Dietary partitioning among three cryptobenthic reef fish mesopredators revealed by visual analysis, metabarcoding of gut content, and stable isotope analysis
Abstract
Understanding how mesopredators partition their diet and the identity of consumed prey can assist in understanding the ecological role predators and prey play in ecosystem trophodynamics. Here, we assessed the diet of three common coral reef mesopredators; Pseudochromis flavivertex, Pseudochromis fridmani, and Pseudochromis olivaceus from the family Pseudochromidae, commonly known as dottybacks, using a combination of (i) visual stomach content analysis, (ii) stomach content DNA metabarcoding (18S, COI), and (iii) stable isotope analysis (δ15N, δ13C). In addition, P. flavivertex is found in two distinct color morphs in the Red Sea, providing an opportunity to analyze intra-morph differences. These techniques revealed partitioning in the dietary composition and resource use among species. Arthropods comprised the main dietary component of P. flavivertex (18S > 60%; COI > 10%) and P. olivaceus (18S = 57.2%), while P. fridmani ingested predominantly mollusks (18S = 51.3%, COI = 24.6%). Despite being small predators, microplastics were found in the gut content of some of these fishes. Stable isotope analysis showed differences in species' isotopic niche breadth and trophic position. Pseudochromis olivaceus presented the largest isotopic niche (SEAC = 1.61‰2), while P. fridmani showed the smallest isotopic niche (SEAC = 0.45‰2) among species. Although the two techniques used for stomach content analysis did not show differences in the diet within color morphs of P. flavivertex, they differed in the isotopic niche and resource use. Despite our limited sampling, our findings provide evidence of species-specific differences in the trophic ecology of dottybacks and demonstrate their important role as predators of cryptic invertebrates and small fishes. This study highlights the importance of combining several approaches (short-term: visual analysis and DNA metabarcoding; and long-term: isotope analysis) when assessing the feeding habits of coral reef fish, as they provide complementary information necessary to delimit their niches and understand the role that small mesopredators play in coral reef ecosystems.
1 INTRODUCTION
Understanding drivers and mechanisms that contribute to the maintenance of tropical biodiversity, and how diversity is partitioned along natural and anthropogenic gradients within ecosystems is important to predicting the existence and persistence of species and the ecological functions they provide (Legendre et al., 2005; Pearman et al., 2018; Schoener, 1974; Socolar et al., 2016). Differentiation of ecological niches promotes species diversity by the reduction of interspecific competition due to any substantial difference in resource use between coexisting species (Chase & Leibold, 2003; Chesson, 2000; Finke & Snyder, 2008; Levine & Hille Ris Lambers, 2009; McKane et al., 2002). Since overlap in resource use may determine the likelihood of competitive interactions among species (Pastore et al., 2021; Schoener, 1974), they may employ differing behaviors or strategies to reduce niche overlap (Heupel et al., 2019; Matley et al., 2017; Schoener, 1974). For example, species may occupy different microhabitats (e.g., spatial niche partitioning), may have asynchronous activity patterns (e.g., temporal niche partitioning), or they may have different feeding habits (e.g., dietary niche partitioning) (Leray et al., 2019; Sale, 1978; Schoener, 1974). Therefore, understanding species' behavior along with dietary preferences is important to delimit species' ecological niches and define their role in the ecosystem. Furthermore, knowledge about species' diet is critical to understanding how species exploit resources available in their environment, and thus predict the effects of their population changes on the ecosystem and, if necessary, design effective conservation strategies (Lazic et al., 2021).
Several studies have identified differences in the use of dietary resources among reef fishes by using different approaches. Some of these methods include analysis of feeding behavior (e.g., Berumen et al., 2005; Brandl & Bellwood, 2014; Fox & Bellwood, 2013), stomach content analysis (e.g., Ashworth et al., 2014; Clarke, 1999), gut content DNA metabarcoding (e.g., Brandl et al., 2020; Casey et al., 2019; Coker et al., 2023; Leray et al., 2015; Nalley, Donahue, Heenan, & Toonen, 2021; Nalley, Donahue, & Toonen, 2021; Takahashi et al., 2020), stable isotope analysis (SIA) (e.g., Matley et al., 2017), and a few incorporating multi-method approaches (e.g., Miller et al., 2019; Nagelkerken et al., 2009). Stomach content analysis provides information related to the diversity and composition of an individual's diet at the time of the capture or over the course of hours to days (short-term picture of diet). However, visual analysis of stomach content, based on the microscopic identification of stomach contents, can underestimate the diversity of the taxa, as they are highly dependent on the digestion state of prey items and the ability to identify organisms from fragments of prey (Berry et al., 2015; Pompanon et al., 2012). On the contrary, SIA provides integrated information on the diet assimilated by an organism and the contribution to the consumer's tissue across the course of weeks to months (long-term dietary picture) (Petta et al., 2020; Phillips et al., 2014). SIA allows an estimate of trophic position and sources of dietary carbon based on the ratios of isotopic nitrogen (δ15N) and isotopic carbon (δ13C), respectively, but lacks a taxonomic resolution of the ingested prey (Petta et al., 2020; Phillips et al., 2014; Pompanon et al., 2012). This challenge can be overcome by applying molecular approaches such as DNA metabarcoding, which combines DNA barcoding and high-throughput sequencing to determine the community composition of a complex mixture of DNA extracted from stomach content or feces (Leray et al., 2013). With the use of universal primers to amplify markers such as portions of the mitochondrial cytochrome c oxidase subunit I (COI) gene or of the small subunit of the nuclear ribosomal RNA (18S rRNA) gene, DNA metabarcoding provides higher taxonomic resolution and higher detection of rare species than approaches based on morphological identification with less reliance on taxonomic expertise (Barbato et al., 2019; Clare, 2014; Deagle et al., 2014; Nalley, Donahue, & Toonen, 2021). These two universal markers (18S and COI) are complementary (Casey et al., 2021; Clarke et al., 2017; Wangensteen et al., 2018), increasing the probability of prey detection in gut content studies (Coker et al., 2023).
Small reef mesopredators, such as dottybacks (family Pseudochromidae), feed mainly on macro-invertebrates and small cryptic species, including fishes, (e.g., Ashworth et al., 2014; Holmes & McCormick, 2006, 2009), and comprise an important component of coral reef fish assemblages (Depczynski & Bellwood, 2003). These small predators are widespread on coral reefs, inhabiting different habitats such as coral slopes, vertical walls, reef flats, patch reefs, sand, and rubble (Lieske & Myers, 2004), and they can modify the composition and abundance of cryptobenthic invertebrates and mutualistic coral species with cascading effects on host corals (Coker et al., 2015; Leray et al., 2015; Stier & Leray, 2014). Therefore, understanding how small mesopredators partition their diet, and the diversity of prey consumed, is key to determining the ecological role these predators play in the trophic web (Harley, 2011; Leray et al., 2015).
