Promoting Community-Led Monitoring of Taonga (Treasured) Species and Freshwater Health Through eDNA Metabarcoding
Funding: This work was supported by the New Zealand Ministry for Business, Innovation and Employment's Our Land and Water (Toitū te Whenua, Toiora te Wai) National Science Challenge, contract C10X1901 as part of the “eDNA as a holistic measure of pastoral landscape effects on taonga species” Rural Professionals Fund’ (2022–2023) and Impact Extension Fund (2023–2024). AC and RC were funded through the AgResearch Strategic Science and Investment Fund—“Food Integrity.”
ABSTRACT
In response to intensified agricultural impacts on Aotearoa New Zealand's river systems, our study sought to demonstrate a novel approach for assessing freshwater quality through environmental DNA (eDNA) and promote a holistic understanding of ecological health. Employing 13 eDNA tree-of-life metabarcoding assays alongside 11 standard physicochemical attributes, we generated a comprehensive temporal and spatial dataset along a 3.5 km section of an upper river catchment. The study catchment moves from a culturally significant native bush area, through sheep and beef farming, to an organic dairy farm, capturing the transition through varying land uses. Our analysis focused on the detections of key culturally significant taonga (treasured) species, known animal sources of fecal contamination, and the taxon-independent community index (TICI) as a measure of ecological health. We identified 479 species including whīo/native blue duck, long and short finned tuna/eels, kaharore bully/cockabully, kōkopu/dwarf galaxias, and kōura/freshwater crayfish. Although Escherichia coli (E. coli) levels did not significantly vary across sites, eDNA sequence counts of cattle and deer were significant predictors of a site's E. coli levels, suggesting that eDNA could be a valuable indicator of fecal contamination sources. TICI scores were strongly correlated with changes in water quality attributes (Adj-R2 = 0.92) and consistently detected subtle declines driven by increased pastoral land use. Community leadership was central to our methodology, enabling local stakeholders, including mana whenua and farmers, to actively participate in water monitoring and data interpretation. This approach not only fulfilled regulatory requirements but also fostered a deep connection with the river, enhancing community-led conservation efforts. By enhancing the mauri (life force) of the community through active participation and capacity building, this approach exemplifies sustainable, collaborative efforts in environmental management and revitalization.
1 Introduction
1.1 The Challenge
Global freshwater environments and biodiversity are increasingly threatened by human activities (Dudgeon et al. 2006; Grooten and Almond 2018; Reid et al. 2019; Vörösmarty et al. 2010) particularly intensive agriculture, which leads to sedimentation, nutrient pollution, and fecal contamination, degrading ecosystems (Allan 2004; Giri and Qiu 2016; Gluckman 2017; Joy et al. 2018; Julian et al. 2017; Larned et al. 2020, 2016; Ministry for the Environment and Stats NZ 2023). In Aotearoa New Zealand, recent legislation (Te Mana o te Wai—The prestige of water) (Ministry for the Environment 2020) and the National Policy Statement—Freshwater Management (NPS-FM, 2020) aims to restore and protect water resources, emphasizing the importance of water in regulatory decisions and the safe harvesting of “Mahinga kai”, Māori traditional foods. However, current monitoring metrics, focused on physicochemical qualities like E. coli and nutrient levels, fail to provide a comprehensive view of ecosystem health (Harmsworth et al. 2011; Karr 1999; Rapport et al. 1998; Ward and Pyle 1997), making them complex and less effective for assessing improvements (Karr and Dudley 1981).
Biotic indices (Fausch and Lyons 1990; Karr 1981; Ward and Pyle 1997), often based on macroinvertebrates (Stark 1985) and fish (Joy and Death 2004), simplify complex ecological data but have limitations (e.g., invasive, not readily scalable and monotrophic). These methods require taxonomic expertise, are costly, and are not ideal for comprehensive waterways modeling (Greenwood et al. 2015; Mächler et al. 2014). Additionally, they may not be the most sensitive indicators of ecological stress (Leese et al. 2018; Pawlowski et al. 2016; Piggott et al. 2015), limiting their effectiveness in assessing ecosystem health. These challenges hinder community involvement in environmental monitoring (as discussed in Peters et al. (2016)), highlighting the need for alternative strategies that meet regulatory needs while supporting local biodiversity, cultural restoration, and broader community engagement for more resilient environmental outcomes.
1.2 New Methodologies
Environmental DNA (eDNA) metabarcoding is a noninvasive molecular monitoring technique that uses microscopic genetic fragments naturally shed by organisms to survey biodiversity (Rees et al. 2014; Stat et al. 2017; Takahashi et al. 2023). Aseptic recovery of eDNA fragments can be collected from water using disposable gloves and a simple, handheld syringe filtration method, accessible for widespread participation. The collected material is preserved, then processed in a laboratory where purified DNA is amplified, sequenced, and compared with genetic reference databases to identify species (as reviewed in Ruppert et al. (2019); Deiner et al. (2017)). These tools for eDNA testing offer advantages for improved sensitivity and spatial coverage (Carraro et al. 2023; Carvalho et al. 2022; David et al. 2021; Mächler et al. 2019; Perry et al. 2024), improved specificity and taxonomic resolution for cryptic species (Beermann et al. 2018; Leese et al. 2018; Lim et al. 2016; Macher et al. 2016), and improved consistency between samplers, while minimizing harm by avoiding the need for physical capture or handling of organisms (David et al. 2021; Hering et al. 2018).
The widespread adoption of eDNA for biological monitoring (Compson et al. 2020; Rees et al. 2014; Takahashi et al. 2023; Valentini et al. 2016) is driven by clear applications in biodiversity, conservation (Department of Conservation and Toitū Te Whenua Land Information New Zealand 2023; Pfleger et al. 2016), biosecurity (Bowers et al. 2021; McDonald et al. 2020; Scriver et al. 2024; Zaiko et al. 2015), and ecological health assessments (Chariton et al. 2015; Pawłowski et al. 2020; Stoeck et al. 2018; Waters et al. 2024). In Aotearoa, the New Zealand Environmental Protection Authority's program, Wai Tuwhera o te Taiao—Open Waters Aotearoa, has empowered over 300 community groups, including iwi, hapū, schools/kura, and farmers, with access to eDNA tools and technical guidance (Bunce and Freeth 2022). These groups have used eDNA results to inform conservation efforts, enhance educational resources, and strengthen local narratives and storytelling, demonstrating grassroots leadership in environmental protection (Crowe 2024) (examples are highlighted through the program website: https://www.epa.govt.nz/community-involvement/open-waters-aotearoa/).
A key limitation of eDNA tools is their dependence on genetic reference databases, which often results in a large proportion of detected DNA remaining unmatched to known taxonomy, particularly lesser studied microorganisms (such as diatoms, bacteria, ciliates, and rotifers) recognized as powerful biological indicators (Caruso et al. 2016; Eiler et al. 2013; European-Committee-for-Standardization 2004; Kelly 1998; Montuelle et al. 2010; Niu et al. 2018; Sagova-Mareckova et al. 2021; Vasselon et al. 2017). This limits the interpretation and value of the eDNA data; however, to address this, taxonomy-free methods (Apothéloz-Perret-Gentil et al. 2017; Cordier et al. 2018; Feio et al. 2020; Pearman et al. 2022), including a newly developed taxon-independent community index (TICI) (Wilkinson et al. 2024), make use of sequence data such as amplicon sequence variants (ASVs) or operational taxonomic units (OTUs) rather than traditional taxonomic assignments to provide a measure of ecological health. The TICI, similar to the NZ Macroinvertebrate Community Index (MCI) (Stark 1985), incorporates 3000 diverse ecological indicators and provides a score (theoretical range from 60 to 140) to assess ecological health, making better use of unidentified eDNA data.
This approach integrates data into actionable scores, empowering communities to monitor ecology effectively. By building capacity and uniting stakeholders, it enhances social networks and resilience, leading to better environmental outcomes (Charles et al. 2020 and as discussed throughout Olsson et al. 2004). Thus, user-friendly and equitable monitoring tools provide actionable data, motivating communities to actively restore and protect their natural environments.
