatal—were reported from Borneo (B. Sideleau, unpublished data).
The lack of knowledge on where any remaining populations of freshwater mussels in Borneo and other tropical regions are located is hampering conservation efforts, including generation of a better understanding of the ecological requirements of species based on presence–absence data (Gallardo et al. 2018). Surveying by environmental DNA (eDNA) could be a powerful alternative or additional tool for collecting these data. Since the early 2010s, the potential and limitations of eDNA for detecting freshwater organisms have been assessed, applying various protocols and with varying success (e.g., Minamoto et al. 2012; Thomsen et al. 2012). The first studies applying eDNA on freshwater mussels were published in 2014–16 and focused on detecting single species in Central Europe and Canada, respectively (Deiner and Altermatt 2014; Cho et al. 2016; Stoeckle et al. 2016). Since then, the annual output of freshwater mussel eDNA studies has increased considerably; yet, this literature is still almost exclusively focused on temperate freshwater systems in Europe (e.g., Wacker et al. 2019; Prié et al. 2021, 2023; Stoeckle et al. 2021) and North America (e.g., Sansom and Sassoubre 2017; Coghlan et al. 2021; Klymus et al. 2021; Dokai et al. 2023). Isolated studies are also available from, for example, Japan (Togaki et al. 2020; Sugawara et al. 2022; Wu et al. 2024) and New Zealand (Steiner et al. 2022, 2023).
Published eDNA studies focused on freshwater mussels from the tropics are currently lacking, highlighting the under-representation of eDNA studies across tropical freshwater taxa in general (Belle et al. 2019; Huerlimann et al. 2020). Applying a protocol to capture, preserve, extract, and amplify eDNA that is suitable to the specific conditions of the study system and target species is crucial to minimize instances of false positives and false negatives. This is particularly important for threatened tropical species, such as many freshwater mussels, for which eDNA detection may be difficult due to high turbidity and temperature, strong UV radiation, and several other factors that interfere with the filtration and/or amplification process (Robson et al. 2016; Wilson et al. 2017; Cilleros et al. 2019; Huerlimann et al. 2020). To maximize detection rates in these challenging conditions, eDNA studies on other tropical taxa have commonly applied sophisticated and expensive equipment, including filtration capsules and portable peristaltic pumps (Bellemain et al. 2016; Cantera et al. 2019; Cilleros et al. 2019). However, as such equipment will often not be available to local scientists and practitioners in the Global South, currently available eDNA protocols may inhibit a wide application of this promising tool on tropical freshwater biodiversity.
The majority of currently available studies on freshwater mussel eDNA applied species-specific markers using quantitative PCR (qPCR) approaches, which offer greater specificity and sensitivity than conventional PCR, as well as the ability to quantify the amount of eDNA in a sample (Klymus et al. 2020). Freshwater mussel eDNA assays are exclusively designed using mitochondrial DNA (mtDNA), which is present in cells in higher copy numbers than most nuclear DNA targets and thus is thought to represent a more abundant target for eDNA (Prié et al. 2021). These include portions of the 12S rRNA (Deiner and Altermatt 2014), 16S rRNA (Stoeckle et al. 2016; Prié et al. 2021), NADH dehydrogenase subunit 1 (ND1) (Sansom and Sassoubre 2017; Gasparini et al. 2020; Lor et al. 2020) and cytochrome c oxidase subunit I (COI) genes (Cho et al. 2016; Currier et al. 2018; LeBlanc et al. 2021; Sugawara et al. 2022). COI is particularly popular for developing species-specific eDNA markers for freshwater mussels and other animal taxa due to the availability of sequences in global databases and suitability for separating species by DNA barcoding (Hebert, Ratnasingham, et al. 2003; Hebert, Cywinska, et al. 2003; Duarte et al. 2023), although low specificity appears to be an issue of COI eDNA metabarcoding primers (Collins et al. 2019).
Available eDNA protocols for temperate freshwater mussels and (other) tropical freshwater animal taxa vary with respect to their methodologies for capturing, preserving, and extracting eDNA. While filtration rather than precipitation has arguably emerged as the more effective method of eDNA capture, filter membrane material, and pore size vary widely across studies, with the latter ranging from ~0.22 μm (e.g., Deiner and Altermatt 2014) to > 1 μm (e.g., Currier et al. 2018; Dysthe et al. 2018; Wacker et al. 2019; Hauck et al. 2023) and even up to 20 μm (Robson et al. 2016). Methodologies for freshwater mussel or tropical freshwater animal eDNA preservation applied thus far include freezing without any preservative (e.g., Deiner and Altermatt 2014; Sansom and Sassoubre 2017; Togaki et al. 2020; Steiner et al. 2022), storing with desiccant (Dysthe et al. 2018; Marshall et al. 2022), and more recently, ethanol-preservation (Doble et al. 2020; LeBlanc et al. 2021; Preece et al. 2021) and lysis buffer-preservation (Wacker et al. 2019; Prié et al. 2021; Dokai et al. 2023). The most popular extraction method applied in freshwater mussel or tropical freshwater animal eDNA studies to date is arguably the Qiagen DNeasy Blood & Tissue Kit or equivalent kit from alternative companies (Stoeckle et al. 2016; Dysthe et al. 2018; Wacker et al. 2019; Sugawara et al. 2022), although some authors have also used more advanced and costly alternatives, such as the Qiagen MoBio PowerWater kit (Currier et al. 2018; Hauck et al. 2023) and SoilKit (Hashizume et al. 2017; Prié et al. 2021; Steiner et al. 2022). In a recent review on aquatic eDNA surveys, Kumar et al. (2020) recommended the use of cellulose-based (e.g., CN) filters (as, e.g., Majaneva et al. 2018), preservation of filter membranes in Longmire's (or other lysis) buffer (as, e.g., Majaneva et al. 2018) and extraction with the Qiagen DNeasy Blood & Tissue or equivalent Kit.
The aim of this study was to develop a reliable and cost-efficient eDNA protocol for surveying freshwater mussels in tropical rivers. We used a hydrolysis-probe-based qPCR approach and selected Rectidens sumatrensis (Dunker, 1852) as our study species, as it is the only native species of northern Borneo that still maintains a considerable number of healthy populations (Zieritz, Bogan, Rahim, et al. 2018). The study was conducted in three stages: (1) in silico primer-probe design, (2) in vitro qPCR assay optimization and validation, and (3) testing of mussel eDNA capture, preservation, and extraction methods using four experimentally generated mussel eDNA samples (reflecting two different mussel densities and two different water types, i.e., a lake and a river). Informed by previous literature, 18 different protocols were selected and trialed, although not in a fully crossed design. To identify the most appropriate protocol for specific conditions (i.e., low vs. high mussel density, lake vs. river water), eDNA detection rates, quantification cycle (Cq-values), and evidence of PCR inhibition were compared across protocols and water samples.