Family Pseudochromidae has the highest rate of endemism within any fish family in the Red Sea (Bogorodsky & Randall, 2019). However, knowledge about their ecology (e.g., habitat use and feeding habits) is still limited, while most of the information available on trophic ecology for this family is based on studies from the Indo-Pacific species Pseudochromis fuscus (e.g., Cortesi et al., 2015; Feeney et al., 2012; Holmes & McCormick, 2009; Messmer, Jones, et al., 2005; Messmer, van Herwerden, et al., 2005). Dottybacks are known for their variation in coloration, with different color morphs reported for several species in the Indo-Pacific (Cortesi et al., 2015; Gill, 2004; Messmer, Jones, et al., 2005; Messmer, van Herwerden, et al., 2005). Pseudochromis flavivertex, Pseudochromis olivaceus, and Pseudochromis fridmani are three common pseudochromid species found in the Red Sea that coexist across a range of reef habitats, and all are endemic to the region.
Here, we assessed the dietary niche breadth and variation in diet composition using a combination of (i) visual gut content analysis, (ii) gut DNA metabarcoding, and (iii) bulk-tissue stable isotope analysis (δ15N, δ13C). We then used this information to assess trophic niche partitioning among different species. In the Red Sea, Pseudochromis flavivertex has been observed to display four different color morphs: (i) sunrise (morph 1, M1), (ii) sunset (M2), (iii) super blue (M3), and (iv) sunshine (M4). Only two of the color morphs of P. flavivertex are commonly found in the study region (M1 and M2) and were included in the study for an intermorph comparison. These findings help us gain a better understanding of the role of dottybacks as small mesopredators in coral reef ecosystems and underscore the importance of combining several approaches (short-term: visual analysis and DNA metabarcoding; and long-term: isotope analysis) when assessing the feeding habits of coral reef fishes.
2 MATERIALS AND METHODS
2.1 Fish and tissue collection
A total of 125 adult individuals from three species and two color morphs of dottybacks (Pseudochromis flavivertex M1 = 48; P. flavivertex M2 = 28; P. olivaceus = 24; and P. fridmani = 25) were collected (Figure 1) in the central Red Sea off the coast of Thuwal, Saudi Arabia (22.2833° N, 39.1000° E) between 9:00 am and 12:00 pm of August and September 2021. Fish collections took place in different reefs along a cross-shelf gradient (coastal, mid-shore, and offshore) to broadly cover all available shallow reef habitats for these species in the central Red Sea, and to maximize the diversity of dietary items detected for each dottybacks species rather than compare habitats. Not all dottybacks species were collected across the different shelves because not all species were found in the same location during the sampling period (see Data S1: Table S1 for individual sample details). Fishes were collected under KAUST IACUC Protocol 19IACUC03.

Fish were caught using a hand net and clove oil solution (1:10 clove oil: ethanol) as an anesthetic and euthanized by immersion in buffered tricaine methanesulfonate (MS-222: 250 mg/L seawater). Fish were photographed on the boat immediately after capture to document the color pattern and preserved on ice in situ. Once in the laboratory, total (TL mm) and standard length (SL mm), wet weight (mg), and sex (when possible) were recorded for each individual (Data S1: Table S1). The gut cavity was opened and the entire intact stomach and intestine were removed using sterile dissection tools from the fish within 3 h after collection and preserved separately in sterile Eppendorf tubes containing 96% ethanol at −20°C for analysis. A section (~1 cm2) of dorsal muscle tissue was removed from each individual and preserved in a sterile vial at −20°C for isotope analysis. All dissecting tools were sterilized between individuals and between tissues within individuals in a three-step process by rinsing and incubating them in a series of falcon tubes containing 1:10 bleach and sterile water, sterile water, and ethanol.
In addition to collected fish, representative samples of potential prey items and basal energetic resources (pelagic: zooplankton and benthic: macroalgae) were collected in the same locations as the fish for stable isotope analysis to examine variability in these basal resources and their relative contribution to species diet (Miller et al., 2019). Zooplankton samples (n = 16; coastal: monument = 3, South Beach = 3, mid-shore: Al Fahal = 6, and offshore: Shib Nazar = 3) were collected with a mesh net (150 μm) by carrying out horizontal tows (<1 m depth) for 5 min at 5 km/h along the edge of the reef and preserved on ice in situ. Once in the lab, zooplankton samples were concentrated on mesh filters (150 and 100 μm) and preserved at −20°C. Brown (Turbinaria sp. and Rosenvingea sp.) and red macroalgae (Hypnea sp.) were collected from reef crest (n = 31; monument = 7; Al Fahal = 11; Shib Nazar = 13) and preserved on ice in situ. Once in the lab, algae samples were rinsed of detritus, large epibionts removed, and preserved at −20°C until preparation for isotope analyses.
2.2 Diet composition determination and trophic niche of dottybacks
2.2.1 Visual gut content analysis
A total of 62 gut tracts (P. flavivertex M1 = 24; P. flavivertex M2 = 14; P. olivaceus = 12; and P. fridmani = 12) were dissected out, and all prey or distinguishable fragments of prey were counted and identified to the lowest taxonomic level possible using a Carl Zeiss stereo microscope, taxonomic keys, illustrated guides, and list of the species for the region (e.g., Al-Aidaroos et al., 2019; Al-Yamani et al., 2011; Amer, 2019; Khalil & Abd El-Rahman, 1997; Ryanskiy, 2020). Digested material with no distinguishable fragments was also sorted, recording its presence and absence as it contributes to gut fullness. The vacuity coefficient (V), which represents the percentage of empty stomachs, was calculated in relation to the total number (n) of stomachs examined. Likewise, the frequency of occurrence (%F) and percentage by number (%N) were calculated for each prey (Cortés, 1997; Hyslop, 1980). Undigested prey items with visible morphological features were photographed using a Leica Z6 APO stereo microscope.
2.2.2 Gut content DNA metabarcoding
DNA extraction
A total of 56 gut tracts (P. flavivertex M1 = 21; P. flavivertex M2 = 13; P. olivaceus = 10; and P. fridmani = 12) were dissected, and the entire gut content (excluding tissue from the host fish to minimize dietary masking by the host DNA) extracted using the DNeasy PowerSoil DNA extraction Kit (QIAGEN) following the manufacturer's protocols with the following modifications: samples were incubated with 12.2 μL of proteinase K and 60 μL of solution C1 at 56°C at 200 rpm overnight, to ensure that all the tissues were completely lysed (Leray et al., 2013) and final DNA elution was done at 50 μL. Extractions were performed under sterile conditions in a dedicated space in the Bioscience Core Laboratory at KAUST to minimize the risk of contamination. Filter tips were used, and negative controls were included in all the extraction rounds. The concentration of DNA extracts was determined using a Qubit dsDNA HS (high sensitivity, 0.2 to 100 ng) Assay Kit with a Qubit 4.0 fluorometer (Thermo Fisher Scientific) according to the manufacturer's protocols. DNA samples were normalized to 5 ng/μL.
