eDNA Replicates, Polymerase and Amplicon Size Impact Inference of Richness Across Habitats
Funding: J.S.R., H.J.dB. and Q.M. acknowledge funding from the Research Council of Norway through RCN INTPART 322457: SAMBA: Scaling Advanced Methods for Biodiversity Assessments; and from HK-Dir through UTF-2017-CAPES-SIU/10022: Transnational training in eDNA for biodiversity assessments and restoration ecology. We also thank Sigma2 HPC through the Biodiversity Research Consortium Brazil-Norway project METABAR NN9813K for providing computing capacities.
ABSTRACT
Environmental DNA-based monitoring has been increasingly used in the last decade to monitor biodiversity in aquatic and terrestrial systems. Molecular-based surveys now allow quick and reliable production of baseline knowledge of species community composition on a large scale, allowing a better understanding of ecosystem function and mitigation of stressors linked to anthropogenic activities. Despite this, technical hurdles often remain, and the impact of replicates, PCR polymerases, and amplicon size on the recovered species richness is still poorly understood. Here, we conducted a large controlled experiment, with bulk samples collected from terrestrial, marine, and freshwater environments to assess the impact of natural and technical replicates, PCR polymerases with different degrees of fidelity or proofreading activity, as well as amplicon size on species richness recovery across habitats. In this study, we consistently found variations in sample species richness depending on PCR polymerase choice. We further demonstrate the dissimilar impacts between natural and technical replicates on species richness recovery and the necessity of increasing natural replications in eDNA-based surveys. We highlight the benefits and limitations of replication strategies, polymerase choice, and amplicon size across terrestrial, marine, and freshwater habitats, and provide recommendations to increase the reliability of future eDNA-based metabarcoding studies.
1 Introduction
In the last decade, environmental DNA (eDNA) metabarcoding has become an increasingly applied method for assessing biodiversity in a variety of substrates and ecological communities (Ruppert et al. 2019; Blackman et al. 2024). eDNA refers to both organismal and extra organismal DNA isolated from the environment, and can be present as (i) dissolved DNA, (ii) organelle, (iii) cell, or (iv) DNA bound to suspended particles (Mauvisseau et al. 2022; Rodriguez-Ezpeleta et al. 2021). Detection of these traces in soil, water, sediment, and even air has revolutionized the monitoring capacity for ecological studies and biodiversity assessments worldwide (Goldberg et al. 2018; Beermann et al. 2021; Bohmann and Lynggaard 2023; Carvalho, Gromstad, et al. 2024). Initial studies investigated the efficiency and reliability of this monitoring method compared to traditional techniques (Shaw et al. 2016; Watts et al. 2019; Hallam et al. 2021; Basset et al. 2022; Keck et al. 2022) and morphology analyses (Berry et al. 2015; Emilson et al. 2017; Schenk et al. 2020; Brantschen et al. 2021), and showed that eDNA could be used to detect biodiversity from environmental substrates that were difficult or impossible to study using morphology, for example, dietary analysis from feces, vegetation reconstruction from ancient sedimentary DNA (Alsos et al. 2016; Guillerault et al. 2017; Alsos et al. 2018; Ribas et al. 2021; de Queiroz et al. 2024; Rosa et al. 2024). Indeed, compared to traditional taxonomic biodiversity screening, DNA metabarcoding is more sensitive, yields more comprehensive taxonomic datasets, is more cost-efficient, and relies less on taxonomic expertise (Ji et al. 2013; Fediajevaite et al. 2021). While the method is not likely to replace botanists making floristic inventories, lepidopterists catching butterflies, or ornithologists spotting birds, it is likely to find increasing adoption and application in assessment and monitoring scenarios where it excels compared to traditional methods (Gogarten et al. 2019; Mas-Carrió et al. 2022; Milla et al. 2022). Coupled with the involvement of citizen science allowing habitat monitoring on a large scale, reanalysis of old samples, or museum collection, it offers a promising avenue for future ecological and biodiversity mapping studies (Bi et al. 2013; Burian et al. 2023; Jeunen et al. 2024).
DNA extracted from environmental samples consists of DNA molecules shed from multiple organisms, many of them present with relatively few molecules, and multiple natural and technical replicates are needed to obtain reliable results and decrease the risks of false negatives (Ficetola et al. 2015, 2016; Erickson et al. 2019; Mauvisseau et al. 2019; Burian et al. 2021; Darling et al. 2021; Acharya-Patel et al. 2024). eDNA sampling protocols vary across environments and studies, and optimal design often differs depending on research questions and cost-efficiency (Smart et al. 2016; Lugg et al. 2018; Wilcox et al. 2018). In the eDNA metabarcoding analytical workflow, DNA extraction and potential inhibitor removal (McKee et al. 2014; Djurhuus et al. 2017; Tsuji et al. 2019; Uchii et al. 2019; Pawlowski et al. 2022) are followed by PCR amplification of the targeted genetic markers. Metabarcoding then relies on the analysis of a relatively short targeted genetic region, a “barcode”, amplified by polymerase chain reaction (PCR) and sequenced using high-throughput sequencing (Valentini et al. 2009; Thomsen and Willerslev 2015; Taberlet et al. 2018).