2 Materials and Methods
2.1 qPCR Primer-Probe Development
We designed a species-specific hydrolysis probe-based qPCR in the mtDNA Female-type (F-type) COI region (i.e., the maternally inherited mtDNA of the Unionidae and several other bivalve groups that exhibit a doubly uniparental mode of mitochondrial inheritance; Breton et al. 2009) of R. sumatrensis. We initially used Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Ye et al. 2012) to identify species-specific primer-binding sites 80–250 bp apart in the COI mtDNA sequence of the target species using all R. sumatrensis F-type COI sequences available on GenBank, resulting in a set of 20 sequences from 13 sites and five river basins, and covering intraspecific variation across the species range (Table 1). Sequences were aligned by ClustalW in MEGA11 (Tamura et al. 2021) to generate a consensus sequence. The developed primers Rec_sumF and Rec_sumR (Table 2) amplify a 204 bp fragment within the COI region of R. sumatrensis, situated at the 3′ end of COI, and overlap with the section amplified by Leray et al.'s (2013) DNA metabarcoding primers. While Primer-BLAST is integrated into BLAST (Basic Local Alignment Search Tool Tool; GenBank, www.ncbi.nlm.nih.gov/blast/) and finds species-specific primers based on available sequences on GenBank, potential affinity of the primers with non-target species was additionally checked by comparing developed primers with all available sequence data with BLAST, and via manual comparison to related mussel species added into the master R. sumatrensis alignment.
| Haplotype number | Voucher number | GenBank Accession number | Sampling location | River basin | PCR primer development | qPCR primer-probe development | qPCR assay optimisation | qPCR assay sensitivity validation |
|---|---|---|---|---|---|---|---|---|
| 3 | x311 | MG591504 | Sungai Tatan near Loagan Bunut, Sarawak, Borneo | Baram | Yes | Yes | ||
| 4 | x325 | MG591505 | Sungai Lunang near Long Bedian, Sarawak, Borneo | Baram | Yes | Yes | ||
| 4 | x326 | MG591506 | Sungai Mam near Long Bedian, Sarawak, Borneo | Baram | Yes | Yes | ||
| N/A | x328 | N/A | Sungai Mam near Long Bedian, Sarawak, Borneo | Baram | Yes | |||
| N/A | x332 | N/A | Sungai Mam near Long Bedian, Sarawak, Borneo | Baram | Yes | |||
| 3 | x337 | MG591507 | Sungai Selulit near Loagan Bunut, Sarawak, Borneo | Baram | Yes | Yes | ||
| 5 | x314 | MG591508 | Sungai Selulit near Loagan Bunut, Sarawak, Borneo | Baram | Yes | Yes | ||
| 2 | x361 | MG591502 | Sungai Sibau near Saremas, Sarawak, Borneo | Suai | Yes | Yes | ||
| N/A | x359 | N/A | Sungai Sibau near Saremas, Sarawak, Borneo | Suai | Yes | |||
| 2 | x347 | MG591501 | Sungai Sebilak near CLC Wilma Segarmas, Sarawak, Borneo | Suai | Yes | Yes | ||
| N/A | x346 | N/A | Sungai Sebilak near CLC Wilma Segarmas, Sarawak, Borneo | Suai | Yes | Yes | ||
| 2 | x369 | MG591503 | Sungai Linau near CLC Wilma Segarmas, Sarawak, Borneo | Suai | Yes | Yes | ||
| 7 | x396 | MG591500 | Sungai Lalut Pelasan near Data Kakus, Sarawak, Borneo | Batang Kemena | Yes | Yes | ||
| N/A | x394 | N/A | Sungai Lalut Pelasan near Data Kakus, Sarawak, Borneo | Batang Kemena | Yes | |||
| N/A | x397 | N/A | Sungai Lalut Pelasan near Data Kakus, Sarawak, Borneo | Batang Kemena | Yes | |||
| 6 | x375 | MG591496 | Sungai Mitik near Long Mitik, Sarawak, Borneo | Rajang | Yes | Yes | Yes | |
| 6 | x386 | MG591492 | Sungai Mitik near Long Mitik, Sarawak, Borneo | Rajang | Yes | Yes | ||
| 7 | x408 | MG591498 | Sungai Mit, Sarawak, Borneo | Rajang | Yes | Yes | ||
| 7 | BIV2993 = x401 | MG591493 | Sungai Mit, Sarawak, Borneo | Rajang | Yes | Yes | ||
| 7 | x412 | MG591495 | Sungai Ranan, Sarawak, Borneo | Rajang | Yes | Yes | ||
| 7 | BIV3010 = x418 | MG591497 | Sungai Ranan, Sarawak, Borneo | Rajang | Yes | Yes | ||
| 7 | BIV3017 = x425 | MG591494 | Sungai Ranan, Sarawak, Borneo | Rajang | Yes | Yes | ||
| 7 | BIV3027 = x435 | MG591499 | Sungai Sekerubong near Nanga Lipus, Sarawak, Borneo | Rajang | Yes | Yes | ||
| N/A | x431 | N/A | Sungai Sekerubong near Nanga Lipus, Sarawak, Borneo | Rajang | Yes | |||
| N/A | x433 | N/A | Sungai Sekerubong near Nanga Lipus, Sarawak, Borneo | Rajang | Yes | |||
| 1 | BIV1659 | KX051314 | Sungai Perak near Kampung Pulau Kemiri, Perak, Peninsular Malaysia | Perak | Yes | Yes | ||
| 1 | BIV1660 | KX051312 | Sungai Perak near Kampung Pulau Kemiri, Perak, Peninsular Malaysia | Perak | Yes | Yes | ||
| 1 | BIV1664 | NC_059765 | Sungai Perak near Kampung Pulau Kemiri, Perak, Peninsular Malaysia | Perak | Yes | Yes |
| Primer name | Unit | Sequence 5′–3′ | Method | Fragment length [bp] |
|---|---|---|---|---|
| Rec_sumF | F | AAT GTT GCG CAT TCT GGT GC | PCR | 204 |
| Rec_sumR | R | CAA TAC AGG CAA TGC CGC AA | PCR | |
| Rec_sumF_475 | F | GGG TTG GTA GCT GAG CGA AT | TaqMan qPCR | 90 |
| Rec_sumR_545 | R | ACC TGC CAA TAC AGG CAA TG | TaqMan qPCR | |
| Rec_sumP_495 | Probe | 56-FAM – CCC TTT ATT/ZEN/TGT ATG GGC TGT GAC TGT – 3IABkFQ | TaqMan qPCR | |
| MIMf | F | GTA TTC CTG GTT TGT AGG TTG AGC | TaqMan qPCR | 143 |
| MIMr | R | ATG TGA CTG GAC TTC CGT ATC G | TaqMan qPCR | |
| IPC probe | Probe | Cy3 – CGA CGG CCA GTG AAT TGT AAT ACG A – BHQ-2 | TaqMan qPCR |
Using the alignment generated above as a starting point, a TaqMan probe was designed in Primer3Plus (Untergasser et al. 2012). The probe design was constrained to the 5' region of the species-specific amplicon, where R. sumatrensis intraspecific variation was low and mismatches were present with respect to other mussel taxa potentially present in the study region. The developed primers Rec_sumF_475 and Rec_sumR_545 amplify a 90 bp fragment of the COI region of R. sumatrensis (Table 2).