Library preparation and sequencing
The Illumina 16S metagenomic sequencing library preparation guide was followed (Illumina, 2019) with modifications. Two sets of universal primers were used to perform the polymerase chain reactions (PCRs), one amplifying a 313 bp segment of the mitochondrial cytochrome c oxidase subunit I (COI) gene (mlCOlintF: 5′—GGWACWGGWTGAACWGTWTAYCCYCC—3′; jgHCO2198: 5′—TAIACYTCIGGRTGICCRAARAAYCA—3′); (Geller et al., 2013; Leray et al., 2013) and the other amplifying a 450 bp segment of the eukaryotic V4 region of the nuclear small subunit ribosomal RNA (18S rRNA) gene (Uni18SF: 5′—AGGGCAAKYCTGGTGCCAGC—3′; Uni18SR: 5′—GRCGGTATCTRATCGYCTT—3′); (Zhan et al., 2013). These primers were selected due to their versatility in detecting a wide range of metazoan prey items, and they work well for the identification of marine invertebrates (Casey et al., 2019; Coker et al., 2023; Geller et al., 2013; Leray et al., 2013; Rey et al., 2020; Zhan et al., 2013).
Three PCR replicates were conducted per sample. Each PCR reaction was run in a total volume of 25 μL: 1 μL of 10 μM forward primer, 1 μL of 10 μM reverse primer, 12.5 μL of KAPA HiFi HotStart DNA Polymerase (HotStart and Ready Mix formulation, KAPA Biosystems), 8 μL of RNA free water, and 2.5 μL of genomic DNA. PCR was performed under the following conditions: Initial denaturation at 95°C for 3 min, followed by 35 cycles: 98°C for 20 s, 46°C (COI) or 50°C (18S) for 60 s, and 72°C for 90 s, and a final extension at 72°C for 5 min. Each PCR contained 61 samples, including 56 gut content DNA samples, 3 extraction negative controls, 1 PCR negative control, and 1 PCR positive control containing genomic DNA from one fish species (Acanthopagrus berda). PCR reactions were verified on 1.0% agarose gels and QIAxcel (QIAGEN) with the QIAxcel DNA Screening Kit, using a QX alignment marker of 15 bp to 3 kb and method AM320. Electropherograms were analyzed with BioCalculator 3.0 (QIAGEN). The PCR reaction performed with negative control extractions confirmed the absence of contaminants (no bands). All successfully replicated PCR reactions were pooled into a single product for a final volume of 75 μL.
PCR products were bead cleaned using Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA) at a concentration of 0.8x vol/vol. For library preparation, dual indices were attached to the amplicon of each sample using the Nextera DNA Library Prep Kit and the Nextera XT Index Kit (Illumina). Each indexing PCR reaction was run in a volume of 50:5 μL of Index Primer 1 (N7xx), 5 μL of Index Primer 2 (S5xx), 25 μL of 2x KAPA HiFi HotStart Ready Mix (KAPA Biosystems), 10 μL of PCR Grade water, and 5 μL of PCR product. The PCR amplification included an initial denaturation at 95°C for 3 min, followed by eight cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 5 min. PCR products were cleaned and normalized using the SequalPrep Normalization Plate (96) Kit (Invitrogen).
Purified libraries were pooled with unique indices and quantified using a Bioanalyzer (Agilent) and a Qubit Fluorometer (Invitrogen) using a dsDNA HS Assay Kit. A Final concentration of the library was calculated using quantitative PCR (qPCR) and normalized to 4 nM, and 20% phiX was used for internal control. Pair-end sequencing (2 × 300 bp) was performed on an Illumina MiSeq platform with a MiSeq v3 Reagent Kit (Illumina). Sequence data were automatically demultiplexed using MiSeq Reporter (V2), and forward and reverse reads were assigned to samples.
Bioinformatics pipeline
The USEARCH-UNOISE3 pipeline (Edgar, 2010, 2016) was used to process 18S rRNA and COI reads. For 18S rRNA gene-based amplicon libraries, raw reads were decontaminated of phiX, quality filtered (QV = 25), and adapter trimmed using the BBDuk tool from the BBMap suite (Bushnell, 2022). Primers were removed using cutadapt (Martin, 2011). USEARCH-UNOISE3 was used to conduct all further reads processing: paired-end reads merging, deduplication, sequence alignment, denoising, chimera removal, clustering, and the generation of a zero-radius OTU (zOTU) table. Input sequences with low abundances (i.e., representing <8 reads) were discarded by the default version of the USEARCH-UNOISE3 pipeline. Filtering of the zOTUs was performed based on BLASTn (Altschul et al., 1990) query coverage analysis (>70% query coverage), the post-clustering curation algorithm LULU (Frøslev et al., 2017), and SortMeRNA v4.3 (Kopylova et al., 2012). Finally, BLASTn and BLAST-QC (Torkian et al., 2020) of the final available zOTU sequences were performed against the latest available NCBI nucleotide database to assign taxonomy. To generate the full NCBI taxonomic lineage information for all of the identified hits, the Entrez Direct (Kans, 2010) and TaxonKit (Shen & Ren, 2021) tools were used. The taxonomy of all zOTU sequences that had >98% identity hits in the nt database was further verified against the curated EukRibo database v2 (Berney et al., 2022). All zOTUs representing bacterial rRNA genes or non-target genes were excluded from the analysis. The relative abundance of zOTU sequences in each library was estimated as a percentage of the whole library.
To process COI amplicon libraries, a similar pipeline was followed as with the 18S rRNA gene amplicons (except for the SortMeRNA filtering step). Apart from the curation of the COI zOTU sequences on LULU, the sequences were additionally filtered based on a visual screening of stop codons and frameshift errors on SeaView (Gouy et al., 2010) after aligning the corresponding amino acid sequences on MACSE (Ranwez et al., 2011). The taxonomy of all fish-affiliating zOTU COI sequences that had >98% identity hits in the nt database was further verified against the curated MitoFish database (Iwasaki et al., 2013) and that of the remainder sequences against the BOLD database (Ratnasingham & Hebert, 2007).
All zOTUs representing fish (Phylum—Chordata; Class—Actinopterygii) were removed from the analysis of the 18S sequences as the 18S rRNA genes representing the host fish species (Pseudochromis flavivertex, P. olivaceus, and P. fridmani) were not available in the NCBI database, and therefore could not be recognized among the other fish zOTUs present in the stomach content. COI sequences belonging to the host fish species were available in the NCBI database. Thus, all zOTUs matching those sequences were removed from the analysis while retaining all other zOTUs assigned to Actinopterygii. These “self-hits” (e.g., all zOTUs identified as P. flavivertex, P. olivaceus, and P. fridmani) were removed to exclude host tissue and to avoid wrong inferences of cannibalism or trophic linkages among the studied species. Although cannibalism of smaller individuals could happen, this likely represents an insignificant fraction of pseduochromids diets. Likewise, zOTUs corresponding to sequences identified as endoparasitic protozoa (e.g., Apicomplexa) were removed from the analysis as these organisms were most likely living in the host gut rather than being eaten by them. Finally, zOTUs that were identified only as Eukarya were labeled as Unidentified along with the zOTUs without matches (<85% identity match) on NCBI.