PCR amplification is highly prone to bias, and significant stochasticity is observed in results (Zinger et al. 2019; Bohmann et al. 2022). This is exacerbated if the DNA concentration and purity of the eDNA template are low (Acharya-Patel et al. 2024). As the driver of the PCR reaction, the polymerase enzyme plays a critical role in the amplification of DNA extracted from environmental samples and can be associated with biases depending on primers used and environments analyzed (Acharya-Patel et al. 2024). Indeed, primers are used to amplify DNA from targeted organisms or groups prior to NGS sequencing, and primer choices might vary depending on the sampled environments or study design, as they will have a great impact on the recovered richness (Freeland 2016; Hajibabaei et al. 2019; Shu et al. 2021; Espinosa Prieto et al. 2024). The resulting sequence data, “the barcodes” are thereafter mapped to publicly available genetic databases for identification of ZOTUs (i.e., Zero Radius Operational Taxonomic Units, also called Amplicon Sequence Variants—ASVs) or OTUs (Operational Taxonomic Units, also called Molecular Operational Taxonomic Units—MOTUs) (Antich et al. 2021). ZOTUs are generated through denoising and refer to all correct biological sequences, while OTUs are generated through clustering and refer to subsets of the correct biological sequences (Edgar 2010, 2013, 2016), but both serve as a molecular proxy to identify a taxon. However, prior to the generation of ZOTUs or OTUs, the DNA metabarcoding workflow consists of several steps and strategic experimental decisions that may all influence the outcome and composition of molecular proxies (Deiner et al. 2017).
Due to these known biases, careful experimental design is necessary to overcome potential limitations (Rees et al. 2015; Zaiko et al. 2018; Kestel et al. 2022; Couton et al. 2023). To mitigate such hurdles, we designed a large controlled experiment to investigate the impacts of both natural and technical replicate numbers, polymerases, and primers (targeting different fragment sizes and genes) on freshwater, marine, and soil bulk samples (Figure 1). While some of these parameters have been studied individually previously, the current study is the first one investigating their combined effect on such a large scale. Here, we tested if (i) polymerases with low fidelity performed better than polymerases with high fidelity to recover richness, (ii) if natural and technical replicates had similar or different impacts on richness recovery, (iii) if the combination of all these variables led to similar results across marine, freshwater, and terrestrial habitats, and (iv) if these effects were consistent using primer sets amplifying shorter and larger DNA fragments targeting different mitochondrial genes.
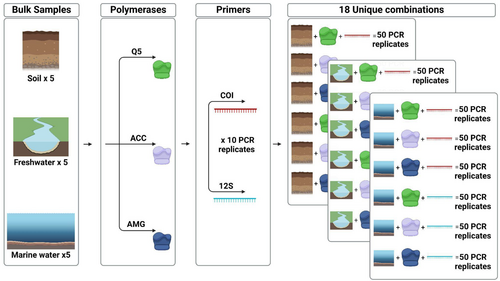
2 Methods
2.1 Sample Collection and Extraction
Freshwater: Freshwater was collected from the surface of the Drengsrudbekken river (59.8346 N, 10.43014E), Asker, Norway, and from the surface of the Akerselva river (59.9166 N, 10.7615E), Oslo, Norway on February 1st 2023. No environmental parameters were collected at these sampling locations. Equal amounts of water from both rivers were pooled and thoroughly mixed together in a 15 L sterile jerrycan to create a freshwater artificial mock community. Then, a total of five independent subsamples (referred to later as natural replicates) of 1 L each were filtered through 0.8 μm Whatman (Cytiva, Germany) Cellulose Nitrate membrane filters (25 mm diameter) using sterile 25 mm Swinnex (Merck Millipore, Germany) Filter Holders and a Vampire Sampler (Bürkle GmbH, Germany) system. Environmental DNA was extracted from the filters using the DNeasy Blood & Tissue Kit (Qiagen) with slight modification (volumes of ATL, proteinase K, AL buffers and ethanol were doubled, and the lysis step was conducted overnight).
Marine: Marine water was collected from 5 locations across a transect in the Oslo fjord (59.6603 N, 10.6051E; 59.6606 N, 10.6100E; 59.6610 N, 10.6144E; 59.6627 N, 10.6181E; 59.6633 N, 10.6230E) on January 18th 2023. An equal amount of water from each sampling location (i.e., 2 L) was pooled and thoroughly mixed together in a 15 L sterile jerrycan to create a marine artificial mock community. Following this, five independent subsamples (i.e., natural replicates) of 1 L each were filtered through 0.8 μm Whatman (Cytiva, Germany) Cellulose Nitrate membrane filters (25 mm diameter) using sterile 25 mm Swinnex (Merck Millipore, Germany) Filter Holders and a Vampire Sampler (Bürkle GmbH, Germany) system. Environmental DNA was extracted from the filters using the same kit and method described earlier regarding the freshwater eDNA samples.