1.3 Our Approach
The Whanganui River, known as “Te Awa Tupua,” was recognized as an “indivisible and living whole” and granted legal personhood through the Te Awa Tupua (Whanganui River Claims Settlement) Act 2017. Inspired by the Māori whakataukī of the Whanganui River iwi, “Ko au te awa, ko te awa ko au”—“I am the river, the river is me,” this approach emphasizes the deep connection Māori have maintained from time immemorial with the waterways regarded as precious taonga (treasure) and vital for the health of the environment, the hapū and the whole community. This connection, grounded in environmental stewardship and cultural links to the land and water (kaitiakitanga) (McAllister et al. 2023), formed the foundation for our partnership formed between researchers, local farmers, Māori environmental groups, and mana whenua to integrate standard physicochemical water quality attributes with eDNA techniques. This collaboration identified taonga species, sources of fecal contamination, and calculated TICI scores, fostering community participation, reviving historical narratives, sharing Mātauranga (indigenous knowledge), and enhancing sustainable water management.
2 Materials and Methods
2.1 Study Site and Sample Collection
Our case study site area was situated within the upper reaches of the Manawatū River located in the lower North Island of Aotearoa New Zealand. Five sites with differing land cover and use profiles were chosen along a 3.5 km stretch of first and second-order streams. Land cover ranged from a pristine reference site in native bush cover, through hill country sheep and beef farming, to an organic dairy farm (see Figure 1 with overlaid catchment polygons and refer to Figure 2A,B for land cover information). The uppermost sites (S1 Reserve and S2 Whare) have high cultural significance and value to local mana whenua (those with authority over tribal land), Te Kāuru Eastern Manawatū hapū collective, and are focus areas for pest mammal trapping and native planting. The Ngāmoko Whare (S2) is one of eight Whare Mātauranga (similar to educational kiosks) built to provide a place for the community to gather and reconnect with the Manawatū River (awa) through the telling of stories of the area's history and whakapapa (genealogy) (read more about Tū Te Manawa here: https://www.manawaturiver.co.nz/activities/tu-te-manawa/). Te Miro Farm is an organic regenerative dairy farm with a herd size of approximately 350 dairy cattle and a dairy platform of 187 ha. Over 38 ha have been retired and planted since 2018 with ~20,000 native plants: 18 ha along the Manawatū River and 20 ha on farm (between S4 and S5, Figure 1).


The distance between sample sites ranged from 700 m to 1.1 km, allowing narrow spatial resolution to interrogate overall stream conditions as dominant land uses changed. These sites were sampled for standard water quality attributes and eDNA bimonthly on eight sampling occasions over the course of 16 months. These samples were collected in December 2022, February 2023, April 2023, June 2023, August 2023, October 2023, December 2023, and February 2024. The sample collection days were chosen to remain consistent with the local maramataka (Māori lunar calendar) (Warbrick et al. 2023) to follow optimal lunar phases for water-based fieldwork and biological activity for the study area. The chosen sampling days also took into account recent rainfall to avoid sampling where more than 10 mm had fallen in the previous 48 h, as recommended in Melchior and Baker (2023). Rainfall data was obtained from the closest Regional Council rainfall monitoring site (Manawatū at Āpiti Track, latitude −40°02′42.43″, longitude 176°08′34.43″) approximately 1.7 km southwest of the S4 Carpark site. Between the February 2023 and April 2023 sampling time points, a severe tropical cyclone, Cyclone Gabrielle, devastated parts of the northeastern side of New Zealand, including the study site, which sits at the southernmost extent of the impacted area. The closest rainfall gauge is thought not to have fully captured the true degree of the flooding, as the ranges anecdotally saw severe rates of flooding off the hills and there were changes to the river channel morphology (Drysdale & A. Paewai, personal communication, February 28, 2023).
Water quality samples were collected by sampling 1 L of the surface of the stream water column, retained below 10°C, and submitted for diagnostic testing within 8 h of sample collection. Water quality was tested once at every site (n = 5) at every time point (n = 8) (n = 40 overall). Standard water quality attribute data were determined for dissolved oxygen, pH, turbidity, nitrate, nitrite, ammonia, total nitrogen, dissolved reactive phosphorus, carbonaceous biochemical oxygen demand (BOD-carbonaceous), and conductivity using standard methods (American Public Health Association 2022) and performed by Central Environment Laboratories (Palmerston North, New Zealand). E. coli from 100 mL stream samples were also retained below 10°C and processed within 8 h of sampling, enumerated using Colilert Quanti-Tray assays (IDEXX Laboratories Inc., Maine, US) and incubated at 35°C for 24 h. Where E. coli MPN > 2419.6/100 mL, water samples were diluted to obtain an E. coli concentration that was within the Quanti-Tray 2000 range with subsequent recalculation to account for dilution.
eDNA samples were collected using custom-made eDNA syringe mini kits, each containing an encapsulated 30 mm diameter, 1.2 μm pore size cellulose acetate capture filter with Luer Lock inlet and outlet fittings, a 60 mL Luer Lock syringe, 400 μL DNA/RNA Shield preservation buffer (Zymo) preloaded in a 3 mL Luer Lock syringe, and a pair of sterile nitrile gloves. Six replicates were collected at each site (n = 5) and each time point (n = 8), following the best practice sampling protocol for characterizing local riverine communities in New Zealand (Melchior and Baker 2023). For each replicate, the 60 mL syringe was used to filter 1 L of stream water through the encapsulated capture filter. Residual water in the capture filter was then purged by forcing 60 cc of air through the filter, using the emptied 60 mL syringe. The material retained on the capture filter was then preserved by injecting the 400 μL DNA/RNA Shield preservation solution (Zymo) from the preloaded 3 mL syringe into the filter inlet with the filter outlet sealed off with a Luer Lock cap. Samples were sent by standard post to the laboratory in Wellington within 3 days of sample collection. Upon receipt, sample lysates were removed from their filters by applying a vacuum with a 3 mL syringe. Lysates were transferred to pre-labeled 1.5 mL low-bind tubes (Eppendorf) and stored at −20°C until further analysis.
DNA extraction, NGS library preparation, sequencing, and bioinformatics including providing TICI scores, were performed by Wilderlab (Wellington, New Zealand) and followed the methods for the “comprehensive metabarcoding panel” outlined in metabarcoding methods v 2.1.0 (Wilkinson et al. 2024; https://s3.ap-southeast-2.amazonaws.com/wilderlab.resources/methods/Wilderlab_metabarcoding_methods_2.1.0.pdf) and Wilkinson et al. (2024). For DNA extraction and purification, 200 μL of each sample lysate were loaded into a Genolution GD141 cartridge and run on the Nextractor NX-48S system (Genolution, Korea) using the standard extraction settings. DNA quality/quantity analysis, adapter-fusion, indexing, and amplification were carried out in single-step PCR reactions on an Applied Biosystems ProFlex PCR instrument. DNA extracts were PCR-amplified in duplicate using fusion-tag mitochondrial assays for the detection of target DNAs (see Table 1 for primer sequences and associated taxon targets). Fusion tag primers included Illumina P5 and P7 adapter sequences, Illumina TruSeq sequencing primer bind site (forward primer only), unique 8 or 9 bp index sequences, and locus specific primers, respectively. All indexes differed from each other by at least 3 bp. Each PCR reaction contained 3 μL MyTaq 2× Red Mix (Bioline) with 2 mg ml−1 BSA (Sigma Aldrich), 0.5 μL forward primer (10 μM), 0.5 μL reverse primer (10 μM), and 1.5 μL template DNA. PCR cycling conditions included an initial denaturation step of 3 min at 95°C, followed by 38 cycles of 5 s at 95°C, 10 s at the assay-specific annealing temperature specified in Table 1, and 15 s at 72°C. At least one negative control reaction containing 1.5 μL of DNase-free water (IDT) in place of the template DNA was included with each sequencing run. Sequencing libraries were pooled, cleaned and double-end size selected using AMPure XP magnetic beads (0.9x and 1.2x for lower and upper size bounds, respectively). The final pooled library concentration was determined using a Qubit 4 Fluorometer (ThermoFisher Scientific) and the concentration adjusted to 50 pM in sterile DNAse/RNAse free water (IDT). Each library was loaded onto an iSeq i1 V2 reagent cartridge with 5% Phi X and run for 200 cycles in a single direction on an Illumina iSeq 100 instrument.