2.2.3 Bulk-tissue stable isotope analysis (δ15N, δ13C)
A total of 96 fish dorsal muscle tissue samples (P. flavivertex M1 = 19; P. flavivertex M2 = 28; P. olivaceus = 24; and P. fridmani = 25) and 47 basal resources samples (zooplankton and macroalgae) were freeze dried for 72 h, then ground into a powder with a mortar and pestle. Between 0.5 and 0.6 g of homogenized fish muscle and zooplankton samples and 2 and 3 mg of homogenized macroalgae of each sample were packed into 3 × 5 mm tin capsules. The δ13C and δ15N values were measured with a Costech ECS 4010 Elemental Analyzer (Valencia, CA, USA) coupled to a Thermo Scientific Delta V Plus isotope ratio mass spectrometer (Bremen, Germany) at the Center for Stable Isotopes, University of New Mexico. The international reference standards Vienna PeeDee Belemnite (carbon) and atmospheric nitrogen (nitrogen) were used to compare isotope ratios. Analytical precision (within run) was measured at ±0.1‰ and ± 0.2‰ for δ13C and δ15N, respectively, using internal protein (casein and tuna) and plant (blue gramma and green chili) standards. Isotope ratios are reported in delta (δ) notation as parts per mil (‰) following the equation δ = (R sample/R standard−1) × 1000, where R sample and R standard are the ratios of the heavy and light isotopes (13C/12C or 15N/14N) in a sample and standard, respectively.
2.3 Data analysis
2.3.1 Gut content analysis: Visual and DNA metabarcoding
To analyze dietary diversity for pseudochromid species and P. flavivertex color morphs, diet diversity indices (richness, Simpson's diversity H′, and Pielou's evenness) were calculated for each individual. Diet composition (relative abundance) of pseudochromid species and morphs was square root transformed to reduce the relative influence of the most frequent prey species, then plotted with non-metric multidimensional scaling ordination (nMDS) in two dimensions using Bray–Curtis similarity matrices. Differences in the diet composition of the species were tested by performing a permutational analysis of variance (PERMANOVA) on the same distance matrix, using “Species” and “Sites” as the two predictor variables with 999 permutations. To analyze which species significantly contributed to the difference, a pairwise test with a Bonferroni correction and 999 permutations was performed. The homogeneity of group dispersion was analyzed by using the PERMDISP routine. Additionally, a similarity percentages (SIMPER) analysis was conducted to determine the average Bray–Curtis dissimilarity between species and to identify the diet items that significantly impacted species differences in diet.
Morisita–Horn index, which ranges between 0 (no overlap) to 1 (complete overlap), was applied to the relative abundances of zOTUs obtained for 18S and COI assays, to assess dietary niche overlap among pseudochromid species and morphs. Rarefaction curves using zOTUs were produced to visualize sampling effort and the cumulative dietary richness, simulating extrapolated projections to 45 individuals for each species using Chao's diversity estimator (Chao et al., 2014). We then generated two bipartite networks to illustrate the interactions among fish species, morphs, and higher-level prey taxa (class and order) obtained for 18S and COI assays, respectively. Due to the high proportion of Unidentified zOTUs found for the COI assay, two bipartite networks including only identified prey items with >90% identity match were also generated for 18S and COI assays.
All analyses and visualizations of gut content data were performed in R version 4.1.3 (R Core Team, 2021), using the packages vegan, bipartite, iNEXT, MASS, and ggplot2.
2.3.2 Bulk-tissue stable isotope analysis (δ15N, δ13C)
Isotopic niche breadth and overlap between species were assessed by Bayesian standard ellipses (Jackson et al., 2011). For that, every individual within a population was plotted in a bivariate stable isotope space (δ13C and δ15N), and the standard isotopic ellipse area corrected for small sample sizes (SEAC) was calculated and represented by the bivariate standard deviation of δ13C and δ15N values, encompassing approximately 40% of the data (Jackson et al., 2011; Matley et al., 2017; Miller et al., 2019). Isotopic niche overlap was calculated as the average percentage of isotopic space shared by species and morphs based on SEAC values and the area of ellipse overlap. Niche overlap was considered significant when the overlap in shared isotopic space between species was >60% (Cybulski et al., 2022; Matley et al., 2017). To statistically compare the area of the ellipse among species, Bayesian models were fitted using uninformative priors to estimate the posterior distributions of the ellipse areas (iterations: 20000; burn-in: 1000, thin 10), and the mode of each distribution and the 95% credible intervals were calculated for each population. Differences in SEAC were considered significant when ≥95% of posterior draws (104) for one species or morphs were different from the other (Matley et al., 2017). To integrate isotopic values of consumers and sources, trophic position and the relative contribution of each source (pelagic: zooplankton; benthic: macroalgae) to each individual pseudochromid and morph was estimated (see Data S1: Methods for more details).
All analyses and visualizations of bulk stable isotope data were performed in R version 4.1.3 (R Core Team, 2021), using the packages SIBER and ggplot2.
3 RESULTS
3.1 Diet breadth and composition based on visual analysis
From the 62 individuals of pseudochromid species analyzed, 82% (51) contained prey items in their guts (stomachs and/or intestines) and were considered for the diet analysis (e.g., F and N). However, from these 51 individuals, only 43 contained prey in their stomachs (intestines excluded), with a vacuity coefficient (V) of 50% for P. flavivertex M1, 14.3% for P. flavivertex M2, 25% for P. olivaceus, and 16.7% for P. fridmani (Data S1: Table S2). A total of 1372 prey items were detected within the gut contents of pseudochromid species and morphs were visually analyzed (mean ± standard error: P. flavivertex M1 = 38.44 ± 14.45, P. flavivertex M2 = 27.46 ± 6.95, P. olivaceus = 6.5 ± 3.56, and P. fridmani = 22.58 ± 4.71). Because most prey items were highly digested, only two prey items were identified at the family level. The remaining prey items were classified to higher taxonomic levels (Table 1; Data S1: Table S3). From the unidentified prey, 43 were classified as morphospecies (Data S1: Figures S1–S3). Microplastics were also detected in the diet of P. flavivertex M1 (11%) and P. olivaceus (13%; Table 1) by visual identification under the stereomicroscope.