Terrestrial: A total of 5 soil samples were collected in Vesterøy, Hvaler, Norway in Summer 2018 (2 samples in 59.0906 N; 10.8994E, one sample in 59.08667 N; 10.86798E and 2 samples in 59.0900 N; 10.8648E) (see full sample description in Ariza et al. 2024). At each location, various debris (i.e., stones, rocks, living or dead plant parts) were removed to expose the upper organic soil layer. Then, a 50 mL falcon tube was inserted into the soil top layer until it was entirely filled and capped before being stored at −20°C back in the laboratory. From each of these 5 initial soil samples, 3 g were merged (15 g total) in a 100 mL grinding chamber (IKA-Werke GmbH & Co) and homogenized using an IKA Tube mill 100 (IKA-Werke GmbH & Co) to create an artificial soil mock community. Following this, five independent subsamples of 250 mg each were extracted using the Qiagen PowerSoil Kit (Qiagen) following the protocol with slight modifications (for the lysis step, a Mini-Beadbeater (Techtum) was used to homogenize the samples for 2 min at 25 Hz).
To ensure the absence of contamination, one negative control (i.e., sample without any biological contents) was also included in each of the extraction workflows of the respective marine, freshwater, and terrestrial samples. DNA concentration of all DNA extracts was measured using a high sensitivity kit on a Qubit 2.0 spectrophotometer (Invitrogen) to assess the extraction success. All DNA samples were then diluted to a concentration of 1–2 ng/μl before conducting PCR amplification.
2.2 PCR Amplification
Amplification targeted a short fragment (approx 106 bp) of the mitochondrial 12S rRNA amongst vertebrate species using the 12S-V5F 5'-ACTGGGATTAGATACCCC-3' and 12S-V5R 5'-TAGAACAGGCTCCTCTAG-3' primers (Riaz et al. 2011; Kelly et al. 2014), and another longer fragment (approx 313 bp) of the mitochondrial COI region amongst metazoans using the mlCOIintF 5'-GGWACWGGWTGAACWGTWTAYCCYCC-3' and Fol-degen-rev 5'-TANACYTCNGGRTGNCCRAARAAYCA-3' primers (Yu et al. 2012; Leray et al. 2013). PCRs were conducted using three different polymerase mastermixes: (i) Q5 High-Fidelity 2X Master Mix (New England Biolabs), a mastermix high fidelity polymerase reported with > 280X higher fidelity than Taq polymerase (Potapov and Ong 2017), referred to as “Q5” throughout the manuscript, (ii) AmpliTaq Gold 360 mastermix (ThermoFisher), a mastermix with a Taq polymerase designed for high sensitivity and requiring a low copy number of target for amplification (Taberlet et al. 2018), and is referred to as “AMG” throughout the manuscript, and (iii) Accustart II PCR Toughmix (QuantaBio), a mastermix designed for degraded and/or challenging material with a high degree of known PCR inhibitors, and referred to as “ACC” throughout the manuscript. The Q5 polymerase used in this study does not require heat activation and, in contrast to AMG and ACC (who are both hotstart enzymes), has a proofreading activity.
2.3 PCR Optimization
Prior to metabarcoding amplification, amplification success for each primer set was assessed through a gradient analysis on a subset of samples. In brief, PCRs were conducted in a 15 μL final volume, including 1X of the polymerase mastermix, 0.3 μM of each primer, and 1.5 μL of template DNA diluted to a concentration of 1–2 ng/μl. It should be noted that both Q5 and AMG mastermixes were provided with a GC enhancer, an additive which prohibits the formation of secondary structures. As a result, PCR reactions were performed in parallel with and without the GC enhancer for the Q5 and AMG mastermixes in order to verify if this reagent improved amplification quality. These initial tests allowed us to identify the annealing temperature for each primer set and polymerase generating the highest amplicon yield without producing extra fragments outside of the target size range. Amplification success was verified using 1.2% agarose gel electrophoresis and visualized using the ImageLab v6.0 software on a GelDoc XR+ system (BioRad). Based on this initial testing, the PCRs were run as follows: Q5 and Accustart: 1X enzyme mastermix, 0.3 μM primers, 1.5 μL of diluted DNA template, and nuclease-free water to a final volume of 15 μL. Similar conditions were used for the Amplitaq Gold master mix, except we also included 20% (v/v) of the provided GC enhancer. Details regarding the final PCR conditions for each primer set and polymerase can be found in Table S1.
Absence of amplification was confirmed through visual observation of target bands in electrophoresis gels, and none of the extraction negative controls (i.e., samples without any biological contents) yielded amplicons during the initial tests aiming to optimize the PCR reactions for each primer and polymerase combination. As a result, these extraction negative controls were not included for amplifications alongside the soil, freshwater, and marine eDNA samples. However, a total of 60 NTC (i.e., 3 polymerases x 2 primer sets x 10 technical replicates) or non-template control (PCR amplification where the DNA template was replaced with ddH2O) was included in the amplification workflow (see Table S2). All PCR amplifications were performed using indexed primers following the dual-index design as in Fadrosh et al. (2014). All indexed primers used in this study were ordered without modification and using desalt purification.