| Assay | Gene | Temp | Forward Primer | Reverse Primer | Target | Ref |
|---|---|---|---|---|---|---|
| WV | mt16S | 58°C | GACGAGAAGACCCTWTGGAGC | CCRYGGTCGCCCCAAC | Vertebrates | [1] |
| RV | 12S-V5 | 58°C | TTAGATACCCCACTATGC | TAGAACAGGCTCCTCTAG | Vertebrates | [2] |
| LG | mt12S | 58°C | CGGCGTAAAGWGTGGTTAGG | CATAGTGGGGTATCTAATCCCAGTTTG | Fish | [3] |
| CI | COI | 45°C | DACWGGWTGAACWGTWTAYCCHCC | GTTGTAATAAAATTAAYDGCYCCTARAATDGA | Insects | [4] |
| BE | 18S-V9 | 52°C | CCCTGCCHTTTGTACACAC | CCTTCYGCAGGTTCACCTAC | General eukaryotes | [5] |
| BU | 18S-V9 | 52°C | TTGTACACACCGCCC | CCTTCYGCAGGTTCACCTAC | Microbes | [6] |
| EA | COI | 55°C | TATATAATGTTATTGTAACRGCGC | CCCARCATCAAAGGAATCAAYCA | Echyridella freshwater mussels | [7] |
| TP | trnL | 52°C | GGGCAATCCTGAGCCAA | CCATTGAGTCTCTGCACCTATC | Vascular plants | [8] |
| MZ | rbcL | 52°C | CTTCTTCAGGTGGAACTCCAG | GTCACCACAAACAGAGACTAAAGCAAGT | Vascular plants | [9] |
| UM | 16S-V5 | 52°C | GGATTAGATACCCTGGTA | CCGTCAATTCMTTTRAGTTT | Microbes | [10] |
| YG | cyt-B | 55°C | CBGAYATCTCYACCGCYTTYTC | AAAGAAAGATGCGCCRTTRGCATG | Galaxias spp. | [11] |
| ZP | mt16S | 55°C | ATATTCTTGTCACCCCAACAAAAC | AGGGACGATAAGACCCTATAAAGC | Paranephrops freshwater crayfish | [7] |
| XG | COI | 55°C | GCAATYTCCCAGTATCARACACC | GCAGCAAGMACGGGGAG | Gobiomorphus bullies | [7] |
- Note: Primer references are as follows: [1] Forward and reverse primers adapted from Nester et al. (2020), [2] Forward and reverse primers from Riaz et al. (2011), [3] Forward primer custom-developed and reverse primer from Miya et al. (2015), [4] Forward and reverse primers from Wilkinson et al. (2024), [5] Forward and reverse primers from Amaral-Zettler et al. (2009), [6] Forward and reverse primers from Amaral-Zettler et al. (2009), [7] Forward and reverse primers custom-developed (unpublished), [8] Forward and reverse primers from Taberlet et al. (2006), [9] Forward and reverse primers from Bradley et al. (2007), and [10] Forward primer from Morey et al. (2006) and reverse primer from Lane et al. (1985). “Temp” indicates PCR annealing temperature for each individual metabarcoding assay.
The sequence fastq files were de-multiplexed in R (R Core Team 2024) using the insect package (v 1.4.0; (Wilkinson et al. 2018)) and trimmed sequences filtered to produce a table of exact ASVs using the DADA2 package (Callahan et al. 2016). ASVs were identified to the lowest possible taxonomic rank using a global reference sequence database primarily compiled of trimmed reference sequences downloaded from GenBank (Benson et al. 2010). Any ASV matching with 100% identity and 100% coverage to at least one reference sequence was assigned at the lowest common ancestor level (LCA; i.e., assigned to genus level if matched to more than one species, or to family level if matched to more than one genus). Unmatched sequences > 50 bp in length were queried against the same reference database using the SINTAX classification algorithm (Edgar 2016) with a conservative assignment threshold of > 0.99 and taxon assignment restricted to genus level or above. Of note, any DNA identified as having human origin was excluded during the taxonomic assignment stage as per local standard operating procedures to alleviate privacy concerns (as raised in Whitmore et al. (2023)).
2.2 Data Exploration and Analysis
To visualize patterns in water quality attribute data across sites and time points, bar plots were generated using “ggplot2” (Wickham 2016), “ggbreak” (Xu et al. 2021), and “ggpubr” (Kassambara 2023) in R (R Core Team 2024). To compare the water quality attributes between sites, the attribute data from all time points were combined and considered as replicates. The data from BOD-carbonaceous and nitrite were excluded from further consideration as the values recorded across all sites and time points were “< 1” and “< 0.0005,” respectively. For each water quality attribute (conductivity, ammonia, nitrate, total nitrogen, dissolved oxygen, dissolved reactive phosphorus, pH, turbidity, and E. coli), an ANOVA was performed to determine whether significant differences occurred between the sites. This was performed using the “aov” function from the stats package in base R (R Core Team 2024). Where a statistically significant difference was found, a Tukey multiple comparisons of mean post hoc test was conducted using the “TukeyHSD” function also from the stats package in base R (R Core Team 2024).
Site TICI scores were calculated for each replicate eDNA sample by multiplying the mean tolerance value of the detected indicator ASVs by 20 (as described in Wilkinson et al. (2024)) falling within a theoretical range of 60–140, with low scores indicating poorer health and higher scores indicating better health. TICI scores from replicate samples were then averaged for further interpretation, though individual sample scores and summary output can be referred to in Appendix F. Line plots were generated using the “ggplot2” package (Wickham 2016) in base R (R Core Team 2024).
Nonmetric multidimensional scaling (NMDS) was performed to visualize the dissimilarity in ASV composition across the different sites and time points. For this, the “metaMDS” function from the “vegan” R package (Oksanen et al. 2022) was used to perform NMDS with Hellinger dissimilarity and a monotone regression (monoMDS also from the “vegan” package). Hellinger dissimilarity was used to reduce the effect of possible differences in sequencing depth between samples. Both site and species scores were calculated using the default parameters from the “scores” function within the “vegan” package (Oksanen et al. 2022) and included on the visualizations. Ellipses were added using the “geom_mark_ellipse” function from the R package “ggforce” (Pedersen 2024) to highlight grouping arrangement by site across the time points.
Multiple linear regression was performed to explore the relationship between site E. coli levels and the detected DNA sequence counts from common fecal source animals known to be in the area (cattle, deer, possum, and sheep; see more detail in Appendix A). Note that humans were excluded from the scope of known sources as human-associated DNA was filtered out of the final eDNA results as per the local standard procedure; however, known human density in the catchment was less than 10 persons in total. The validity of the regressions was determined by assessing the test assumptions, namely that the residuals follow a normal distribution, the residuals show constant variance (homoscedasticity), and that there are no influential points. Normality was tested using the Shapiro–Wilk normality test (“shapiro.test” function from base stats package in R (R Core Team 2024), homoscedasticity was tested using the studentized Breusch–Pagan test (‘bptest’ function from the ‘lmtest’ R package (Hothorn et al. 2015)), and influential points were investigated using Cook's distance (“cooks.Distance” function from base R package, “stats” (R Core Team 2024)). A natural logarithmic transformation was applied to E. coli counts due to the residuals not following the normal distribution. The measure from S3 Upstream at the February 2023 time point was shown as very likely to be influential based on the Cook's distance value (Cook's distance > 1.5) and was therefore removed from further regression analysis.