| Prey items | P. flavivertex M1 | P. flavivertex M2 | P. olivaceus | P. fridmani | ||||
|---|---|---|---|---|---|---|---|---|
| %F | %N | %F | %N | %F | %N | %F | %N | |
| Arthropoda | ||||||||
| Crustracea | ||||||||
| Decapoda | ||||||||
| Brachyura | – | – | 7.69 | 0.28 | – | – | 8.33 | 0.37 |
| Megalopa | 5.56 | 0.14 | – | – | – | – | – | – |
| Paguroidea | 5.56 | 0.14 | 15.38 | 0.56 | – | – | – | – |
| Caridea | 5.56 | 0.14 | 7.69 | 0.28 | 25.00 | 3.85 | 8.33 | 0.74 |
| Alpheidae | 5.56 | 0.14 | – | – | – | – | – | – |
| Zoea | – | – | 7.69 | 0.28 | – | – | – | – |
| Isopoda | ||||||||
| Gnathiidae | 5.56 | 0.14 | – | – | – | – | – | – |
| (Other isopods) | 11.11 | 0.43 | 15.38 | 1.12 | ||||
| Tanaidacea | 50.00 | 2.31 | – | – | – | – | 16.67 | 7.75 |
| Amphipoda | 16.67 | 0.43 | 7.69 | 0.28 | – | – | – | – |
| Copepoda | ||||||||
| Cyclopoida | 61.11 | 56.50 | 38.46 | 26.89 | – | – | 16.67 | 10.70 |
| Harpacticoida | 5.56 | 0.14 | – | – | – | – | 8.33 | 0.37 |
| (Other copepods) | 50.00 | 9.39 | 61.54 | 36.97 | 62.50 | 78.85 | 75.00 | 11.81 |
| Ostracoda | 27.78 | 1.30 | 30.77 | 1.40 | 12.50 | 1.92 | 8.33 | 0.37 |
| Parts of crustaceans | 44.44 | – | 15.38 | – | 25.00 | – | 25.00 | – |
| Chelicerata | ||||||||
| Pycnogonida | 5.56 | 0.14 | – | – | – | – | – | – |
| Mollusca | ||||||||
| Bivalvia | 11.11 | 0.29 | 7.69 | 0.28 | 25.00 | 5.77 | 75.00 | 20.30 |
| Gastropoda | 55.56 | 21.68 | 84.62 | 19.33 | – | – | 91.67 | 40.96 |
| Echinodermata | ||||||||
| Ophiuroidea | – | – | 7.69 | 0.28 | – | – | – | – |
| Annelida | ||||||||
| Polychaeta | 11.11 | 0.87 | 7.69 | 0.28 | – | – | – | – |
| Nematoda | 5.56 | 0.14 | – | – | – | – | 16.67 | 1.11 |
| Sipuncula | 5.56 | 0.14 | 7.69 | 0.56 | – | – | – | – |
| Foraminifera | 11.11 | 0.29 | 23.08 | 0.84 | – | – | 25.00 | 1.85 |
| Chordata | ||||||||
| Vertebrata | ||||||||
| Actinopteri | ||||||||
| Fish scales | 5.56 | 0.14 | 30.77 | 9.80 | 12.50 | 7.69 | 25.00 | 2.58 |
| Others | ||||||||
| Gelatinous material | 16.67 | 0.87 | 7.69 | 0.28 | – | – | 16.67 | 0.74 |
| Spines | 5.56 | 2.89 | 7.69 | 0.28 | – | – | – | – |
| Algae | 5.56 | 0.14 | – | – | – | – | – | – |
| Microplastic | 11.11 | 0.43 | – | – | 12.50 | 1.92 | – | – |
| Sand and rocks | 5.56 | 0.43 | – | – | – | – | – | – |
| Unidentified | 11.11 | 0.29 | – | – | – | – | 8.33 | 0.37 |
- Note: %F = Frequency of occurrence, that is, the percentage of individuals' stomachs in which a prey taxon was identified. %N = percentage by number, that is, the proportion of the specific taxa compared to the total number of identified prey items across all stomachs (n = 1372 prey items). Bold illustrates diet items with %F or %N > 50%.
Mean diversity metrics (± SE) per species revealed the highest diet richness for Pseudochromis fridmani (0.67 ± 0.07) and the lowest for P. olivaceus (0.20 ± 0.10). Conversely, the highest evenness diet was observed for P. olivaceus (0.67 ± 0.03) and the lowest was for P. flavivertex (0.52 ± 0.02; Figure 2). The comparison of the mean diet diversity metrics showed significant differences in the Simpson's diversity index, richness, and evenness at the species level (Table 2, Figure 2). However, average diet diversity metrics did not differ between P. flavivertex color morphs (Table 2; Figure 2). More than 50% of P. fridmani diet was represented by mollusks (bivalves and gastropods), while crustaceans (e.g., copepods) comprised more than 50% of P. flavivertex and P. olivaceus diet (Figure 3). Although no substantial differences in dietary diversity were found between the two color morphs of P. flavivertex, P. flavivertex M1 consumed a higher proportion of cyclopoid copepods in its diet compared to P. flavivertex M2 (Table 1, Figure 3).

| Analysis | Index | Species | Morphs | |||
|---|---|---|---|---|---|---|
| K-W chi-square | p | W | t | p | ||
| Visual | Simpson D | 10.51 | 0.005 | 134.00 | – | 0.507 |
| Richness | 10.61 | 0.004 | – | 0.478 | 0.636 | |
| Pielou's J | 6.15 | 0.046 | – | −1.441 | 0.163 | |
| 18S | Simpson D | 20.54 | 0.000 | 175.00 | – | 0.181 |
| Richness | 20.81 | 0.000 | 179.00 | – | 0.136 | |
| Pielou's J | 20.15 | 0.000 | 193.00 | – | 0.463 | |
| COI | Simpson D | 6.87 | 0.032 | 176.50 | – | 0.161 |
| Richness | 19.65 | 0.001 | 174.00 | – | 0.371 | |
| Pielou's J | 0.05 | 0.976 | 215.50 | – | 0.154 | |
- Note: K-Wchi-square = Kruskal–Wallis test; W = Mann–Whitney test; t = Unpaired T-test. Significant p values indicate differences.
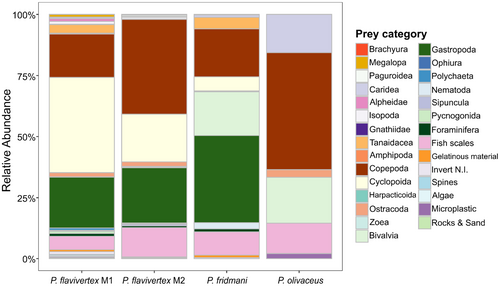
Non-metric multidimensional scaling ordination (nMDS) revealed clustering for all three pseudochromid species. A pairwise test did not show differences between the diet composition of P. flavivertex color morphs (M1 and M2) (Data S1: Table S4); therefore, these were tested at the species level for the following. The clusters of species showed differences in the diet composition with a significant explanatory power for species as a grouping variable but not for sites (Table 3, Figure 4). However, species and sites showed non-homogeneous dispersion in ordination space based on their centroids, with the variances of species and sites contributing to observed differences in PERMANOVA (Table 3). The differences in the diet composition at the species level were driven by the high abundance of copepods and gastropods in the diet (Data S1: Table S5). Copepods contributed 26.4% to the difference between P. flavivertex and P. olivaceus, while gastropods contributed 20.08% to the difference between P. flavivertex and P. fridmani, and 28.9% to the difference between P. olivaceus and P. fridmani (Data S1: Table S5, Figure 3).