2.2 Assay Optimisation and Validation
The qPCR assay was optimized and validated in vitro using DNA extracts of nine sumatrensis tissue samples collected from 6 sites and four river basins, including the same site where specimens for the eDNA experiments were collected (i.e., Sungai Sekerubong, Sarawak, Malaysia, see details below) (Table 1). The specificity of the assay was additionally tested against four other freshwater mussel species from the study region (i.e., Sarawak), representing all unionid genera reported from Sarawak (Zieritz et al. 2024) (Table 3). Genomic DNA for this purpose was extracted using the Qiagen DNeasy Blood & Tissue Kit following the manufacturer's protocol. Total DNA concentration was measured using a Qubit Fluorometer (Thermo Fisher Scientific) with a dsDNA HS assay.
| Species | Voucher number | GenBank Accession number of F-type COI sequence from same population | Number of mismatches per Rec_sum-primer/probe (see Table 2) compared to GenBank sequence | Sampling location | River basin | DNA concentration [ng/μL] | Amplification above LOD [namplified/nreplicates] | Amplification below LOD [namplified/nreplicates] | C q | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F_475 | R_545 | P_495 | |||||||||
| Ctenodesma mawonae Zieritz, Rahim, Lopes-Lima & Pfeiffer in Zieritz et al. (2024) | FRST_MFw_x674 | PP697618 | 2 | 4 | 5 | Sungai Sebua Jebung, Jambusan | Sarawak River | 2.5 | 0/2 | 0/2 | N/A |
| 0.25 | 0/2 | 1/2 | 43.30 | ||||||||
| 0.025 | 0/2 | 1/2 | 44.82 | ||||||||
| Lens lugens (Drouët & Chaper, 1892) | FRST_MFw_x663 | PP697585 | 3 | 3 | 3 | Sungai Nyanuh, Kampung Lobang Batu, Silabur, Serian | Batang Sadong | 2.5 | 0/2 | 2/2 | 43.22 |
| 0.25 | 0/2 | 0/2 | N/A | ||||||||
| 0.025 | 0/2 | 0/2 | N/A | ||||||||
| Pseudodon walpolei (Hanley, 1871) | FRST_MFw_x667 | PP697595 | 4 | 4 | 4 | Sungai Nyanuh, Kampung Lobang Batu, Silabur, Serian | Batang Sadong | 2.5 | 0/2 | 1/2 | 43.70 |
| 0.25 | 0/2 | 0/2 | N/A | ||||||||
| 0.025 | 0/2 | 0/2 | N/A | ||||||||
| Sinanodonta pacifica Heude, 1878 | FRST_MFw_x664 | PP697614 | 3 | 3 | 4 | Sungai Redan, Kampung Lobang Batu, Silabur, Serian | Batang Sadong | 2.5 | 0/2 | 0/2 | N/A |
| 0.25 | 0/2 | 2/2 | 39.84 | ||||||||
| 0.025 | 0/2 | 2/2 | 38.70 | ||||||||
| Rectidens sumatrensis (Dunker, 1852) | FRST_MFw_x685 | MG591499 | N/A | N/A | N/A | Sungai Skibang 1, Serikin, Sibau | Sungai Sambas Besar | 2.5 | 4/4 | 0/4 | 22.66 |
| 0.25 | 4/4 | 0/4 | 25.78 | ||||||||
| 0.025 | 4/4 | 0/4 | 28.98 | ||||||||
- Note: Amplification above and below LOD, respectively, is given as the number of amplified samples (namplified) per number of replicate samples (nreplicates). FRST—Faculty of Resource Science & Technology, Universiti Malaysia Sarawak, Kota Samarahan, Sarawak, Malaysia.
Optimal annealing temperature for the qPCR primer pair (Table 2) was determined using a gradient PCR on nine 1:10 diluted genomic R. sumatrensis DNA samples (Table 1) in duplicate. Reactions consisted of 2x GoTaq PCR Mastermix (Promega), 0.1 mM of each Rec_sumF_475 and Rec_sumR_545 primers (Table 2), 2 μL template DNA, and nuclease-free water to 20 μL. Thermal cycling conditions were: initial denaturation of 5 min at 95°C, followed by 35 cycles of 45 s at 95°C, 45 s at annealing temperature ranging from 55°C to 66°C in 1°C intervals, and 45 s at 72°C, and a final extension of 10 min at 72°C. Amplicons were run on a 2% gel electrophoresis (25 min at 100 V) and reviewed visually.
Sensitivity of the qPCR assay was tested on a 10-fold dilution series of R. sumatrensis genomic DNA (Table 1; 6.38 ng/μL to 6.38 ng/μL × 10−9), run with 2 replicates for dilutions 6.38 ng/μL to 6.38 ng/μL × 10−2, 22 replicates for dilutions 6.38 ng/μL × 10−3 to 6.38 ng/μL × 10−9, and four negative PCR controls (nuclease-free water).
Specificity of the qPCR assay was tested using the four non-target freshwater mussel species (Table 3). qPCRs were run with a 10-fold dilution series of each of these species and R. sumatrensis (all 2.5 ng/μL to 2.5 ng/μL × 10−2) run with 2 replicates, 2 positive eDNA controls, and 4 negative PCR controls (nuclease-free water).
All subsequent qPCR reactions were setup in 96-well MicroAmp Fast Optical Plates (Thermo Fisher Scientific Inc., Waltham, MA) with reactions containing 2x GoTaq Probe qPCR Master Mix (for qPCR primer-probe; containing GoTaq Hot Start Polymerase, MgCl2, dNTPs and a proprietary reaction buffer and 2 μL/mL CXR Reference Dye, Promega), 0.5 mM of each primer, 0.25 mM of the Rec_sumP_495 probe (Table 2), 2 μL template DNA, and nuclease-free water to a total reaction volume of 10 μL. Thermal cycling conditions were: initial denaturation of 2 min at 95°C, followed by 55 cycles of 3 s at 95°C and 30 s at 60°C. Reactions were analyzed on a 7500 Fast Real-Time PCR System version 2.3 (Applied Biosystems).
2.3 Experimental Setup
Tank experiments to test the performance of a range of protocols to capture, preserve, and extract eDNA were conducted on 21 and April 22, 2022 at the Aquatic Biology laboratory at the Faculty of Resource Science and Technology, Universiti of Malaysia Sarawak, Kota Samarahan, Borneo, at 25°C–27°C room temperature. To test the performance of eDNA protocols under different environmental conditions, on 17/04/2022, mussel eDNA-free water was collected using a pump and 25 L-containers from two sites with differing land use and water chemistry, i.e., the man-made Prau Lake situated within the Universiti Malaysia Sarawak campus (1.4602 N, 110.4534 E) and Sungai (River) Beri Gru'Ong situated within a forest reserve (1.3512 N, 110.3864 E). Sites were known to be devoid of freshwater mussels, including upstream sections of the river. In the laboratory, four 80 L-capacity glass tanks each were set up with 40 L of lake and river water, respectively (Figure 1A). All equipment, including tanks, containers, and pump, had beforehand been sterilized with 2% sodium hypochlorite (NaOCl) active (bleach) and thoroughly rinsed with water. Tanks were aerated with an airstone until the start and throughout the experiment.