Multiple linear regression was also used to explore how different water quality attributes affected a site's average TICI score. First, a mixed-effects model was fitted with site as a random effect, and all nine water quality attributes and time included as fixed effects. Model selection via bidirectional stepwise linear regression was then used to determine which water quality attributes may be good predictors of the average TICI score. The “lme4::lmer” function (Bates et al. 2015) was used to fit the mixed-effects models. Stepwise linear regression was carried out with the “step” function from the “stats” package in base R (R Core Team 2024). Due to limitations in this function, random effects were removed when carrying out the stepwise linear regressions, but site as a random effect was still included in the final model. Both Akaike (AIC) and Bayesian (BIC) information criteria were used to select the most informative attributes to include in the model, where lower AIC and BIC scores indicate a more balanced model that is neither over nor under fit.
3 Results
3.1 Water Quality Attributes
Water quality attributes were more variable and indicative of reduced quality at the lower sites (S3–S5) compared to the upper sites (S1 and S2). This was particularly observable in levels of turbidity (Figure 3B), total nitrogen (Figure 3C), and nitrate (Figure 3D). This pattern was further supported by one-way ANOVAs, which showed statistically significant differences between the sites in conductivity (F4,35 = 33.76, p < 0.001), turbidity (F4,35 = 4.78, p = 0.00351), total nitrogen (F4,35 = 22.92, p < 0.001), and levels of nitrate (F4,35 = 29.43, p < 0.001). There was no statistically significant difference between the sample sites for ammonia (F4,35 = 0.046, p = 0.996), dissolved oxygen (F4,35 = 0.11, p = 0.978), dissolved reactive phosphorus (F4,35 = 2.612, p = 0.052), pH (F4,35 = 1.04, p = 0.403), or E. coli (F4,35 = 2.494, p = 0.061). Conductivity was significantly higher at S1 compared with all other sites (p < 0.001), and S5 similarly had significantly higher conductivity than S2 (p < 0.001) and S3 (p = 0.002). Turbidity levels at S1 were significantly lower than both S4 (p = 0.030) and S5 (p = 0.011). Total nitrogen levels at S5 were significantly higher than at S1 (p < 0.001), S2 (p < 0.001), and S3 (p < 0.001). S4 also had significantly higher total nitrogen levels than both S1 (p < 0.001) and S2 (p < 0.001). When considering nitrate levels, Tukey's HSD test showed significantly elevated levels at S5 compared to all other sites (p < 0.001). Similarly, S4 showed higher levels than both S1 (p < 0.001), and S2 (p < 0.001).
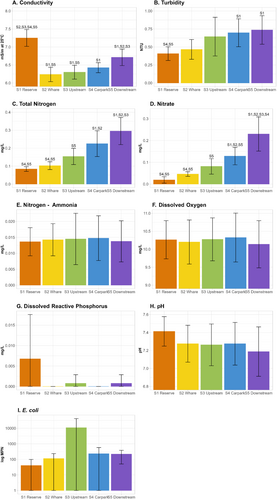
Although E. coli levels showed neither visibly consistent pattern across the sites (Figure 3I), nor were there any statistically significant differences between sites (F4,35 = 2.494, p = 0.061), the February 2023 time point had elevated E. coli levels across all sites with a substantially higher measure of E. coli detected at S3 (86,640 MPN/100 mL). As noted in earlier methods, this was identified as influential by Cook's distance, breaking the test assumptions and therefore not included in further regression analyses.
3.2 Environmental DNA
A total of 240 eDNA samples were collected and analyzed from the five sample sites (six replicates per site) over eight sampling time points. These samples were each analyzed using 13 distinct metabarcoding assays (see Table 1 and Appendix C) resulting in 15.1 million sequence reads passing the DADA2 (Callahan et al. 2016) quality filter (all raw sequence data uploaded to https://www.ncbi.nlm.nih.gov/bioproject/1100206). Of these, 2.6 million reads were unable to be taxonomically identified to the phylum level. The remaining 12.5 million reads were made up of 28,633 unique amplicon sequence variants (ASVs), representing 60 phyla, 144 classes, 359 orders, 701 families, 912 genera, and 479 identifiable species (see a portion of this displayed in Figure 4, links to all online sample reports in Appendix D). The eDNA results detected a wide range of local taonga species, both aquatic and terrestrial, including whīo/native blue duck (Hymenolaimus malacorhynchos), kōtare/sacred kingfisher (Todiramphus sanctus), long and short finned tuna/eels (Anguilla dieffenbachii and Anguilla australis), kaharore bully/cockabully (Gobiomorphus mataraerore), kōkopu/dwarf galaxias (Galaxias divergens), kōura/freshwater crayfish (Paranephrops planifrons), kererū (Hemiphaga novaeseelandiae), tūī (Prosthemadera novaeseelandiae), ruru/morepork (Ninox novaeseelandiae), pīwakawaka/fantail (Rhipidura fuliginosa), māhoe (Melicytus ramiflorus), miro (Prumnopitys ferruginea), mānuka (Leptospermum scoparium), tutu (Coriaria), kōtukutuku/tree fuchsia (Fuchsia excorticata), putaputawētā (Carpodetus serratus), harakeke (Phormium tenax), makomako/wineberry (Aristotelia serrata), and hangehange (Geniostoma ligustrifolium).

The NMDS analysis of the eDNA data (see Figure 5) revealed a clear ecological gradient in the composition of ASVs along the first NMDS axis (NMDS1). This gradient of change corresponds to the increase in pastoral land cover from S1 to S5, with the sites ordered from right to left on NMDS1. The sample sites formed distinct groups, highlighted by the ellipses along NMDS1, with the greatest distinction observed between sites S1 and S5. Along the second NMDS axis (NMDS2), the data showed varied clustering of sampling time points. Some of the time points formed tight bands indicating similar ASV profiles across sites within the same time point, while others were more dispersed along the axis suggesting different ASV compositions between sites within the same time point. Further interrogating this pattern, we looked at the contributing ASVs in the ordination and their broad taxonomic groups (see Figure 6A,B). In these additional ordination plots, no clear pattern emerged whereby any single group of organisms was driving the separation between the sites, that is, there is no clear presence or absence of a group that is driving the observed separation in Figure 5. Interestingly, there was a wide spread of ASVs of unknown taxonomy across both axes of the plot, meaning that a large amount of unknown diversity was also contributing to the separation of the sites.


Multiple linear regression of natural log-transformed E. coli levels using the DNA sequence counts from typical sources of animal feces revealed that cattle and deer sequence counts were significant predictors of E. coli levels (p < 0.001 and 0.0440, respectively). Both these effects were positive; that is, an increase in cattle or deer DNA sequence counts is associated with higher E. coli counts, also demonstrated with analysis of the raw data with combined cattle and deer reads compared with log E. coli MPN/100 mL (Appendix E).
3.3 Ecological Health
There were consistent decreases in site-averaged TICI scores from S1 through S5, with only a small overlap observed between S2 and S3 from the August 2023 data (Figure 7). The TICI scores ranged from a minimum of 106.89 at S5 in April 2023 to a maximum of 128.55 from S1 in October 2023. The number of TICI indicator sequences incorporated into the average scores ranged from a minimum of 195 indicators at S1 in April 2023 to a maximum of 616 indicators at S5 in February 2024. Site TICI scores across the 6-replicate samples generally had low variability (max 1.66 ± SD) (Figure 7, see full TICI summaries in Appendix F and Appendix G). Two-way ANOVA demonstrated that some differences in average TICI score were statistically significant between both sample sites (F4,28 = 94.117, p < 0.001) and time points (F7,28 = 5.819, p < 0.001). Further interrogation with Tukey's HSD revealed that, with the exception of the S3 and S4 pairwise comparison (p = 0.090), all sites showed significant differences from each other (all p < 0.001).