2.4 Metabarcoding Analysis
All unique eDNA samples analyzed in this study were amplified using ten PCR replicates (see Figure 1). Together with the analysis of 18 unique combinations (i.e., three habitats, three polymerases, two amplicon sizes) with 50 PCR replicates per combination (i.e., five natural replicates analyzed each with 10 PCR replicates), this resulted in the analysis of 960 unique samples (900 eDNA samples and 60 NTCs). All amplicons were visualized through a 1.2% agarose gel electrophoresis, and relative quantities of each amplicon were estimated using the software ImageLab v6.0 (BioRad). Equimolar amounts of each amplicon were merged into separate pools based on marker and enzyme used in amplification using a Biomek4000 liquid handling robot (Beckman Coulter). The pools were cleaned using 10% of Illustra ExoProStar (Cytiva) and AMPure XP Beads (Beckman Coulter) (0.8X ratio COI, 1.2X ratio 12S). Quality control of the cleaned amplicon pools was performed on a Fragment Analyzer system (Agilent) and revealed amplification of homogenous fragment lengths in the two primer pairs. Consequently, targeted size selection to remove longer PCR fragments was not performed. Because of the different fragment lengths of the two markers, we sequenced amplicons from each primer set on two separate Illumina MiSeq flow cells (COI: MiSeq V3 300 PE, 12S: MiSeq V2 250 PE).
2.5 Bioinformatics
Bioinformatics data processing, filtering, and cleaning was performed as in (Carvalho, Gromstad, et al. 2024; Carvalho, Pazirgiannidi, et al. 2024) with slight modification depending on the amplicon's libraries. Detailed code can be found in https://github.com/fabricioA14/Bioinformatics. In brief, forward and reverse raw sequencing reads were merged using PEAR 0.9.3 (Zhang et al. 2014) and demultiplexing was performed using the ngsfilter algorithm from OBITools (Boyer et al. 2016). The obigrep algorithm from OBITools was used to remove fragments > 420 bp from the COI library and > 200 bp from the amplicons of the 12S library. Additional filtering was conducted using the USEARCH algorithm (Edgar 2010), and sequences < 100 bp were removed from both 12S and COI amplicon libraries, as well as sequences showing less than 10 occurrences in the datasets. Finally, 12S and COI datasets were denoised using the UNOISE algorithm (Edgar 2016) to retrieve ZOTUs and clustered using the UPARSE algorithm (Edgar 2013) to retrieve OTUs, both using the default 97% identity threshold in USEARCH (Edgar 2010, 2013, 2016). Both clustering and denoising were performed to retrieve subsets of the correct biological sequences (OTUs) as well as all correct biological sequences (ZOTUs) to assess later the effect of polymerases and proofreading activities on OTU/ZOTU richness. As we created artificial mock communities to assess the impacts of replication, polymerase, and amplicon size on eDNA samples from various habitats, this manuscript mainly focuses on raw OTU/ZOTU richness (i.e., without taxonomic assignment). However, taxonomic assignment and similar downstream analyses were additionally performed and can be found in (Figures S1–S4, Table S3, File S1). We performed strict filtering to remove any potential false positive detections across the 12S and COI datasets. We first investigated whether each ZOTU/OTU was detected in any of the 60 negative controls (NTCs). If any reads were observed for a given ZOTU/OTU in the negative controls, we then retained the highest number of reads for this ZOTU/OTU in the negative controls and subtracted this number from all PCR replicates from all samples across the dataset. This conservative approach was performed for all ZOTUs/OTUs across both 12S and COI datasets. This was done to ensure the absence of contamination or other sequencing errors.