| Analysis | Test | df | F | R 2 | p |
|---|---|---|---|---|---|
| Visual | PERMANOVA | ||||
| Species | 3 | 3.39 | 0.17 | 0.001 | |
| Site | 2 | 1.51 | 0.05 | 0.108 | |
| PERMDISP | |||||
| Species | 3 | 3.06 | – | 0.037 | |
| Site | 3 | 7.63 | – | 0.001 | |
| 18S | PERMANOVA | ||||
| Species | 3 | 1.96 | 0.11 | 0.001 | |
| Site | 2 | 1.04 | 0.04 | 0.346 | |
| PERMDISP | |||||
| Species | 3 | 1.87 | – | 0.150 | |
| Site | 3 | 7.48 | – | 0.000 | |
| COI | PERMANOVA | ||||
| Species | 3 | 1.55 | 0.08 | 0.001 | |
| Site | 2 | 1.12 | 0.04 | 0.120 | |
| PERMDISP | |||||
| Species | 3 | 8.18 | – | 0.000 | |
| Site | 3 | 53.69 | – | 0.000 |
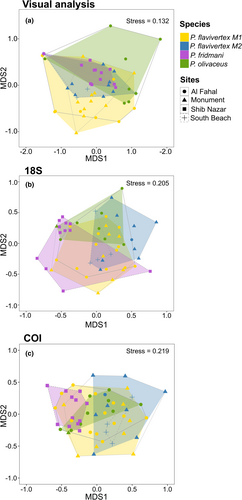
3.2 Diet breadth and composition based on DNA metabarcoding
DNA metabarcoding successfully detected prey items within the gut contents of 56 individuals using 18S and COI. The initial zOTU table included a total of 6,256,906 reads and 782 zOTUs for COI and 919,624 reads and 404 zOTUs for 18S. The COI dataset had an average of 111,730 reads per library (sequencing depth range from 32,461 to 615,297) while that of 18S had an average of 14,597 reads (sequencing depth range from 361 to 41,163). Although rarefaction curves did not reach saturation, zOTUs accumulation for both markers seemed to slow down mainly for 18S (Data S1: Figure S4). At the host species level, the COI table contained 1,720,624 reads for P. fridmani, 1,414,993 reads for P. olivaceus, and 3,121,289 reads for P. flavivertex while that of 18S had 255,846 reads for P. fridmani, 163,011 reads for P. olivaceus, and 500,767 reads for P. flavivertex. Nearly 83% and 96% of the total reads in the COI and 18S zOTU tables, respectively, were assigned a phylum-level taxonomy, while around 66 and 75% of the total reads in the COI and 18S zOTU tables, respectively, were species-level assignments (at >98% identity and 1e−50 e-value cutoff). Close to 17 and 0.07% of the total reads in the COI and 18S tables, respectively, remained unclassified at any taxonomic level.
After removing all the “self-hits” and endoparasitic protozoa from the dataset, gut content DNA metabarcoding resulted in 347 zOTUs for 18S and 496 zOTUs for COI, from which 223 zOTUs and 251 zOTUs were taxonomically assigned, respectively. The highest total number of zOTUs across individuals was found for P. flavivertex M1 (18S = 230; COI = 265), followed by P. fridmani (18S = 186, COI = 202), P. flavivertex M2 (18S = 157; COI = 150), and P. olivaceus (18S = 73; COI = 74). Mean diversity metrics (± SE) per species obtained for 18S and COI revealed the highest diet richness for P. fridmani (18S = 58.8 ± 7.1; COI = 39.5 ± 6.5), followed by P. flavivertex (18S = 28.9 ± 3.20; COI = 19.7 ± 1.7), and P. olivaceus (18S = 12.3 ± 5.1; COI = 10.7 ± 1.4; Table 2; Figure 2). Similarly, the highest average diversity (mean per species ± SE) was observed for P. fridmani (18S = 0.9 ± 0.0; COI = 0.7 ± 0.05) and the lowest was for P. olivaceus (18S = 0.8 ± 0.04; COI = 0.4 ± 0.1; Table 2; Figure 2). However, the mean diet diversity metrics did not differ between morphs of P. flavivertex (Table 2; Figure 2).
The non-metric multidimensional scaling ordination (nMDS) for both COI and 18S markers showed clustering of the three pseudochromid species and the two color morphs of P. flavivertex (Stress: 18S = 0.21, COI = 0.22), but not a clear separation among the sites (Figure 4). For 18S, the generated clusters were supported by the PERMANOVA, which showed differences in the diet composition with a significant explanatory power for species as grouping variable but not for sites (Table 3). These differences were caused by differences between P. flavivertex (M1) and P. fridmani and between P. flavivertex (M2) and P. fridmani (Data S1: Table S4). However, the pairwise test did not show differences between the two color morphs of P. flavivertex (M1 and M2), indicating that diet composition is similar between the two morphs of this species (Data S1: Table S4). Species but not sites showed homogeneous dispersion in ordination space based on their centroids (Table 3), and therefore the variances of sites contributed to observed differences in PERMANOVA. Similarly, the species cluster generated for COI was supported by the PERMANOVA, showing differences in the diet composition with a significant explanatory power for species as a grouping variable but not for sites (Table 3). Although these differences in the diet composition between species were caused by marked differences between the two morphs of P. flavivertex and P. olivaceus and P. fridmani, the pairwise test did not show differences at the color morph level within P. flavivertex (M1 and M2; Data S1: Table S4). Species and sites showed non-homogeneous dispersion in ordination space based on their centroids (Table 3), and therefore the variances of species and sites contribute to observed differences in PERMANOVA. Overall, Morisita–Horn indices for both 18S and COI showed a high overlap between the two color morphs of P. flavivertex (18S = 0.52; COI = 0.28) and limited overlap between species (Data S1: Table S6). The mean (± SE) dietary niche overlap across species for 18S and COI were 0.26 ± 0.06 and 0.16 ± 0.03, respectively.
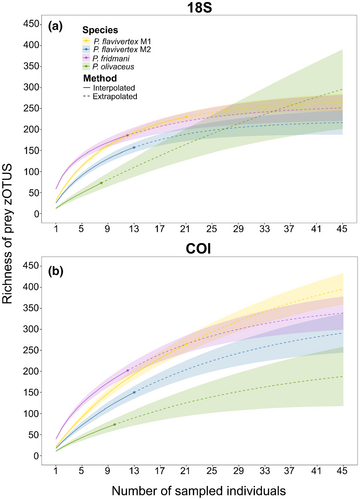
Sample-based rarefaction for 18S and COI showed variance in slopes across species; however, none of the species reached the asymptote for 18S or COI, indicating that no species were sampled exhaustively enough to obtain complete coverage of their diet (Figure 5). In particular, the P. olivaceus curve was still climbing steeply for 18S, suggesting that sampling effort was not exhaustive, and the diet is likely much higher than detected. Therefore, more sampling would be needed to reach an asymptote. Both 18S and COI rarefaction analyses with extrapolations to 45 individuals per species revealed that the richness of prey would continue to increase with increased sampling for all the species. For COI, both the observed and estimated values from extrapolation models showed P. flavivertex M1 with the highest prey richness (COI: 512 zOTUs, lower 95% confidence interval, LCI = 415 and upper 95% confidence interval, UCI = 609) and P. olivaceus with the lowest prey richness (236, LCU = 83 and UCI = 389). For 18S, extrapolated models with a sampling depth of up to 45 individuals per species showed P. olivaceus with the highest prey richness (18S: 633, LUC = 73 and UCI = 1456) and P. flavivertex M2 with the lowest prey richness (220, LUC = 186 and UCI = 253) (Figure 5).