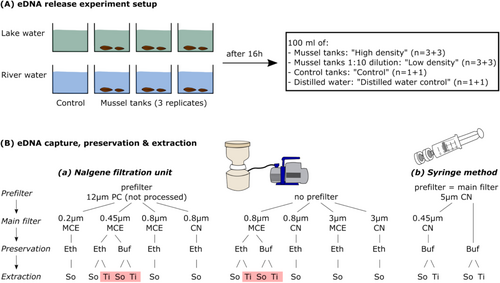
On 19/04/2022, 12 specimens of R. sumatrensis were collected from Sungai Sekerubong, Rajang River basin, Kanowit, Sarawak, Borneo, Malaysia, by hand, and the following day, transported to the laboratory in original river water and immediately placed in an additional aerated tank with river water. Mussels were arranged in two groups of “small” and “large” specimens, respectively.
The eDNA release experiment with the “Lake” water was conducted on April 20, 2022, and the “River” water experiment 24 h later. “Lake” and “River” experiments were conducted in different tanks and with different mussel specimens. On each occasion, one small and one large R. sumatrensis specimen was placed in each of three “Lake” or “River” tanks to attain similar mussel eDNA release (average total mussel wet weight per tank = 19.23 ± 1.96 g). All mussels were verified to be actively filtering during the incubation and were removed after 16 h using a sterilized net. One control tank per experiment was left without mussels (Figure 1A).
After incubation, four sample types were generated from both “Lake” and “River” experiments (Figure 1A). In each case, 100 mL of water was filtered (1) directly from the mussel incubation tanks (“high density” treatment, i.e., reflecting a population density of 2 individuals per 40 L, and thus, 50 individuals m−3 or m−2 assuming 1 m water depth); (2) after a 1:10 dilution of the mussel incubation water (“low density” treatment, i.e., reflecting a population density of 2 individuals per 400 L, and thus, 5 individuals m−3 or m−2 assuming a 1 m water depth); (3) from the incubation tank with no mussels (“controls”); and (4) from a source of distilled water (“distilled water controls”).
In total, 18 different eDNA protocols were tested on replicate 100 mL samples of each of the 16 water sources, respectively (Figure 1). Not all variables were tested in all combinations, due to factors such as the unavailability of all pore-size membranes in all membrane materials. The variables tested are summarized in Figure 1B and varied with respect to (1) eDNA capture method, i.e., filtration through (1a) a Thermo Scientific Nalgene Reusable Filter Unit and vacuum pump using 47 mm-diameter filter membranes (ca. 17.35 cm2 surface area), or (1b) a Terumo 50 mL Luer lock syringe using Sartorius 25 mm diameter (ca. 4.9 cm2 surface area; polycarbonate) filter holders and filters; (2) filter type, i.e., (2a) Mixed Cellulose Ester (MCE; all MF-Millipore) or (2b) Cellulose Nitrate (CN; 47 mm diameter—all Whatman, 24 mm diameter—all Sartorius); (3) filter membrane pore size (0.2, 0.45, 0.8, 3 and 5 μm); (4) pre-filters (i.e., Whatman Nucleopore Hydrophilic Polycarbonate Membrane 12 μm (PC) or 5 μm (Sartorius CN)) vs. no pre-filter; (5) preservative, i.e., 1 mL of 100% ethanol at −20°C vs. 1 mL Longmire's buffer (hereafter, “buffer”) at 4°C (constant storage temperatures following Mauvisseau et al. (2021)); and (6) DNA extraction kit, i.e., Qiagen DNeasy Blood & Tissue Kit vs. Qiagen PowerSoil ProKit (Figure 1).
The Nalgene filtration unit and tubing were sterilized between each sample by submerging in 2% NaOCl active (bleach) for 5 min, followed by thorough rinsing in water and subsequent sterilization under UV light for 20 min on each side. Syringes and filter holders were sterile and not reused. Ethanol-preserved filters were stored at −20°C, and buffer-preserved filters at 4°C until processing, with the exception of a ~36 h period of transportation from Malaysia to the University of Nottingham Life Science laboratories, UK, where samples were processed.
The 5 μm (pre-filter) and 0.45 μm membranes generated with the syringe filtration method were stored and extracted separately. After extraction, a subsample of eDNA from each size fraction extract was pooled equimolar and analyzed alongside the extracts from separate size fractions. The much larger 12 μm pre-filter membranes generated with the vacuum pump filtration method were not extracted.
2.4 eDNA Extraction
Ethanol-preserved filter membranes were removed from the ethanol and placed in sterile 1.5 mL Eppendorf tubes covered with pierced tin foil in an incubator in a fume hood at 56°C for 12 h to allow ethanol to evaporate while minimizing potential cross-sample contamination. The original ethanol collection tubes were retained at −20°C until the following day (i.e., the day of extraction) when they were centrifuged at 10,000 g and 4°C for 40 min to pellet remaining DNA in solution. Ethanol was poured out, and filter membranes were placed back in their original tubes and into the incubator for a further 3 h to remove any residual ethanol. 47 mm-diameter filter membranes were thereby cut into smaller pieces using sterilized microscissors upon replacement in their original tubes to optimize DNA lysis (also see e.g., Baudry et al. 2021). eDNA from buffer-preserved filter membranes was extracted without prior treatment except for cutting 47 mm-diameter filter membranes as explained above.
DNA extraction from filter membranes using the Qiagen DNeasy Blood & Tissue Kit followed the manufacturer's protocol with following modifications: The only modification for ethanol-preserved membranes was a 3-fold increase in ATL lysis buffer volume to completely cover filter membranes. For samples preserved originally in 1 mL of Longmire's buffer, buffers ATL and AL were omitted. Instead, to increase GuHCL concentration in the buffer-preserved samples, 8 M GuHCL (50 μL per 100 μL volume of Longmire's buffer) and 100% ethanol (100 μL per 100 μL Longmire's buffer) were added. Custom Proteinase K solution (10 μL per 100 μL Longmire's buffer) was used to achieve active unit concentration equivalent to kit specification. For all sample types, DNA was eluted twice with 50 μL of AE buffer with 5 min incubations at 37°C. DNA extraction using the Qiagen PowerSoil ProKit followed the manufacturer's protocol with Longmire's buffer replacing the CD1 buffer in buffer-preserved samples.
All samples were extracted in a dedicated eDNA extraction-only room in a UV-sterilized laminar flow hood to minimize cross-contamination. The qPCR Master Mix preparation, eDNA addition, and qPCR analysis were conducted in separate hoods to further control and minimize contamination. Negative laboratory controls were extracted regularly (i.e., with each batch) and amplified via qPCR as below.
2.5 qPCR For eDNA Detection
To amplify R. sumatrensis eDNA, qPCRs were carried out as detailed above (Section 2.2). Five technical replicates were run for each eDNA extract. For Lake-Syringe kit samples only, we additionally tested the performance of combined eDNA extracts from 5 μm and 0.45 μm pore-size filter membranes. For that purpose, equal parts of the two paired DNA extracts per sample were combined and concentrated by including 4 μL of pooled template per reaction. Reaction volumes were adjusted to retain the same concentrations as above.
To ensure comparability of data across plates, a dilution series of R. sumatrensis genomic DNA (Table 1; 6.38 ng/μL to 6.38 × 10−4 ng/μL) was run in duplicate, along with two replicate eDNA samples (positive controls; one from each low density-lake and river tank, respectively; both captured with Nalgene filter unit, preserved in buffer, and extracted with Qiagen DNeasy Blood & Tissue Kit) and two negative controls (nuclease-free water; all 2 μL) per plate.