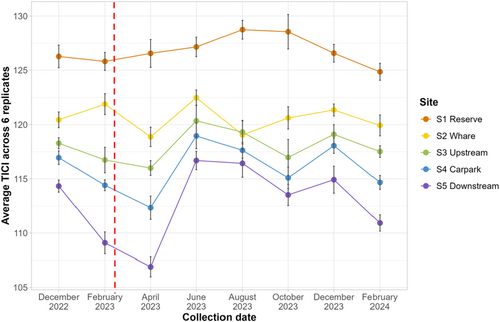
When considering temporal trends, the highest TICI scores were seen in June 2023, December 2023, and August 2023, while the lowest scores were seen in February 2023, April 2023 and February 2024. Significant differences were observed between these groupings of time points, with June 2023 being significantly higher than February 2023 (p = 0.021), February 2024 (p = 0.020), and April 2023 (p < 0.001). April 2023 TICI scores were also significantly lower than those in June 2023 (p < 0.001), August 2023 (p = 0.005), and December 2023 (p = 0.009).
A stepwise regression model with site as a random effect and all water quality attributes (conductivity, ammonia, total nitrogen, nitrate, dissolved oxygen, dissolved reactive phosphorus, pH, turbidity, and E. coli, AIC = 163.46, BIC = 195.54) was reduced to conductivity, total nitrogen, nitrate, pH, turbidity, and E. coli as predictors of average TICI score informed by the decreasing AIC (AIC = 152.06). These same predictors were also selected when considering BIC as the selection criterion (BIC = 167.26). Site was kept as a random effect in the final reduced model, and it did not explain any remaining variance after considering the water quality fixed effects in the reduced model.
4 Discussion and Conclusions
4.1 Understanding Water Quality Insights
This case study is the first to comprehensively investigate the (i) relationship between various conventional physicochemical water quality assessment attributes relied upon by water managers to identify and monitor for human and ecological health as part of national regulatory activities, (ii) contemporary taxa-independent biodiversity metrics incorporating sites of contrasting land use with clear descriptions of land cover and livestock numbers, and (iii) taxa-independent biodiversity assessments and exploration of ecosystem resilience as part of the impact from a significant rainfall event (Cyclone Gabrielle). The data analysis also provided an opportunity to identify potential relationships between E. coli concentrations and eDNA reads, with cattle/deer data providing preliminary evidence of the utility of eDNA analysis for fecal source tracking purposes. The water quality measures obtained over eight separate visits showed a general decrease in water quality as the waterway flowed from the upper bush-covered sites through to the lower pastoral-covered sites. E. coli concentrations trended toward significance (F4,35 = 2.494, p = 0.061) across sites, with a general increase from S1 downstream through farmland to S5; further visits and a larger sample size would likely assist with establishing statistical significances (p < 0.05). According to national regulations (NPS-FM, 2020) which explore the variability of water quality attributes such as E. coli concentrations, the minimum record length for grading a site is the median of 5 years of at least monthly samples (at least 60 samples). Aside from the point source contamination of E. coli at the S3 Upstream site in February 2023, water quality attributes across all sample sites and all time points were within safe bounds (Ministry for the Environment 2020) and reflected the local understanding of the water and habitat quality. Lower sites did, however, consistently exhibit higher levels of nutrient enrichment and turbidity, which are consistent with the changing land use pressures (Larned et al. 2020). Some attributes, such as nitrate and turbidity, showed marked worsening in the levels as they moved through the catchment. Some attributes, such as conductivity and pH, had more complex patterns across the sites and time points, making it more difficult to deduce trends in water quality without further summary statistics. The similarity between the upper vegetated sites (S1 and S2) versus lower pastoral sites (S3, S4, and S5) was also reinforced in the ordination plots (Figure 5), indicating that the transition in land use was detectable in both the water quality attributes and the ecological community.
The variability in the detected E. coli concentrations is likely due to environmental factors (such as rainfall) and different animal movements and sources along the waterway (Farnleitner et al. 2010; McDowell 2008; Moinet et al. 2024). Red deer (Cervus elaphus) are known to occur in high numbers and are commonly found grazing in both the uppermost S1 Reserve and S2 Whare sites, causing disruption to the planted riparian sections and native forest understory monitored regularly by mana whenua (those with tribal authority) and Māori environmental groups. Common brushtail possums (Trichosurus vulpecula) are also known to occur commonly in this section of bush and are key targets for trapping efforts, with high kill counts recorded on monthly trap lines. In the lower pastoral sites, however, the fecal contributions are more likely to be due to farmed animals, as sheep (Ovis aries) and cattle (Bos taurus) are more dominant in these sites. The impact of accidental access of these animals to the waterway and point source impact was highlighted in site S3 in February 2023 sampling. This recorded spike in elevated E. coli levels (86,640 MPN/100 mL, Figure 3I) coincided with substantially higher detected levels of cattle DNA (197,061 sequences) as well as elevated turbidity levels (Figure 3D). The additional exploration in the multiple linear regression did suggest that both cattle and deer DNA sequence counts were significant predictors of a site's E. coli levels. This suggests that there could be valuable insights to be further investigated, considering DNA sequence counts as indicators of fecal sources.
Although links with human contributions of E. coli were not possible to consider in the scope of this work, careful consideration and review of known sewage sources with the local residing community members have marked this as a low-risk area. Future studies interrogating linkages between known fecal sources and eDNA counts may wish to consider this further in other environments.
4.2 Insights Into Local Taonga Species and Biodiversity
The eDNA results uncovered a wealth of biodiversity in the catchment from important taxa groups including macroinvertebrates, fish, mammals, birds, plants, algae, and microorganisms. Among the 912 genera and 479 species identified, there were many fish, bird, and plant local taonga represented. Many of the native plants are species cultivated in the community nursery hosted at Te Miro Farm and are focus plants for reforesting and riparian planting along the study area. Some of the fish and crustacean species, notably the tuna/eels and kōura/freshwater crayfish, can occasionally be seen by the locals when spending time near the waterway. However, some of the less conspicuous species like the kaharore bully/cockabully and kōkopu/dwarf galaxias are often much more difficult to spot visually and many community members had never observed them previously.
The process of field sampling with the community allowed for meaningful storytelling opportunities for kaitiaki (local hapū resource managers) and the reconnection of mana whenua (those with tribal authority over land) to their local waterways. For example, the S2 Whare (Ngāmoko Whare) site is a known breeding area of taonga kōkopu/dwarf galaxias, and during the fieldwork, the team was able to discuss the oral history of the fish and have active leadership in the monitoring of this taonga in a safe and noninvasive way. The representation of so many native birds also showed evidence that the extensive trapping efforts that the community undertakes are having a positive impact on the preservation of native bird life, reinforcing the need for this continued protection.
When looking at the detections more deeply, some unexpected insights prompted further investigation by kaitiaki (local hapū resource managers). For example, whīo/blue duck was not previously known to inhabit this catchment, though a small population was known to reside over the ridge in a separate catchment area under the ongoing surveillance of mana whenua. Its presence in the eDNA results (on 3 time points in the S1 Reserve) suggests that individual whīo may be moving into new territory, warranting additional surveillance to better understand factors that could be driving this migration and inform the extension of pest trapping initiatives to protect what could be a small, expanding population. Another notable result was the consistent absence of kaharore bully/cockabully DNA at the upper S1 Reserve site, despite its presence in all other samples from the other four sites across all time points. This absence could indicate a physical or ecological barrier preventing the cockabully's from reaching the upper section of the catchment and has prompted a separate project for the catchment community to walk the section of river between S2 Whare through to S1 Reserve to better understand the connectivity and inform next management steps.
Unique DNA (ASVs) profiles were detected across the different sample sites and between time points, suggesting that the composition of the ecosystem shifted both spatially and temporally (as highlighted in the NMDS, Figure 5). These shifts followed a consistent pattern of progression from the uppermost vegetated site down through to the lowermost pastoral sites, demonstrating a gradual shift in the ecological community corresponding to changes in land use. Although variations in DNA profiles were detected over time within each site (as illustrated by the spread along NMDS2 axis, Figure 5), the overall similarity between the sites remained consistent over time. Further analysis of broad taxa groups contributing to this pattern (Figure 6A) revealed that no single taxa group predominated in driving the differences among sites. Instead, a diverse range of taxa collectively influenced the distinct ecological profiles of each site, including a large proportion of ASVs belonging to microorganismal groups. There was also a large quantity of ASVs spread across both axes that were unable to be assigned to any known taxonomy (Figure 6B), underscoring the presence of undiscovered biodiversity in the samples both between the sites and time points.