2.6 Statistical Analysis
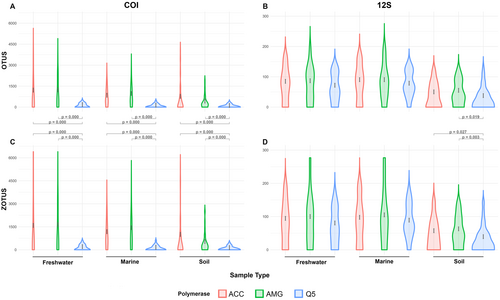
Statistical analyses and data visualization were performed using R software (R Foundation for Statistical Computing 2021). Comparison between OTU/ZOTU richness obtained for each combination was done using the Kruskal-Wallis test from the stats package (version 4.4.1), followed by Dunn's post hoc test using the dunn. test package (version 1.3.6). The Kruskal-Wallis test is a non-parametric method used to assess statistically significant differences between the medians of three or more independent groups, in this case, the three types of DNA polymerase: ACC, AMG, and Q5. After identifying an overall difference, Dunn's test was applied as a post hoc analysis to perform pairwise comparisons, determining which specific polymerase pairs showed significant differences in OTU/ZOTU richness (Harper et al. 2023). A Bonferroni correction was used to adjust for multiple comparisons, reducing the chances of type I errors by dividing the significance threshold by the number of comparisons (Steinke et al. 2022). A significance level of 0.05 was used for both tests. To visually represent the OTU/ZOTU richness across polymerases, a violin plot was created using the ggplot2 package (version 3.5.1) (Wickham 2016), with significant p-values highlighted on the plot to emphasize differences between polymerase groups. Violin plots provide a clear visualization of richness values by showing the data distribution and density, alongside key statistical measures such as centralization and dispersion, thus facilitating the detection of any unusual patterns or extreme values (Casals and Daunis-i-Estadella 2023). Rarefaction curves investigating the impacts of natural and technical replicates for each combination were generated using the “specaccum” function from the vegan package (version 2.6.8). The number of natural replicates ranged from one to five, with richness values being aggregated accordingly. For each natural replicate, the 10 technical replicates were processed for each DNA polymerase combination. These rarefaction curves were then plotted using the ggplot2 package (version 3.5.1) (Wickham 2016), visualizing richness across the technical replicates for each polymerase combination.
3 Results
A total of 14,297,880 and 10,283,473 raw reads were obtained following metabarcoding amplification and sequencing of the 12S and COI libraries. Following bioinformatics processes, including data cleaning and denoising, as well as removing singletons and ZOTUs showing less than 10 reads across the datasets, a total of 8,286,609 reads for 541 unique ZOTUs and 5,424,606 reads for 13,143 unique ZOTUs remained for both 12S and COI datasets, respectively. The clustering approach resulted in a total of 8,306,467 reads for 403 unique OTUs and 5,390,422 reads for 9063 unique OTUs remaining for both 12S and COI datasets, respectively, removing singletons and OTUs showing less than 10 reads across the COI and 12S datasets. Following strict additional filtering of the datasets using negative controls to remove potential false positive detection, a total of 6,941,654 reads with 541 unique ZOTUs remained for the 12S dataset, and a total of 5,191,140 reads with 13,143 unique ZOTUs remained for the COI dataset. In comparison, the OTU production pipeline resulted in a total of 6,953,229 reads with 403 unique OTUs for the 12S dataset, and a total of 5,145,307 reads with 9063 unique OTUs for the COI dataset. A higher number of OTUs can be expected, as ZOTUs would generate clusters with low read counts, which would be filtered out when removing unique reads below 10 singletons. Additional information regarding retained ZOTUs and OTUs for both COI and 12S datasets following taxonomic assignment can be found in (Figures S1 and S2). OTUs and ZOTUs tables can be found in (Tables S4–S11).
Focusing on the COI dataset, the mean (± SD) number of OTUs for polymerase ACC was 9142 ± 14,876 in Freshwater samples, 10,361 ± 13,222 in Marine samples, and 5261 ± 9468 in Soil samples. For polymerase AMG, Freshwater samples had 28,191 ± 55,818 OTUs, Marine samples 15,426 ± 23,026, and Soil samples 33,336 ± 68,004. By contrast, Q5 polymerase yielded the lowest OTU counts, with means of 377 ± 351 (Freshwater), 347 ± 388 (Marine), and 334 ± 360 (Soil). Under the ZOTU-based pipeline for COI, ACC produced 9333 ± 15,130 (Freshwater), 10,546 ± 13,412 (Marine), and 5313 ± 9525 (Soil). AMG reached 28,393 ± 56,125 (Freshwater), 15,557 ± 23,178 (Marine), and 33,478 ± 68,246 (Soil), whereas Q5 yielded 390 ± 359 (Freshwater), 352 ± 394 (Marine), and 340 ± 365 (Soil). For the 12S dataset, the mean (± SD) number of OTUs under the ACC polymerase was 28,940 ± 43,296 in Freshwater, 23,054 ± 32,834 in Marine, and 4462 ± 8046 in Soil. AMG resulted in 17,487 ± 21,335 (Freshwater), 19,927 ± 28,578 (Marine), and 6786 ± 10,140 (Soil). Q5 showed 21,893 ± 27,178 (Freshwater), 13,291 ± 16,618 (Marine), and 3224 ± 8488 (Soil). In the ZOTU-based pipeline for 12S, ACC presented 28,854 ± 43,147 OTUs in Freshwater, 23,012 ± 32,739 in Marine, and 4465 ± 8031 in Soil. AMG reached 17,407 ± 21,218 (Freshwater), 19,928 ± 28,535 (Marine), and 6780 ± 10,107 (Soil). Lastly, Q5 averaged 21,867 ± 27,127 OTUs in Freshwater, 13,296 ± 16,596 in Marine, and 3223 ± 8458 in Soil. These results illustrate both the influence of polymerase choice and the variability among sample types for both the 12S and COI markers.