2.6 Inhibition Tests
Inhibition of the qPCR assay was tested using an exogenous internal positive control (IPC) following Carraro et al. (2017) on 14 eDNA extracts (i.e., five lake samples, five river samples, and four distilled water-control samples), covering all filter pore sizes, preservation, and extraction methods tested (Figure 1), run in duplicates and with four negative PCR controls (nuclease-free water). Reactions were run with 5 μL 2x GoTaq Probe qPCR Master Mix, 0.05 mM of each MIMf and MIMr primer, 0.25 mM of the IPC probe, 1 μL of the IPC template (1 × 10−7 mM), 2 μL DNA, and nuclease-free water to equal a total reaction volume of 10 μL. Thermal cycling regime and reaction analysis followed Carraro et al. (2017). Inhibition was considered significant if the amplification of the IPC template in a reaction with an eDNA sample added showed a shift in the quantification cycle number (Cq) of three or more relative to the reactions with only PCR-grade water added (Hartman et al. 2005).
2.7 Data Analysis
Limit of detection (LOD) and limit of quantification (LOQ) were calculated based on Cq-values obtained from qPCRs on the dilution series of R. sumatrensis genomic DNA (see above). LOQ was determined as the lowest template concentration that could be reliably quantified at 95% confidence (Armbruster and Pry 2008). For this purpose, two log10-regression models were fitted to the DNA concentration vs. Cq-values obtained from the genomic dilution series and compared. Firstly, the “predictive” model was obtained by fitting data for dilutions 6.38 ng/μL to 6.38 × 10−2 ng/μL and projected to 6.38 × 10−9 ng/μL. Secondly, the “observed” model was obtained by fitting data across the whole dilution series. LOQ was set where the observed values started to divert from the predictions and fell outside of the corresponding 95% confidence interval. Beyond this limit, the assay can no longer reliably quantify samples with a lower concentration.
To assess the LOD, we examined the success rate of amplifications across all replicates at a given dilution. Any observed amplification was classed as a success, and no amplification was regarded as a failure. The absolute LOD was set at the concentration where at least one of five replicates was successfully amplified (20%). We then ran another qPCR using the same conditions and only the tissue dilutions 6.38 × 10−6 ng/μL and 6.38 × 10−7 ng/μL, alongside a 1:5 dilution of 6.38 × 10−6 ng/μL for a concentration between these two dilutions, with 20 replicates per dilution. The LOD was set where at least 5% of these replicates were successfully amplified. The efficiency of the assay was calculated as [10(−1/slope) – 1] × 100%.
A sample was considered as positively detecting R. sumatrensis eDNA if (1) at least 80% (i.e., four of five) of qPCR replicates amplified above the LOD (i.e., Cq < 38.4; see Section 3); (2) amplification curves showed a regular and clear transition into a steady exponential phase from a uniform and stable baseline (with an even normalization to the reference dye (ROX)); and (3) there was no amplification in the corresponding laboratory or qPCR controls. Detection rates for samples amplifying below the LOD (i.e., at Cq > 38.4) are also reported below as “sub-LOD” amplifications.
To assess statistically significant differences in Cq-values among different water sources, mussel densities, and methodologies of DNA capture and extraction, General Linear Models (GLMs) were run on data collected from buffer-preserved samples in R-version 3.6.3, fitting Cq-values (including those from sub-LOD amplifications) as the response variable and (1) DNA extraction method, (2) filter membrane pore size, (3) water type, and/or (4) mussel density as factors with two levels, respectively.
3 Results
3.1 qPCR Assay Development, Optimization, and Validation
Gradient PCR revealed strong amplification across the dilution series of genomic DNA. qPCRs were therefore run with a 60°C annealing temperature, which is the optimal annealing temperature recommended for the polymerase/Master Mix formulation used. There was no evidence for a shift in Cq value in IPC amplification when eDNA was introduced to the reactions, indicating that eDNA samples were not inhibiting the assay.
There were considerable numbers of mismatches between the developed primer/probe sequences and available COI sequences of non-target taxa (Table 3). No amplification of non-target taxa occurred above the LOD in any of the tissue extractions, although sub-LOD amplification occurred in all four species in one to four of six replicates (Table 3). Based on our occurrence criteria, none of these detections would have been regarded as positive for R. sumatrensis.
qPCR assay efficiency was 102%. The LOQ for the assay was determined as 6.38 × 10−6 ng/μL or a Cq of 36.72. The LOD was determined as a concentration of 6.38 × 10−7 ng/μL, referring to a Cq of 38.4.
3.2 Variation in Rates of False Positives, eDNA Detection Rates and Cq-Values Across Protocols
No amplification was observed in any of the negative laboratory controls.
Only one out of the 120 samples for which eDNA was captured with the Nalgene unit using one of the 10 protocols involving an ethanol-preservation step (at −20°C) fulfilled the requirements for positive R. sumatrensis eDNA detection (i.e., amplifying at Cq < 38.4 in at least four out of five qPCR replicates) (Table 4). In addition, one qPCR replicate of a corresponding distilled water sample, which had been preserved in ethanol and was extracted with the Qiagen DNeasy Blood & Tissue Kit, amplified above the LOD threshold (Table 4).