The bipartite network of prey items generated for 18S and COI showed a different relative prey contribution to the diet of P. flavivertex (M1 and M2), P. fridmani, and P. olivaceus at taxonomic level from phylum to order (Figure 6a, Data S1: Figure S5). Based on 18S, P. flavivertex M1 diet was mainly comprised of arthropods (62.2%). Although half of its diet sequences were not classified beyond the phylum level (43%), a high proportion of the arthropod-based diet was composed of copepods from the order Cyclopoida (14.3%; Figure 6a). Dietary components of P. flavivertex M2 were divided mainly between mollusks (43%) and arthropods (36%), with a high proportion of gastropods from the order Neogastropoda (30%). Pseudochromis fridmani ingested predominantly mollusks (51.3%), mainly bivalves from the orders Mytiloida (6.2%), Arcoida (5.8%), and Ostreida (5.3%), gastropods from the order Neogastropoda (9.8%), and calanoid copepods in less proportion (7.8%). Arthropods comprised the main dietary resource for P. olivaceus (57.2%), with a high proportion of ostracods from the order Podocopida (22%) and taxa unidentified beyond the phylum level (30.6%; Figure 6a).
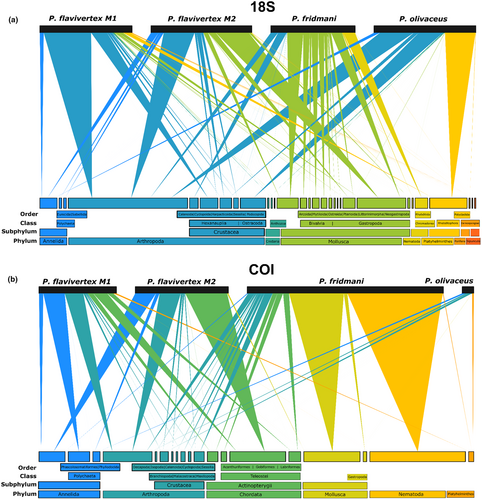
On the contrary, a large proportion of zOTUs were unidentified in the COI dataset for P. flavivertex M1 (67%), P. flavivertex M2 (60.3), P. fridmani (15.44%), and P. olivaceus (95%) (Data S1: Figure S5). The identified prey based on COI (Figure 6b) in P. flavivertex M1 and M2 diet was mainly comprised of annelids (10.9 and 10.3%, respectively) and arthropods (9.64 and 10.7%, respectively), whereas P. fridmani ingested mainly mollusks (24.6%) and arthropods (10.34%). In addition, the bipartite network generated for COI included Actinopterygii taxa, which were part of the diet of the two color morphs of P. flavivertex (M1 and M2) and P. fridmani. Pseudochromis flavivertex ingested fish predominantly from the order Gobiiformes (11.8%), whereas P. fridmani ingested more fish from the order Labriformes (2.5%; Figure 6b). Both 18S and COI detected the nematodes and platyhelminthes, both possible parasites, in the gut content of pseudochromid fish, with a higher proportion of nematodes in P. fridmani (18S = 11.8%, COI = 40.4%) and a higher proportion of Platyhelminthes in P. olivaceus (18S = 27.9%; Figure 6).
3.3 Isotopic niche and resource use
Pseudochromis olivaceus presented the largest isotopic niche (SEAC = 1.61‰2), followed by P. flavivertex M1 and M2 (SEAC M1 = 1.11‰2, SEAC M2 = 1.10‰2) while P. fridmani showed the smallest isotopic niche (SEAC = 0.45‰2) among the species (Data S1: Table S7, Figure 7). According to the comparison between the posterior distributions of the Bayesian standard ellipses (SEAB), only P. fridmani had a significantly smaller SEAB compared to P. olivaceus and P. flavivertex M1 and M2, while all other species pairs showed overlapping posterior distributions (Data S1: Figure S6). Shared isotopic niche overlap among standard ellipses of P. flavivertex M1 and P. flavivertex M2 was not significant (<60% shared overlap; Data S1: Table S7), although P. flavivertex M1 shared only 18% of its isotopic niche with P. flavivertex M2. There was no overlap of SEAC among P. flavivertex (M1 and M2), P. fridmani, and P. olivaceus (Data S1: Table S7).
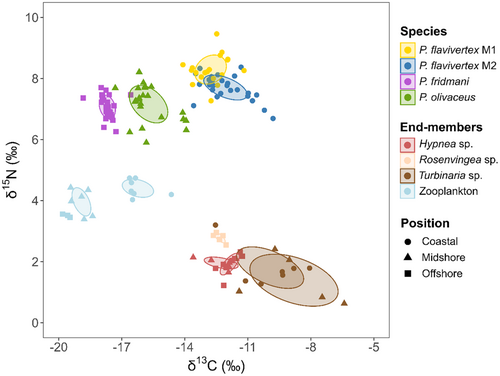
Pseudochromis flavivertex, P. fridmani, and P. olivaceus differed when compared against δ13C and δ15N values (Table 4). Similarly, the comparison of the mean δ13C and δ15N values showed significant differences between P. flavivertex morphs (Table 4). Potential food sources (pelagic: zooplankton; benthic: macroalgae) differed significantly when compared against δ13C and δ15N values (Table 4). Pseudochromis fridmani assimilated predominantly resources from pelagic sources (relative contribution of pelagic resource, PRC = 0.75 ± 0.05, vs. benthic resources, BRC = 0.26 ± 0.05), whereas P. flavivertex M1 and P. olivaceus showed more even use of both energy channels (P. flavivertex M1: PRC = 0.45 ± 0.12 vs. BRC = 0.55 ± 0.12; P. olivaceus: M1: PRC = 0.61 ± 0.11 vs. BRC = 0.39 ± 0.11). Pseudochromis flavivertex M2 assimilated predominantly carbon from benthic resources (BRC = 0.66 ± 0.16 vs. PRC = 0.34 ± 0.16). The comparison of the mean relative contribution of these energy pathways between pseudochromid species showed significant differences in the pelagic and benthic food-web reliance estimates between species and morphs (Table 4; Data S1: Figure S7a,b).
| Contrast | K-W chi-square | p | W | p |
|---|---|---|---|---|
| Muscle tissue | ||||
| δ 13 C | ||||
| Species | 79.81 | 0.001 | – | – |
| Morphs | – | – | 155.50 | 0.050 |
| δ 15 N | ||||
| Species | 50.49 | 0.001 | ||
| Morphs | – | – | 436.00 | 0.001 |
| Pelagic and Benthic sources | ||||
| δ13C | 38.27 | 0.001 | – | – |
| δ15N | 36.51 | 0.001 | – | – |
| Relative contribution of sources | ||||
| PRC | ||||
| Species | 67.97 | 0.001 | – | – |
| Morphs | – | – | 358.00 | 0.020 |
| BRC | ||||
| Species | 67.97 | 0.001 | – | – |
| Morphs | – | – | 155.00 | 0.020 |
| Trophic position | ||||
| Species | 62.70 | 0.001 | – | – |
| Morphs | – | – | 311.50 | 0.224 |
- Note: K-Wchi-square = Kruskal–Wallis test. W = Mann–Whitney test. Significant p values indicate differences.