Out of the 900 unique amplicons (i.e., 450 amplicons per primer set after the exclusion of negative controls), amplification success varied across polymerases, with ACC showing higher read numbers across all environments using the COI primer set, but with more similar read numbers across all polymerases and environments using the 12S primer set (Figure S3). This was consistent across both the OTU and ZOTU approaches. We found that Q5 retrieved significantly lower OTU and ZOTU richness than ACC and AMG for large COI amplicons (p-values ≤ 0.001) across all environments. We found a similar effect with shorter 12S amplicons using the ZOTU approach in the soil environment (p-values ≤ 0.05). A significant difference was also found between Q5 and AMG using the OTU approach in the soil environment (p-values ≤ 0.05). However, no significant differences were found between ACC and AMG for both short 12S amplicons (p-value = 0.57) and larger COI amplicons (p-value = 0.99) (Figure 2). Due to the inherent variation of species composition across marine, freshwater, and terrestrial environments, and primer efficiency (i.e., primer sets designed to target different organisms, genes, and DNA fragment sizes) we did not compare the species richness retrieved across habitats and markers. However, details regarding significant differences across polymerases per habitat and per primer sets can be found in Table S3, for both OTU and ZOTU approaches.
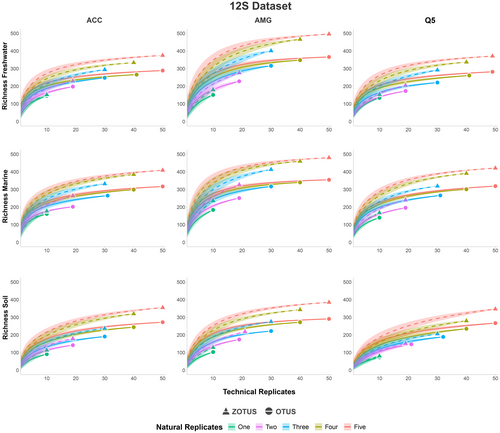
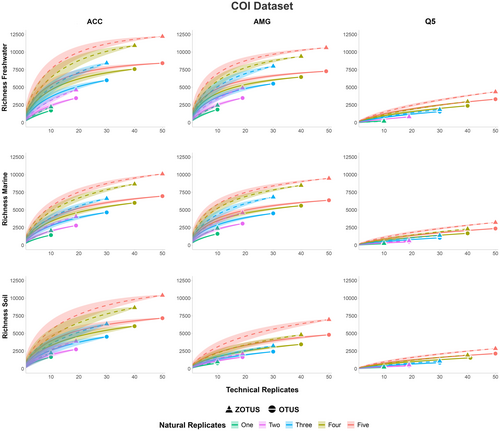
Finally, we found that across all tested combinations (i.e., three polymerases across three habitats), the number of natural replicates (i.e., number of filter or soil samples) had a dissimilar impact compared to the number of technical replicates (i.e., PCR replicate) on the recovery of OTU and ZOTU richness. The increase of natural replicates allowed a greater recovery of OTU and ZOTU richness than the increase of technical replicates (Figures 3 and 4). This effect was consistent with primers amplifying both the short fragment of the 12S gene and the larger fragment of the COI gene (Figures 3 and 4). Using the 12S primer set, we found that the OTU and ZOTU richness recovered was consistently lower than when using the COI primer set, an expected outcome due to the primer design targeting specific groups of organisms. Despite this, we also found that in the analysis of five natural replicates, including ten PCR replicates each, for a total of 50 PCR replicates for each mock community, we were not able to recover the whole OTU or ZOTU richness in any of the tested combinations, suggesting that a high number of natural replicates would be necessary to reach the plateau phase of the rarefaction curves (Figures 3 and 4). This was further exacerbated with the use of the primer set amplifying a larger fragment of the COI gene, which allowed the recovery of a higher ZOTU richness (Figure 4). Finally, we found that across specific habitats, the use of AMG polymerase significantly improved the OTU and ZOTU richness recovery (Figure 4) (Table S3).
4 Discussion
eDNA-based assessment is increasingly used worldwide for ecological studies and biodiversity monitoring (Thomsen and Willerslev 2015; Taberlet et al. 2018; Deiner et al. 2021; Huang et al. 2022), and currently used in freshwater, marine, terrestrial, and even air environments, allowing us to quickly and efficiently monitor large areas and include citizen sciences (Agersnap and Thomsen 2022; Bohmann and Lynggaard 2023; Carvalho, Pazirgiannidi, et al. 2024). While previous work has investigated the impact of false positives and false negatives (Chambert et al. 2015; Ferguson et al. 2015; Ficetola et al. 2016; Guillera-Arroita et al. 2017; Pinfield et al. 2019; Burian et al. 2021; Darling et al. 2021), payoffs of both natural and technical replicates (Ficetola et al. 2015; Beentjes et al. 2019; Mauvisseau et al. 2019; Fukaya et al. 2021), and the impact of primer sets on the either recovered OTU and/or ZOTU richness (Rojahn et al. 2021; Shu et al. 2021; Xiong et al. 2022; Macher et al. 2023), most studies often focus on only one set of these parameters, or on one habitat only, hampering our understanding of the combined parameters across systems.