| Filtration | Prefix. (μm) | Main filter (μm) | Preservation | Extraction | Lake | River | C-Lake | C-Riv | DWLake | DWRiv | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low density | High density | Low density | High density | |||||||||||||||||
| Nalgene unit & vacuum pump | 12PC | 0.2-MCE | Ethanol | Soil | 0 (0.2) | 0.2 | 0 (0.2) | 0 | 0 | 0 | 0 (0.4) | 0 | 0 | 0.2 | 0 | 0 (0.2) | 0 | 0 | 0 | 0 |
| 12PC | 0.45-MCE | Ethanol | Soil | 0 (0.2) | 0 | 0 (0.2) | 0 | 0 | 0 (0.4) | 0.4 (0.4) | 0 | 0 | 0 | 0 | 0.2 | 0 (0.2) | 0 | 0 | 0 | |
| 12PC | 0.45-MCE | Ethanol | Tissue | 0 (0.2) | 0.2 (0.2) | 0.4 (0.2) | — | — | — | 0 | 0.8 (0.2) | 0 | — | — | — | 0 (0.2) | 0 (0.4) | 0.2 (0.2) | 0 | |
| 12PC | 0.45-MCE | Buffer | Soil | 0.6 | 0.4 | 1.0 | — | — | — | 0.6 | 1.0 | 1.0 | — | — | — | 0 | 0 | 0 | 0 | |
| 12PC | 0.45-MCE | Buffer | Tissue | 0.8 | 1.0 | 1.0 | — | — | — | 1.0 | 1.0 | 1.0 | — | — | — | 0 | 0 | 0 | 0 | |
| 12PC | 0.8-MCE | Ethanol | Soil | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0.4) | 0 | 0 | 0 | 0 | 0 | |
| 12PC | 0.8-CN | Ethanol | Soil | 0 | 0.2 | 0.2 | 0 | 0.2 | 0 | 0 | 0.2 | 0.2 | 0 (0.2) | 0 | 0 | 0 | 0 | 0 | 0 | |
| None | 0.8-MCE | Ethanol | Soil | 0 | 0 | 0 | 0 (0.2) | 0.4 | 0 | 0 | 0 | 0 | 0 (0.2) | 0 (0.2) | 0 | 0 | 0 | 0 | 0 | |
| None | 0.8-MCE | Ethanol | Tissue | 0 | 0.2 | 0 (0.4) | — | — | — | 0 (0.6) | 0.4 (0.6) | M | — | — | — | M | 0 (0.4) | 0 | M | |
| None | 0.8-MCE | Buffer | Soil | 0.6 (0.4) | 1.0 | 0.8 (0.2) | — | — | — | 0.4 | 1.0 | 1.0 | — | — | — | 0 | 0 | 0 | 0 | |
| None | 0.8-MCE | Buffer | Tissue | 1.0 | 1.0 | 1.0 | — | — | — | 1.0 | 1.0 | 1.0 | — | — | — | 0 | 0 | 0 (0.6) | 0 | |
| None | 0.8-CN | Ethanol | Soil | 0 | 0 | 0 | 0 | 0 (0.2) | 0 | 0 (0.4) | 0 | 0 | 0 | 0 | 0 (0.6) | 0 | 0 | 0 | 0 | |
| None | 3.0-MCE | Ethanol | Soil | 0 (0.2) | 0 | 0 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0.2 | 0 (0.2) | 0 | 0 | 0 | 0 | 0 | |
| None | 3.0-CN | Ethanol | Soil | 0 (0.2) | 0 | 0 (0.4) | 0 | 0 | 0 | 0 (0.2) | 0 (0.2) | 0 | 0 (0.4) | 0 (0.2) | 0 | 0 (0.2) | 0 | 0 | 0 | |
| Syringe kit | None | 5-CN | Buffer | Tissue | 1.0 | 0.8 (0.2) | 0.8 (0.2) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0 | 0 (0.4) | 0 (0.2) | 0 |
| None | 5-CN | Buffer | Soil | 0.6 | 0.8 (0.2) | 0.4 (0.2) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.8 (0.2) | 1.0 | 1.0 | 1.0 | 0 | 0 | 0 | 0 | |
| 5-CN | 0.45-CN | Buffer | Tissue | 0.4 (0.2) | 0 (0.2) | 0.4 (0.4) | 1.0 | 1.0 | 1.0 | 1.0 | 0.8 (0.2) | 0.2 (0.6) | 1.0 | 1.0 | 1.0 | 0 | 0 | 0 | 0 (0.2) | |
| 5-CN | 0.45-CN | Buffer | Soil | 0.4 (0.2) | 1.0 | 0 (0.6) | 1.0 | 1.0 | 0.6 (0.2) | 0.6 (0.4) | 1.0 | 0.6 (0.4) | 1.0 | 1.0 | 0.8 | 0 | 0 | 0 | 0 (0.4) | |
| 5-CN + 0.45-CN | Buffer | Tissue | 0.6 (0.4) | 0 (0.4) | 0.4 (0.6) | 1.0 | 1.0 | 1.0 | — | — | — | — | — | — | 0 | — | 0 | — | ||
| 5-CN + 0.45-CN | Buffer | Soil | 0.2 (0.4) | 0.6 (0.4) | 0.2 (0.2) | 1.0 | 1.0 | 1.0 | — | — | — | — | — | — | 0 | — | 0 | — | ||
Buffer-preservation in Nalgene-unit samples resulted in a considerably higher eDNA detection rate than ethanol-preservation and no false positives above the LOD (Table 4). Although buffer-preservation was tested only on low-density samples, R. sumatrensis eDNA was positively detected in 19 out of 24 samples (79%). A 100%-detection rate was achieved across all buffer-preserved samples extracted with the Qiagen DNeasy Blood & Tissue Kit, for which eDNA was collected either with a 12 μm-PC pre-filter followed by a 0.45 μm-MCE filter or a 0.8 μm-MCE filter without pre-filter. However, three qPCR replicates of one of the DW controls (Lake, 0.8 μm-MCE) that were extracted with the Qiagen DNeasy Blood & Tissue Kit also amplified, albeit below the LOD threshold (Table 4). No amplification in negative controls was observed in buffer-preserved samples extracted with the Qiagen PowerSoil ProKit, which recovered 50% and 67% detection rates in 0.45 μm-MCE and 0.8 μm-MCE samples, respectively.
Across all buffer-preserved samples of the Nalgene experiment, R. sumatrensis eDNA amplification success rate was higher in Tissue Kit- compared to Soil Kit-extracts, River- compared to Lake-samples, and 0.8 μm-MCE compared to pre-filtered 0.45 μm-MCE samples, respectively (Table 5a). The type of DNA extraction method, filter membrane pore size, and water type all significantly affected Cq-values (GLM: F3,105 = 43.25, p < 0.0001, R2adjusted = 0.54; see Table 6a for results on each factor). Significantly lower Cq-values were recovered for samples (1) for which DNA was extracted using the Tissue- compared to the Soil Kit, (2) for which eDNA was collected with a pre-filtered 0.45 μm compared to a 0.8 μm-MCE filter membrane, and (3) from river compared to lake water (Table 5a, Figure 2a). Thus, although a greater proportion of 0.8 μm-MCE compared to pre-filtered 0.45 μm-MCE samples amplified above the LOD, those 0.45 μm-MCE samples that amplified above the threshold did so earlier (i.e., at lower Cq-values).
| n | % amplified | % amplified above LOD (i.e., at Cq < 38.4) | Cq-values (Mean ± SD across all amplified samples) | |
|---|---|---|---|---|
| (a) Dataset: Nalgene filtration unit eDNA capture only | ||||
| Soil | 60 | 83.3 | 78.3 | 36.77 ± 1.20 |
| Tissue | 60 | 98.3 | 98.3 | 35.63 ± 1.19 |
| 0.45-pf | 60 | 86.7 | 86.7 | 35.9 ± 1.32 |
| 0.8 | 60 | 95 | 90 | 36.39 ± 1.27 |
| Lake | 60 | 90 | 85 | 36.94 ± 1.12 |
| River | 60 | 91.7 | 91.7 | 35.39 ± 1.01 |
| (b) Dataset: Syringe eDNA capture only | ||||
| High | 120 | 98.3 | 97.5 | 34.31 ± 1.65 |
| Low | 120 | 86.7 | 69.2 | 37.14 ± 1.48 |
| Soil | 120 | 91.7 | 81.7 | 35.83 ± 2.03 |
| Tissue | 120 | 93.3 | 85.0 | 35.44 ± 2.18 |
| 0.45-pf | 120 | 88.3 | 74.2 | 36.23 ± 1.99 |
| 5 | 120 | 96.7 | 92.5 | 35.10 ± 2.08 |
| Lake | 120 | 86.7 | 75.8 | 36.51 ± 1.63 |
| River | 120 | 98.3 | 90.8 | 34.87 ± 2.19 |
- Note: High, high mussel density samples; Lake, samples from lake-water tank; Low, low mussel density samples; River, samples from river-water tank; Nalgene, eDNA captured using Nalgene filtration unit with vacuum pump; Soil, eDNA extracted using Qiagen PowerSoil ProKit; Syringe, eDNA captured using Luer lock-Syringe method; Tissue, eDNA extracted using Qiagen DNeasy Blood & Tissue Kit; 0.45-pf, eDNA collected by filtering through a pre-filter and 0.45 μm-MCE (for Nalgene-samples) or -CN (for syringe-samples) filter membrane; 0.8, eDNA collected by filtering through a 0.8 μm-MCE filter membrane; 5, eDNA collected by filtering through a 5 μm-CN filter membrane.