4.3 Tracking River Ecological Health
The consistent ASV pattern observed across sites was also seen in the site-averaged TICI scores (as seen in the line plot, Figure 7), with a gradual decrease from native bush sites through to the pastoral sites. Given eDNA can be transported ~4 km in river systems (Melchior and Baker 2023; Perry et al. 2024; Seymour et al. 2018), it is likely that DNAs from upper sites appear in lower sites due to their close proximity. Despite this, the increased prevalence of low-value TICI indicators in the lower sites was sufficient to consistently reduce their averaged TICI scores (see more detailed site facet plots in Appendix G). This suggests that despite the influx of DNAs from upstream, this method can effectively detect changes in ecological health across small spatial scales. This is a significant feature, as most biological and ecological metrics have difficulty resolving complex landscape and quality information, so they are often not used for small spatial comparisons (Boulton 1999; David et al. 2021; Harding et al. 1999; Meador et al. 2008; Springe et al. 2007).
The stepwise regression showed that water quality attributes were significant drivers of TICI values where neither site (fitted as a random effect) nor time point explained any additional variability. In practice, this suggests that the TICI scores are robust to inherent spatial and temporal changes and are highly responsive to changes in water quality. These findings suggest that the TICI could be a meaningful way to understand multiple land use stressors on rivers across small spatial scales, enhancing any insights gained from water quality attributes alone. The lower pastoral dominated sites (S3, S4, and S5) experienced the highest variability in TICI values within sites and between time points. This coincides with high fluctuations in the water quality attributes for these sites (see full water quality data in Appendix B). In contrast, the TICI scores in the upper sites were comparatively more stable over the course of the study. Notably, in the wake of the large flooding event during Cyclone Gabrielle where the lower sites experienced their lowest average TICI scores for the length of the study, the upper sites appear to have been less impacted by the heavy rainfall event. This apparent higher resilience to disruption in the more naturally vegetated systems is likely multifactorial, potentially influenced by factors such as overall catchment area and total water volumes, but suggests a greater ecological stability than more pastorally dominated sites, consistent with broader concepts of ecological resilience and stability (Ives and Carpenter 2007; Ouyang et al. 2024; Pimm 1984) and previous land use impact studies (Grime et al. 2000; Liao et al. 2022; Montuelle et al. 2010; Piggott et al. 2015; Wu 1999). As microorganisms respond quickly to shifts in water quality metrics (Gilbert et al. 1998; Grime et al. 2000; Jassey et al. 2011; Nuy et al. 2018; Sagova-Mareckova et al. 2021), their utilization in indices such as the TICI (as further described in Wilkinson et al. (2024)) can improve the sensitivity and rigor of ecological health assessments (as argued in Pawlowski et al. (2016)). The gradual recovery of site TICI scores post-cyclone is encouraging, suggesting that the scores can distinguish phases of recovery in a practical way for non-specialists. Future studies should consider extending the time series to better investigate recovery patterns, allowing normal seasonal ecosystem fluctuations to be distinguished and disentangled from ecosystem resilience and recovery after heavy rainfall events. A limitation of this study, however, is that the high-resolution analysis of ecological health and other water quality metrics in this case study has been focused in one catchment, which makes inferences regarding the upstream/downstream gradient difficult to disentangle. However, bar and density plots of individual TICI indicators and their corresponding tolerance value clearly indicated the increased evidence of low-value indicators with movement downstream (Appendix G). Further investigations are required in other catchments to demonstrate whether the observations and associations between, for example, eDNA reads from known fecal sources and E. coli counts, are able to be extrapolated to other sites and land uses with different spatial and temporal scales.
4.4 Community Empowerment and Revitalization of Mauri
Throughout the project, a genuine partnership among farmers, mana whenua, researchers, and environmental groups emerged, united by a shared vision and commitment to strengthen community connections to the environment and build new capability and capacity to safeguard their local waterways. During the field sampling days, 18 community members were able to learn and undertake the eDNA sample collections and experience standard water quality assessments. Ten of these kaimahi (workers) were mana whenua (those with tribal authority to land) from three separate Māori environmental groups (Taiao Ora Contracting, Mauri Oho, and Rangitāne Wai Warriors), four were tertiary students (Pūhoro STEMM students), two were researchers (AgResearch staff), and two were local farmers. The field days provided a valuable opportunity to reconnect with each other and build new relationships; sharing stories and experiences with their different places around the catchment naturally brought out a wealth of local knowledge, observations, and Mātauranga (local indigenous knowledge) that reinforced the data collected and provided valuable context to enrich the catchment's story.
Importantly, the act of physically collecting samples served as a means of reconnection to the environment for the community, fostering a deeper sense of attachment and responsibility toward the waterway. It refocused the goals of ongoing restoration efforts and encouraged positive narratives around land management in agriculture. The simplicity and inclusivity of the eDNA sampling methods were key in promoting broad community participation. These methods were accessible and easy to understand, empowering individuals to actively engage in the process of environmental data collection in their local waterways. The analysis and interpretation of the eDNA data were streamlined through online summaries that provided easy navigation and exploration of the detailed biodiversity (see all links to summaries in Appendix D). The team came together on numerous occasions online and in person to discuss the results in workshops, collaborating to uncover the meaning of the results and implications in a place-based context. By bridging the gap between scientific research and traditional knowledge systems, the study not only generated valuable insights but also fostered a sense of connection, pride, and responsibility among local communities toward their environment. This integrated approach serves as an example for promoting sustainable development that respects and preserves the rich cultural heritage and ecological integrity of the land and water resources.
Reflecting on the project, study partners consider that the outcomes extended beyond scientific inquiry to encompass holistic and tangible improvements across environmental, social, and cultural dimensions. Through their active leadership in monitoring taonga species and ecosystem health in the catchment, mana whenua were able to reaffirm kaitiakitanga (care and guardianship) over the waterway in a safe and noninvasive way, enhancing the mana (similar to prestige) within themselves and their wider community. This sense of ownership and guardianship, deeply intrinsic in kaitiakitanga, underscores the importance of partnerships led by mana whenua and local communities in preserving and protecting the land and water resources. This is growing in recognition in other exemplar bicultural case studies across Aotearoa New Zealand environments (examples throughout in Clapcott et al. 2018 and Henwood and Henwood 2011). Furthermore, the collaborative efforts and coming together of Māori and non-Māori partners with a common cause and shared purpose described in the present study were seen as contributing to the enhancement of the mauri (life force) of the upper Manawatū River catchment. This concept recognizes the interconnectedness between environmental health and spiritual well-being, highlighting the intrinsic value of the land and water beyond their mere utility. By acknowledging and respecting the mauri (life force) of the environment, this study emphasized the importance of holistic and sustainable approaches to environmental management across diverse landscapes that honor both scientific understanding and indigenous wisdom. Through the sharing of water quality aspirations, the vision of environmental restoration outcomes, commitment to action, and equitable sampling methodologies, catchment community partnerships can lead to improved ecological health and enhanced environmental resilience.
Author Contributions
A.A.G., A.P., P.D., and A.C. conceived and designed the experiments. A.A.G., A.P., P.D., E.M., H.D., R.C., and A.C. performed the experiments. A.A.G., S.A.W., S.P.W., and A.C. analyzed the data. A.A.G., S.A.W., A.P., P.D., and A.C. prepared figures and tables and/or authored the drafts. All authors (A.A.G., S.A.W., A.P., P.D., S.P.W., E.M., H.D., H.M., R.C., and A.C.) reviewed and approved the final draft. A.A.G. and A.C. should be considered joint first authors and listed as joint corresponding authors.