Here, the creation of marine, freshwater, and terrestrial bulk samples combined with a structured analytical design allowed us to mitigate the effects of considering results from studies that focused only on one aspect of variation at a time. However, it should be noted that metabarcoding analysis often leads to the generation of artifact sequences, leading to OTU/ZOTU inflation. We therefore conducted our analyses using both OTU/ZOTU approaches, with and without performing the taxonomic assignment, to avoid potential biases due to artifacts as these would be excluded following taxonomic identification of the sequences. Overall, using long COI amplicons, we found no significant differences between ACC and AMG polymerases, and observed that Q5 consistently retrieved lower OTU and ZOTU richness. In addition to the poor amplification rates with this combination of primer and polymerase, such results could be explained by the proofreading activity displayed by Q5, leading to a decrease of amplification errors and reduction of alpha diversity (Mahé et al. 2015; Vermeulen et al. 2016; Taberlet et al. 2018). However, this observation remained consistent with and without performing the taxonomic identification of sequences, ruling out PCR errors and artifacts as a driver of these differences. Such proofreading polymerase can additionally remove mismatches at the 3′ end of primers and lead to non-specific PCR products (Mahé et al. 2015; Vermeulen et al. 2016; Taberlet et al. 2018). These PCR products could display incorrect lengths compared to the expected amplicons, and therefore be discarded following library preparation and fragment size selection as they will be either shorter or larger than the expected amplified fragment, leading to the observed decrease of OTU/ZOTU richness. Such bottlenecks would occur prior to sequencing and bioinformatic processes, and their effect would not be able to be mitigated during the post-processing steps. Here, we used a proofreading polymerase without particular modification of the PCR protocol (i.e., without the addition of three to five phosphorothioate bonds between the nucleotides and 3′ end of the primers (Mahé et al. 2015; Vermeulen et al. 2016; Taberlet et al. 2018)). This is expected to lead to an important loss of specificity due to the generation of non-specific PCR products, and potentially explain the differences observed between the different enzymes used in this study. Additionally, we didn't observe significant differences between AMG and ACC enzymes. As ACC is designed to work with degraded samples with a high amount of inhibition, this indicates that there were no biases due to potential inhibition in our study. While we observe significant differences between Q5 and the other tested polymerases using the long amplicon size targeting the COI gene, we did not observe this using smaller amplicons targeting the 12S gene. This difference is likely due to the lower OTU/ZOTU richness obtained using the 12S primer set compared to the COI primer set (Figure 2). However, it should be noted that we didn't formally compare results between the different primer sets tested. Indeed, these primer sets have been designed to amplify different targets (Freeland 2016; Schenekar et al. 2020; Burian et al. 2023). As a result, any comparison of OTU/ZOTU richness retrieved with these different sets would have been inherently biased. Additionally, such difference could potentially be linked to a collateral amplification of sequences by the degenerate COI primers. Indeed, the COI primers used here have degenerate bases on both forward and reverse fragments to maximize the taxonomic recovery of metazoan diversity, and this could also have led to an artificial inflation of amplified and sequenced fragments. To the contrary, the non-degenerate 12S primers would have therefore recovered a more accurate picture of the OTU/ZOTU composition independently of polymerase effects. However, such effects would need a different experimental design to disentangle potential analogous effects. Here, we used degenerate primers to recover as much metazoan diversity as possible to achieve a much broader scope than just vertebrate taxa and help to understand whether methodological choices influenced recovery curves at different scales and in different habitats that naturally vary in the relative proportion of taxa belonging to distinct taxonomic groups. Future studies should therefore conduct preliminary testing using various polymerases and mock samples of their targeted environment to identify their potential limitations depending on the primer sets (with and without degeneracy) used. While this current study provides extensive testing of polymerases across environments and primer sets, various other parameters, including but not limited to inhibition, acidity, salinity, temperature, and volume of water sampled are also expected to impact the recovery of OTU/ZOTU richness (Bylemans et al. 2018; Deiner et al. 2018; Holman et al. 2022; Jo 2022; Koziol et al. 2019; Pukk et al. 2021; Ruppert et al. 2019; Seymour et al. 2018). The environments tested in our study (freshwater, marine and terrestrial) have been sampled in Norway, and while we did not record environmental parameters susceptible to impact the recovered richness, it is expected that similar types of environments sampled elsewhere could lead to different richness recovery.