| Factor | t | p |
|---|---|---|
| (a) Dataset: Nalgene filtration unit eDNA capture only | ||
| DNA extraction method (Qiagen DNeasy Blood & Tissue Kit vs. Qiagen PowerSoil ProKit) | −6.512 | < 0.0001 |
| Filter membrane pore size (0.45 μm-MCE with 12 μm-pre-filter vs. 0.8 μm-MCE filter) | 2.105 | 0.0377 |
| Water type (lake vs. river) | −8.845 | < 0.0001 |
| (b) Dataset: Syringe eDNA capture only | ||
| DNA extraction method (Qiagen DNeasy Blood & Tissue Kit vs. Qiagen PowerSoil ProKit) | −1.273 | 0.205 |
| Filter membrane pore size (0.45 μm-CN with 5 μm-pre-filter vs. 5 μm-CN filter) | −1.020 | 0.309 |
| Water type (lake vs. river) | −10.856 | < 0.0001 |
| Mussel density (high vs. low) | 17.297 | < 0.0001 |

All samples processed with the syringe kit were preserved in buffer. R. sumatrensis eDNA was positively detected in 37 out of 48 single-membrane samples (77%; i.e., not including pooled 5 μm- and 0.45 μm-CN membrane extracts), with no false positives above the LOD (Table 4). A 100%-detection rate was achieved across samples collected with a 5 μm-CN filter and extracted with the Qiagen DNeasy Blood & Tissue Kit (Table 4). However, at least one qPCR replicate of one or two of the negative control samples also amplified—albeit below the LOD—for both protocols involving the Qiagen DNeasy Blood & Tissue Kit and one protocol involving the Qiagen PowerSoil ProKit, respectively. No amplification in negative controls was observed in the 5 μm-CN—Qiagen PowerSoil ProKit combination, which recovered an 83% detection rate across all samples.
At high mussel densities, detection rates for pooled 5 μm- and 0.45 μm-CN membrane extracts (i.e., 5-CN + 0.45-CN samples in Table 4) were similar to extracts from > 5 μm- (i.e., 5-CN Main filter) and 5–0.45 μm fractions (i.e., 5 μm prefilter and 0.45 μm main filter). At low mussel densities, the pooled extracts failed to detect R. sumatrensis in all replicate tanks, with detection rates being considerably lower than for the > 5 μm-fraction and similar to the 5–0.45 μm fraction (Table 4).
Across all samples of the Syringe experiment (not including pooled 5 μm- and 0.45 μm-CN membrane extracts), R. sumatrensis eDNA amplification success rate was higher in high-density compared to low-density samples, Tissue Kit compared to Soil Kit extracts, River compared to Lake samples, and 5 μm-CN compared to 0.45 μm-CN samples, respectively (Table 5b). Cq-values were significantly affected by mussel density and water type but not by the type of DNA extraction method or filter membrane pore size (GLM: F4,217 = 100.1, p < 0.0001, R2adjusted = 0.64; see Table 6b for results on each factor). Significantly lower Cq-values were recovered for samples (1) from high-density compared to low-density treatments, and (2) river compared to lake water (Table 5b, Figure 2b,c).
4 Discussion
4.1 A qPCR Protocol for Detecting the Tropical Freshwater Mussel Rectidens sumatrensis
Our study presents the first qPCR assay for a tropical freshwater mussel, i.e., the Southeast Asian species R. sumatrensis. Our validation procedure has shown that the assay is specific, efficient, and able to detect and quantify R. sumatrensis DNA at concentrations above 6.38 × 10−7 ng/μL and 6.38 × 10−6 ng/μL, respectively.
4.2 Effects of eDNA Capture, Preservation, and Extraction Methods on eDNA Detection Rates
Of the 18 different protocols for capturing, preserving, and extracting freshwater mussel eDNA tested in our study, eight resulted in a high eDNA detection rate by subsequent qPCR with no to minimal occurrence of positive controls. The single unifying characteristic across these successful protocols was the preservation of filter membranes in Longmire's buffer rather than absolute ethanol. In general, the amplification success rate was particularly high and Cq-values were particularly low for filter membrane pore sizes > 0.45 μm (without pre-filtering) and when extracting DNA with a Tissue- rather than Soil Kit. The protocols performed well across both tropical water types tested, albeit generally better in river- compared to lake samples, and at densities as low as 5 individuals m−3 or m−2 assuming a 1 m water depth. While this may still be considered a relatively high density for many freshwater mussel species (see e.g., Smith 2006; Simeone et al. 2021), considering that only a comparatively low amount of water was filtered in our experiments (i.e., 100 mL), our protocols can be expected to perform well at even lower population densities when filtering larger water volumes (commonly 0.5 L up to > 30 L; e.g., Dysthe et al. 2018; Gasparini et al. 2020; Preece et al. 2021; Prié et al. 2021; Marshall et al. 2022; Prié et al. 2023). Importantly, the protocols presented here are cost- and time-efficient, utilizing equipment that can be at least partially reused and is readily available in most tropical countries.
Our study applied two different types of filtering equipment, i.e., a reusable filter unit with a vacuum pump vs. a Luer lock syringe with filter holders. Due to differences in pre-filter pore sizes (i.e., 12 μm for filter unit and 5 μm for syringe-system) and material in our experiment, our results do not allow for a direct comparison of the performance of these systems. However, both performed well, achieving detection rates of 77%–79% for buffer-preserved samples. A major advantage of the syringe approach compared to the filter unit approach is that sterilized syringe kits (including syringes, a pair of gloves, filter holders, filter membranes, forceps to handle filter membranes, and vials with buffer) can be pre-prepared for each field outing, thereby reducing the post-fieldwork time required to process collected samples and minimizing the risk of cross-contamination of samples from different sites.
Our results indicate the advantages of using filter-pore sizes of 0.8 μm for detecting tropical freshwater mussels through eDNA. Filter pore sizes > 0.45 μm (commonly > 1 μm) were also found to be highly effective in detecting freshwater mussel eDNA in, for example, North American rivers (Currier et al. 2018; Dysthe et al. 2018; Marshall et al. 2022) and Japanese lakes (Togaki et al. 2020; Sugawara et al. 2022). A trend of larger filter membrane pore sizes retrieving higher eDNA concentrations was also observed for Margaritifera margaritifera in Norwegian rivers, although this result did not account for the reduced water volume filtered through smaller pore sizes (Wacker et al. 2019). Regardless, considering the commonly high sediment loads and turbidity in tropical rivers and lakes (Dudgeon 1999) and the excellent performance of 0.8 and 5 μm filter membranes in our study, we recommend using large pore-size filters for future tropical freshwater mussel eDNA surveys, which will allow for filtering comparatively large water volumes, thus further reducing rates of false negatives.