Acknowledgments
We thank the Te Miro Farm whānau—Penelope, Blair, Joe, and Billie. Taiao Ora kaimahi—Jahnique Whaitiri, Hineatatu Dorset, and Arapera Paewai. Mauri Oho kaimahi—Erana Mōtū, Pigg, Jenny Mauger, Lilia Wakefield, and Taranaki Nepe Apatū. Our wider neighbors and the tamariki of Norsewood and Districts School for joining the kaupapa on community water days and nursery days. Rangitāne Wai Warriors kaimahi. Pūhoro STEMM Charitable Trust students and staff—Navarone Watson, Mosiah Igatia, Izzy Rewiri-Wharerau, Leland Ruwhiu, Jasmine Gavas, and Cody Garton. A special thank you to the Rākautātahi Marae for nurturing this community. Open access publishing facilitated by AgResearch Ltd, as part of the Wiley - AgResearch Ltd agreement via the Council of Australian University Librarians.
Ethics Statement
All submitting authors confirm that the manuscript and conduct of the research meet New Zealand's ethical guidelines. All authors also confirm that this manuscript has only been submitted to Environmental DNA as an original article of novel research.
Conflicts of Interest
S.A.W. and S.P.W. are current employees of Wilderlab, a commercial eDNA processing laboratory. A.A.G. is a former employee of Wilderlab. P.D. is one of the owners of Te Miro Farm, an organic, regenerative dairy farm. A.P. owns and is the principal scientist of Taiao Ora Contracting, an environmental consultancy. A.C. and R.C. are scientists and researchers at AgResearch, a Crown Research Institute.
Appendix A: Taxonomy categories used to determine levels of DNA sequences associated common fecal source animals. Note that humans are not included in the scope of this work as human-associated DNA is filtered out of the final results as per local standard procedure.
| TaxID | Name | Common name | Rank |
|---|---|---|---|
| 9337 | Trichosurus vulpecula | Common brushtail possum | Species |
| 9850 | Cervidae | Deer | Family |
| 9859 | Cervus | Deer | Genus |
| 9860 | Cervus elaphus | Red deer | Species |
| 9913 | Bos taurus | Cattle | Species |
| 9935 | Ovis | Sheep | Genus |
| 9940 | Ovis aries | Sheep | Species |
| 34,878 | Cervinae | Deer | Subfamily |
Appendix B: Results of water quality attributes across all sites and all time points.
| Variable (units) | Sample date | S1 Reserve | S2 Whare | S3 Upstream | S4 Carpark | S5 Downstream |
|---|---|---|---|---|---|---|
| BOD-carbonaceous (mg/L) | December 2022 | < 1 | < 1 | < 1 | < 1 | < 1 |
| February 2023 | < 1 | < 1 | < 1 | < 1 | < 1 | |
| April 2023 | < 1 | < 1 | < 1 | < 1 | < 1 | |
| June 2023 | < 1 | < 1 | < 1 | < 1 | < 1 | |
| August 2023 | < 1 | < 1 | < 1 | < 1 | < 1 | |
| October 2023 | < 1 | < 1 | < 1 | < 1 | < 1 | |
| December 2023 | < 1 | < 1 | < 1 | < 1 | < 1 | |
| February 2024 | < 1 | < 1 | < 1 | < 1 | < 1 | |
| Conductivity (mS/m at 25°C) | December 2022 | 7.4 | 6.5 | 6.5 | 6.6 | 6.8 |
| February 2023 | 7.3 | 6.4 | 6.5 | 6.6 | 7.1 | |
| April 2023 | 7.0 | 6.1 | 6.3 | 6.4 | 6.7 | |
| June 2023 | 7.6 | 6.5 | 6.5 | 6.5 | 6.8 | |
| August 2023 | 7.5 | 6.2 | 6.3 | 6.4 | 6.8 | |
| October 2023 | 7.1 | 6.1 | 6.1 | 6.4 | 6.6 | |
| December 2023 | 7.1 | 6.1 | 6.2 | 6.3 | 6.6 | |
| February 2024 | 7.0 | 6.0 | 6.0 | 6.2 | 6.3 | |
| Nitrogen-Ammonia (g/m3 NH3-N) | December 2022 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 |
| February 2023 | 0.015 | 0.015 | 0.014 | 0.016 | 0.015 | |
| April 2023 | 0.005 | 0.005 | 0.005 | 0.005 | 0.006 | |
| June 2023 | 0.011 | 0.009 | 0.011 | 0.013 | 0.015 | |
| August 2023 | 0.015 | 0.014 | < 0.005 | < 0.005 | < 0.005 | |
| October 2023 | 0.016 | 0.019 | 0.018 | 0.015 | 0.19 | |
| December 2023 | 0.015 | 0.017 | 0.025 | 0.20 | 0.016 | |
| February 2024 | 0.020 | 0.020 | 0.026 | 0.025 | 0.024 | |
| Nitrogen-Total (mg/L) | December 2022 | 0.07 | 0.10 | 0.15 | 0.15 | 0.32 |
| February 2023 | 0.07 | 0.10 | 0.17 | 0.20 | 0.34 | |
| April 2023 | 0.09 | 0.14 | 0.24 | 0.31 | 0.41 | |
| June 2023 | 0.10 | 0.10 | 0.17 | 0.20 | 0.31 | |
| August 2023 | 0.08 | 0.10 | 0.11 | 0.36 | 0.32 | |
| October 2023 | 0.09 | 0.11 | 0.16 | 0.21 | 0.27 | |
| December 2023 | 0.10 | 0.11 | 0.14 | 0.18 | 0.23 | |
| February 2024 | 0.07 | 0.06 | 0.09 | 0.19 | 0.16 | |
| Nitrite (mg/L NO2-N) | December 2022 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 |
| February 2023 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| April 2023 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| June 2023 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| August 2023 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| October 2023 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| December 2023 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| February 2024 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| Nitrate (mg/L NO3-N) | December 2022 | < 0.005 | 0.05 | 0.02 | 0.11 | 0.22 |
| February 2023 | 0.023 | 0.053 | 0.116 | 0.175 | 0.309 | |
| April 2023 | 0.015 | 0.052 | 0.127 | 0.176 | 0.324 | |
| June 2023 | 0.033 | 0.062 | 0.111 | 0.170 | 0.283 | |
| August 2023 | 0.038 | 0.042 | 0.054 | 0.113 | 0.265 | |
| October 2023 | 0.012 | 0.040 | 0.086 | 0.122 | 0.183 | |
| December 2023 | < 0.005 | 0.027 | 0.062 | 0.078 | 0.147 | |
| February 2024 | 0.032 | 0.045 | 0.071 | 0.084 | 0.109 | |
| Dissolved Oxygen (mg/L) | December 2022 | 9.9 | 9.5 | 9.5 | 10.0 | 9.7 |
| February 2023 | 9.7 | 9.6 | 10.0 | 9.5 | 9.3 | |
| April 2023 | 10.2 | 10.6 | 10.6 | 10.3 | 10.3 | |
| June 2023 | 11.1 | 11.1 | 11.0 | 11.1 | 11.0 | |
| August 2023 | 10.8 | 10.7 | 11.0 | 11.4 | 11.0 | |
| October 2023 | 9.9 | 9.8 | 9.8 | 9.8 | 9.7 | |
| December 2023 | 9.8 | 9.7 | 9.7 | 9.8 | 9.6 | |
| February 2024 | 10.7 | 10.6 | 10.6 | 10.7 | 10.5 | |
| Dissolved Reactive Phosphorus (mg/L PO4-P) | December 2022 | 0.01 | < 0.005 | < 0.005 | < 0.005 | 0.01 |
| February 2023 | 0.006 | < 0.005 | 0.006 | < 0.005 | < 0.005 | |