Here, we further investigated the impacts of the number of natural and technical replicates across primer sets, environments, and polymerases on the recovered OTU and ZOTU richness (Figures 3 and 4). We found consistent results across short and long amplicon sizes (Figures 3 and 4). Rarefaction curves using both OTUs and ZOTUs showed a steep increase in the recovered richness when increasing the number of natural replicates, and a moderate increase when increasing the number of technical replicates (Figures 3 and 4). This shows the dissimilar impacts of the replication levels and suggests that including a higher number of natural replicates would decrease false negative detection rates. However, while the combination of five natural replicates with ten technical replicates each allowed us to reach the plateau phase of richness recovery using the short 12S amplicon, this was not the case using longer COI amplicons. This could highlight discrepancies in the taxonomic coverage, and hence the sequencing depth required per sample, for these distinct primer sets, or arise from unspecific amplification rates due to the degeneracy of the COI primer set. These effects were consistent across environments and polymerases despite the impacts identified earlier (Figures 3 and 4). This suggests that a higher number of natural and technical replicates would be needed to ensure an optimal diversity recovery. Previous studies have highlighted similar findings (Ficetola et al. 2015; Hinlo et al. 2017; Beentjes et al. 2019; Mauvisseau et al. 2019; Pearman et al. 2021; Shirazi et al. 2021; Stauffer et al. 2021). However, the inclusion of such a number of samples and replicates could be counterproductive, as it would decrease the number of investigated locations in ecological studies due to the analytical costs. Furthermore, additional replication covering small spatial scale and temporal heterogeneity in the sampled location might have a limited effect on the overall recovery of diversity depending on the sampling design and habitat sampled. However, other factors could mitigate the negative impacts of a low replication level. In aquatic environments, the filtration of large volumes of water would allow us to retrieve an increased amount of eDNA which could be diluted or have a stochastic distribution due to the species ecology (Mächler et al. 2015; Valentini et al. 2016; Peixoto et al. 2020; Wu et al. 2024). Here, we filtered 1 L of water per natural replicate, and filtering a larger volume could have impacted the results of the study. However, the filtration of a larger volume of water is also associated with inhibition, potentially leading to false negatives and potential trade-offs (McKee et al. 2014; Jane et al. 2015; Goldberg et al. 2018; Uchii et al. 2019; Baudry et al. 2021; Dubreuil et al. 2021).
Finally, other tools not tested in this study, such as occupancy modeling or other analytical pipelines, can also allow mitigating potential false negative issues and increase the reliability and efficiency of eDNA based monitoring in future work (Ferguson et al. 2015; Dorazio and Erickson 2018; Martel et al. 2020; Fukaya et al. 2021; Burian et al. 2021, 2023; Buxton et al. 2022). Here, we found that AMG and ACC polymerase perform equally well in marine, freshwater, and terrestrial environments using longer amplicons. Based on these findings, we recommend future studies investigate the effects of potential polymerase on the sampled environment and primers prior to the analysis of large datasets. We also found that a combination of five natural replicates with 10 technical replicates each allowed a greater species richness recovery across environments. As a result, we recommend increasing the number of natural replicates when designing future sampling, potentially combined with other strategies including the filtration of larger volumes in aquatic environments, inhibitor removal, and analytical frameworks when analyzing the sequencing results. This does not necessarily mean that future studies should include 10 technical replicates, but care should be taken to balance the level of natural and technical replication to increase results reliability within a given sequencing run. While eDNA based detection offers new opportunities for species monitoring, it should be noted that this method is associated with its own benefits and biases, and care should be taken during study design to overcome potential limitations (Rees et al. 2015; Zaiko et al. 2018; Kestel et al. 2022; Couton et al. 2023).
Author Contributions
Conceptualization: Jarl Andreas Anmarkrud, Jonathan Stuart Ready, Hugo J. de Boer, Quentin Mauvisseau. Sampling: Jarl Andreas Anmarkrud, Audun Schrøder-Nielsen, Quentin Mauvisseau. Laboratory analysis: Jarl Andreas Anmarkrud, Lisbeth Thorbek, Audun Schrøder-Nielsen. Bioinformatics: Quentin Mauvisseau. Data curation: Fabricio dos Anjos Santa Rosa, Quentin Mauvisseau. Statistical analysis: Fabricio dos Anjos Santa Rosa, Quentin Mauvisseau, Jonathan Stuart Ready. Original draft: Jarl Andreas Anmarkrud, Lisbeth Thorbek, Audun Schrøder-Nielsen, Quentin Mauvisseau, Jonathan Stuart Ready. Review and editing: Jarl Andreas Anmarkrud, Lisbeth Thorbek, Audun Schrøder-Nielsen, Fabricio dos Anjos Santa Rosa, Silvana Melo Sviggum, Jonathan Stuart Ready, Hugo J. de Boer, Quentin Mauvisseau. Funding acquisition: Jonathan Stuart Ready, Hugo J. de Boer, Quentin Mauvisseau.
Acknowledgments
We would like to thank William Gromstad, Mari Engelstad and María Ariza for their valuable support with the collection of the soil and marine eDNA samples, and the Norwegian Sequencing Center for their valuable expertise.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Raw sequencing data can be found here: https://doi.org/10.5281/zenodo.14025716, and additional information can be found in the Supporting Information.