Our study indicates that preservation in Longmire's buffer is by far the preferred method of preserving eDNA of tropical freshwater mussels compared to ethanol preservation. This result is in accordance with Majaneva et al. (2018), Kumar et al. (2020), and Huerlimann et al. (2020), who recommend buffer- rather than ethanol-preservation for tropical and other aquatic eDNA. Apart from resulting in a higher eDNA detection rate, buffer-preservation has a number of additional advantages to ethanol-preservation, including easier handling and transport (including via airplane) and removing the ethanol-evaporation step in the laboratory. Majaneva et al. (2018) highlighted that this step may increase the risk of contamination, which may have been the reason for the false positive in one qPCR replicate of one of our ethanol-preserved, Tissue Kit-extracted samples. Our results also confirm that buffer-preservation at 4°C maintains high-quality eDNA, with some studies showing evidence for effective protection against degradation even at temperatures of up to 45°C for up to 2 weeks (Renshaw et al. 2015; Majaneva et al. 2018). This provides a further argument for applying buffer-preservation on eDNA samples collected under challenging fieldwork conditions and remote locations in the tropics. An alternative preservation methodology not tested here is drying with silica gel, which has shown promising results in other studies (Dysthe et al. 2018; Majaneva et al. 2018).
The Qiagen DNeasy Blood & Tissue Kit is arguably the most commonly used kit for freshwater mussel eDNA extraction (see above). Our results confirm the excellent performance of this kit for extracting tropical freshwater mussel eDNA preserved in Longmire's buffer. This kit performed better than the Qiagen PowerSoil ProKit in terms of amplification success rates and Cq-values and positively detected R. sumatrensis across all mussel tanks in all buffer-preserved filter-unit samples and all buffer-preserved syringe-system samples captured with 5 μm-filter membranes. However, instances of amplification in distilled water and control tanks—albeit all below the LOD—were also more than twice as high in Tissue Kit- compared to Soil Kit samples (i.e., 7 vs. 2 qPCR replicates). Thus, the Qiagen PowerSoil ProKit, which includes an Inhibitor Removal Technology that increases the purity of isolated DNA (Qiagen 2013–2024b), maybe the preferred extraction method in some instances due to its lower susceptibility to false positives whilst retaining relatively high amplification and detection rates, particularly in combination with membrane pore sizes ≥ 0.8 μm (positively detecting R. sumatrensis in 14 out of 18 (78%) buffer-preserved samples). However, the costs per sample are more than twice as high for the Qiagen PowerSoil ProKit (Qiagen 2013–2024b) compared to the Qiagen DNeasy Blood & Tissue Kit (Qiagen 2013–2024a).
4.3 Effects of Water Type and Inhibition
No PCR inhibition was observed in our samples, which were taken from a tropical lake and river, respectively. However, PCR inhibition is a common problem in eDNA from tropical water bodies, due to organic compounds from phytoplankton and/or terrestrial input, particularly during the monsoon season (Matheson et al. 2010; Robson et al. 2016; Huerlimann et al. 2020). A commonly applied strategy in these instances is the dilution of eDNA extracts (Cantera et al. 2019; Cilleros et al. 2019; Gasparini et al. 2020), which, however, reduces eDNA concentrations in the PCR. Alternatives include post-extraction inhibitor-cleanup processes, cleanup columns (McKee et al. 2015; Robson et al. 2016), and the Zymo OneStep PCR inhibitor (Zymo Research) (Dokai et al. 2023). The Qiagen PowerSoil ProKit or similar kit eliminates inhibitors already in the course of the eDNA extraction process.
Whilst eDNA detection rate was higher and Cq-values were lower in river- compared to lake-water, respectively, it is difficult to identify the reason(s) for this discrepancy. Potential explanations for this pattern include differences in eDNA shedding rates and differences in eDNA particle binding to suspended solids in the two water types (Stoeckle et al. 2017). Specifically, eDNA shedding rates were potentially higher in river water because it likely provided conditions more similar to the natural habitat of R. sumatrensis than the lake water (Zieritz and Lopes-Lima 2018).
4.4 Conclusions and Outlook
Whilst our study provides the first eDNA protocols specifically targeted towards detecting tropical freshwater mussels, many questions remain unanswered. Due to the requirement of live specimens in tank experiments, for ethical reasons, we here focused on the most common species in Borneo. Thus, the application of the eDNA protocols on other tropical mussel species will require the development of new qPCR assays. Multi-species approaches, such as eDNA metabarcoding (e.g., Prié et al. 2021) or multiplex assays (e.g., Rodgers et al. 2020) may thereby be more cost-effective than species-specific approaches depending on the study system and aim. Field studies comparing eDNA detection rates to data from traditional hand surveys will be needed to validate eDNA protocols in the field. These should be conducted at different times of the year, as eDNA concentrations and degradation rates of freshwater mussels have been shown to vary significantly across seasons, partly due to temporal patterns in larval release periods (Wacker et al. 2019; Lor et al. 2020; Sugawara et al. 2022). Freshwater mussel eDNA transport has been shown to be extremely variable across previous studies in temperate freshwater systems, ranging from < 1 m to dozens of km (downstream) downstream of populations (Deiner and Altermatt 2014; Stoeckle et al. 2016; Lor et al. 2020; Steiner et al. 2022), but no data are available for tropical systems. The spatial resolution of sampling sites and the number of replicate samples per site will need to be adjusted depending on the specific conditions at the site and the season of sampling. An exciting prospect for future surveying of tropical freshwater mussel eDNA is passive samplers, which have been shown to adsorb mussel eDNA from temperate freshwater environments (Kirtane et al. 2020). Whilst eDNA is an exceptionally promising tool for tropical freshwater mussel and other biodiversity monitoring, traditional surveys using hand sampling will still be required to monitor population demography and certain other conservation-relevant data (Stoeckle et al. 2016).
Author Contributions
A.Z., J.-J.W., and H.H. conceptualized and designed the study. A.Z., H.F.b.K., F.M., K.A.A.R., T.R., J.J.W., and H.H. contributed to the acquisition, analysis, and interpretation of the data. A.Z., F.M., K.A.A.R., T.R., J.J.W., and H.H. wrote the manuscript.
Acknowledgments
This project was funded by Technology Grant NGS-61128T-19, granted to A.Z. and K.A.A.R. by the National Geographical Society. Fieldwork was conducted under Permit No (153)JHS/NCCD/600-7/2/107 by the Sarawak Forest Department. We thank the local community at Gru'Ong who gave us permission to collect water from their stream for laboratory experiments. Our warmest thanks go to “Uncle” Jawan and the Iban community at Kanowit for guiding us through their forest and giving us permission to sample mussels from their river. We also thank Syaifudin bin Bojeng and Muhammad Iqbal Hafizi bin Khairul Adha for their invaluable support during field and laboratory work.
Ethics Statement
Fieldwork for this project was conducted under Permit No (153)JHS/NCCD/600-7/2/107 by the Sarawak Forest Department. The research meets the ethical guidelines of the study country (i.e., Malaysia and the UK).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