| April 2023 | 0.008 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| June 2023 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| August 2023 | 0.006 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| October 2023 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| December 2023 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| February 2024 | 0.032 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | |
| pH | December 2022 | 7.1 | 7.1 | 7.0 | 7.0 | 7.0 |
| February 2023 | 7.3 | 6.9 | 6.9 | 6.9 | 6.7 | |
| April 2023 | 7.5 | 7.4 | 7.5 | 7.3 | 7.3 | |
| June 2023 | 7.5 | 7.5 | 7.3 | 7.6 | 7.6 | |
| August 2023 | 7.5 | 7.3 | 7.4 | 7.5 | 7.4 | |
| October 2023 | 7.3 | 7.2 | 7.4 | 7.2 | 7.1 | |
| December 2023 | 7.5 | 7.3 | 7.1 | 7.3 | 7.1 | |
| February 2024 | 7.6 | 7.5 | 7.5 | 7.4 | 7.3 | |
| Turbidity (NTU) | December 2022 | 0.4 | 0.60 | 0.72 | 0.81 | 0.84 |
| February 2023 | 0.38 | 0.35 | 1.20 | 0.69 | 0.77 | |
| April 2023 | 0.40 | 0.43 | 0.78 | 0.95 | 1.01 | |
| June 2023 | 0.62 | 0.28 | 0.49 | 0.48 | 0.53 | |
| August 2023 | 0.34 | 0.50 | 0.61 | 0.36 | 0.46 | |
| October 2023 | 0.33 | 0.41 | 0.35 | 0.68 | 0.86 | |
| December 2023 | 0.34 | 0.45 | 0.40 | 0.84 | 0.55 | |
| February 2024 | 0.45 | 0.71 | 0.60 | 0.77 | 0.87 | |
| E. coli (MPN) | December 2022 | 7.4 | 131.4 | 110.6 | 193.5 | 435.2 |
| February 2023 | 156.5 | 344.8 | 86,640 | 1046.2 | 344.8 | |
| April 2023 | 17.5 | 15.6 | 47.1 | 68.9 | 55.6 | |
| June 2023 | 3.1 | 42.6 | 24.6 | 20.3 | 12.1 | |
| August 2023 | 19.5 | 18.7 | 29.2 | 56.5 | 172 | |
| October 2023 | 4.1 | 21.8 | 46.5 | 95.9 | 275.5 | |
| December 2023 | 6.3 | 85.7 | 59.1 | 75.9 | 512 | |
| February 2024 | 108 | 238.2 | 195.6 | 290.9 | 387.3 |
Appendix C: Mean ± SE sequence depth for each of the 13 metabarcoding PCR assays included in the study.
| Assay | Target | Average sequence count | Standard error |
|---|---|---|---|
| WV | Vertebrates | 10146.6 | 575.9 |
| RV | Vertebrates | 7060.3 | 244.4 |
| LG | Fish | 2795.2 | 127.8 |
| CI | Insects | 10861.7 | 358.1 |
| BE | General Eukaryotes | 2900.4 | 119.0 |
| BU | Microbes | 3211.8 | 118.7 |
| EA | Echyridella freshwater mussels | 348.7 | 51.8 |
| TP | Vascular plants | 8791.4 | 240.6 |
| MZ | Vascular plants | 3207.5 | 74.2 |
| UM | Microbes | 5417.6 | 185.1 |
| YG | Galaxias spp. | 231.7 | 24.4 |
| ZP | Paranephrops freshwater crayfish | 4967.1 | 389.2 |
| XG | Gobiomorphus bullies | 5175.8 | 315.9 |
Appendix D: All eDNA results from all sites and all time points. From online sample report html files.
Appendix E: Combined cattle and deer eDNA reads were compared to log E. coli MPN (most probable number) per 100 mL water counts. A weak linear relationship was observed which was reduced with the removal of the single high E. coli Concentration outlier from Site 3, February 2023 (R2 = 0.14).
Appendix F: TICI summary information for all sites and all time points. Columns include TICI mean, TICI standard deviation (SD), mean number of TICI sequences detected, SD of number of TICI sequences, and the overall quality rank.
| Site | Sample date | TICI Mean | TICI SD (±) | Mean no. TICI seqs | No. TICI seqs SD (±) | Quality rank |
|---|---|---|---|---|---|---|
| S1 Reserve | December 2022 | 126.27 | 1.05 | 255 | 14 | Pristine |
| February 2023 | 125.81 | 0.83 | 228 | 20 | Pristine | |
| April 2023 | 126.55 | 1.29 | 195 | 12 | Pristine | |
| June 2023 | 127.15 | 0.90 | 271 | 22 | Pristine | |
| August 2023 | 128.74 | 0.86 | 223 | 21 | Pristine | |
| October 2023 | 128.55 | 1.59 | 237 | 22 | Pristine | |
| December 2023 | 126.57 | 0.82 | 338 | 20 | Pristine | |
| February 2024 | 124.85 | 0.78 | 395 | 29 | Pristine | |
| S2 Whare | December 2022 | 120.46 | 0.73 | 427 | 23 | Pristine |
| February 2023 | 121.91 | 0.97 | 329 | 22 | Pristine | |
| April 2023 | 118.88 | 0.89 | 254 | 19 | Excellent | |
| June 2023 | 122.50 | 0.72 | 304 | 27 | Pristine | |
| August 2023 | 119.06 | 1.29 | 218 | 18 | Excellent | |
| October 2023 | 120.63 | 1.03 | 314 | 28 | Pristine | |
| December 2023 | 121.37 | 0.55 | 451 | 15 | Pristine | |
| February 2024 | 119.94 | 0.97 | 527 | 28 | Excellent | |
| S3 Upstream | December 2022 | 118.29 | 0.51 | 414 | 16 | Excellent |
| February 2023 | 116.74 | 1.18 | 250 | 57 | Excellent | |
| April 2023 | 116.00 | 0.68 | 289 | 23 | Excellent | |
| June 2023 | 120.36 | 1.44 | 290 | 36 | Pristine | |
| August 2023 | 119.32 | 1.09 | 241 | 5 | Excellent | |
| October 2023 | 116.98 | 1.66 | 327 | 20 | Excellent | |
| December 2023 | 119.11 | 0.90 | 503 | 16 | Excellent | |
| February 2024 | 117.52 | 0.54 | 576 | 49 | Excellent | |
| S4 Carpark | December 2022 | 116.95 | 0.63 | 436 | 23 | Excellent |
| February 2023 | 114.41 | 0.50 | 387 | 42 | Excellent | |
| April 2023 | 112.34 | 1.07 | 314 | 53 | Excellent | |
| June 2023 | 118.97 | 1.18 | 309 | 25 | Excellent | |
| August 2023 | 117.64 | 0.73 | 199 | 21 | Excellent | |
| October 2023 | 115.09 | 1.00 | 351 | 36 | Excellent | |
| December 2023 | 118.06 | 0.70 | 526 | 13 | Excellent | |
| February 2024 | 114.67 | 0.65 | 601 | 22 | Excellent | |
| S5 Downstream | December 2022 | 114.33 | 0.56 | 412 | 28 | Excellent |
| February 2023 | 109.09 | 1.01 | 413 | 31 | Good | |
| April 2023 | 106.89 | 0.91 | 349 | 16 | Good | |
| June 2023 | 116.69 | 0.84 | 343 | 27 | Excellent | |
| August 2023 | 116.43 | 1.29 | 198 | 17 | Excellent | |
| October 2023 | 113.51 | 0.98 | 370 | 21 | Excellent | |
| December 2023 | 114.92 | 1.25 | 543 | 25 | Excellent | |
| February 2024 | 110.92 | 0.75 | 616 | 24 | Excellent |
Appendix G: Bar and density plots of individual detected TICI indicators and their corresponding tolerance value. Values range from 1 (orange, tolerant indicators) to 9 (green, sensitive indicators). Sites are plotted in rows and time points in columns. Dotted lines indicate mean tolerance value.
Open Research
Data Availability Statement
Raw sequence data are available from https://www.ncbi.nlm.nih.gov/bioproject/1100206. All online sample reports and their links have also been included in Appendix D. All water quality results have been recorded in Appendix B.