Pseudochromis flavivertex (M1 and M2) presented the highest trophic position (P. flavivertex M1: TP = 3.56 ± 0.18; P. flavivertex M2: TP = 3.48 ± 0.11) compared to P. olivaceus (TP = 3.20 ± 0.16) and P. fridmani which presented the lowest trophic position (TP = 3.14 ± 0.11). Pseudochromid species significantly differed when mean trophic positions were compared (Table 4). However, P. flavivertex morphs (M1 and M2) did not differ in their TP (Table 4; Data S1: Figure S7c).
4 DISCUSSION
Understanding dietary preferences and resolving differences in congeneric small predator species may provide important information related to selective pressures and adaptive responses of species (Brandl et al., 2020; Longenecker, 2007; Razgour et al., 2011). Through a multimethod approach combining visual and high-resolution molecular techniques (DNA metabarcoding) for gut content analysis and bulk-tissue stable isotope analysis (δ15N, δ13C), this study provides evidence of dietary partitioning and the first holistic trophic assessment of three common pseudochromid species in the Red Sea. We reveal that Pseudochromis flavivertex, P. fridmani, and P. olivaceus present different dietary specializations and low dietary niche overlap among species. Visual and metabarcoding assessments revealed similar diet composition patterns but different taxonomic resolution. Furthermore, stable isotope analysis revealed differences in the isotopic niche and utilization of different energy channels. Although stomach content analysis did not show intraspecific differences in the diet composition at the color morph level within P. flavivertex, suggesting similar dietary preferences within the same species, stable isotope analysis showed different isotopic niche and resource use between morphs.
In the present study, visual and metabarcoding analysis of gut content showed that Pseudochromis flavivertex (M1 and M2), P. fridmani, and P. olivaceus in the Red Sea mainly feed on small invertebrates (>70%), including crustaceans, mollusks, and annelids, as well as small fishes and larvae of coral reef fishes with marked differences among species but not between morphs for P. flavivertex. The presence of planktonic organisms, like copepods, and early life stages of crustaceans in their diet suggest these fishes feed upon both benthic and planktonic prey in different proportions. For example, cyclopoid and harpacticoid copepods, found in the P. flavivertex diet, are abundant in hard corals and the epilithic algal matrix in coral reefs (Cheng et al., 2016; Kramer et al., 2012, 2013). Due to their slow movements relative to pelagic copepods, harpacticoid copepods are likely easier to capture as they can be hunted without leaving the protection of the benthos, providing a safer resource (Kramer et al., 2013). Conversely, calanoid copepods found in the diet of P. fridmani sometimes form dense stationary aggregations in the water column (Clarke, 1999; Davis et al., 1992). This provides fish inhabiting cavities of coral walls to prey on the zooplankton within the relative safety of their sheltered environment (Depczynski & Bellwood, 2004). Small fishes (e.g., Gobiiformes) and larvae of coral reef fish (e.g., Acanthuriformes and Labriformes) were also found in the diet of some pseudochromid species but to a lesser extent. Predation on small fish has been documented previously for other pseudochromid species (e.g., Pseudochromis fuscus), resulting in a high level of juvenile mortality with high selectivity on damselfish and cryptobenthic fish (e.g., Gobiidae and Tripterygiidae) (Feeney et al., 2012; Goatley et al., 2017; McCormick & Holmes, 2006; Palacios et al., 2016). This highlights the role of these species as predators of small fish and invertebrates and potential regulators of small fish community composition on coral reefs, especially in degraded reef habitats (Coker et al., 2009).
Despite being small mesopredators, microplastics were also found in the gut tract of some individuals. In the Red Sea, microplastics have been extensively found in the digestive tracts and the gills of fishes, with a higher amount of microplastic ingested by benthic fish (Baalkhuyur et al., 2018). Microplastics are easy to ingest by fishes due to their small size (<5 mm), coloration, and buoyancy (Huang et al., 2023), which makes them attractive to fish, which confuse these for prey (Cole et al., 2011). Likewise, microplastic ingestion may result from eating lower trophic organisms that have themselves consumed microplastics (Cole et al., 2011). Either direct or indirect consumption, microplastic is a critical threat to fish and other marine organisms as it can accumulate through different trophic levels in the food chain (Huang et al., 2023). Therefore, these findings also provide evidence of the impact of plastic pollution on a coral reef mesopredator, demonstrating a path for bioaccumulation of these particles in larger, fisheries species.
Overall, the average dietary niche overlap of 0.26 and 0.16 among the three pseudochromids species for 18S and COI datasets in our study is low compared to the 0.6 threshold, indicating niche overlap in studies based on visual identification of prey items, limiting taxonomic resolution and masking potential differences in fish diet (Castellanos-Galindo & Giraldo, 2008; Lawton et al., 2012). Here, low trophic niche overlap values can be explained by the higher prey taxonomic resolution obtained by DNA metabarcoding, allowing us to detect dietary differentiation among species that cannot be detected by visual analysis. However, low values may also be the result of the diversity of prey available to these fishes, the low sample size that can decrease the overlap among species, and the high number of unidentified prey items obtained with COI due to the lack of well-curated databases for the Red Sea (Carvalho et al., 2019). In general, the lack of well-curated databases is one of the main bottlenecks in metabarcoding studies (van der Loos & Nijland, 2021). Although some of the most commonly used databases (e.g., BOLD and NCBI GenBank) are well curated and undergo strict quality checks, a large proportion of their data is private (e.g., ~40% in BOLD), limiting data accessibility (van der Loos & Nijland, 2021). Furthermore, these databases differ in their accuracy of species identification due to coverage gaps in some taxonomic groups (van der Loos & Nijland, 2021; Weigand et al., 2019). While our study had a sufficiently large sample size to show the patterns in dietary differences, the rarefaction curves indicate that the study's coverage was not strong for all species. Therefore, differences in sample size for pseudochromid species may mask potential dietary overlap.
Although similar patterns in diet composition of dottybacks were detected using different approaches (visual and metabarcoding analysis), the visual gut content analysis revealed a lower taxonomic resolution of prey items in comparison with the metabarcoding assessment. However, our results show key advantages of including morphological analysis in dietary studies. In the present study, the visual analysis allowed the detection of different life stages of crustaceans (e.g., Decapoda: zoea and megalopa) in the dottybacks diet that was not recognized by the metabarcoding approach, which highlights the need to further improve DNA barcode libraries. According to Clare (2014), different life stages of prey groups can only be distinguished through a morphological approach, which can discriminate another level of dietary differentiation (e.g., consumption of adult vs. larvae forms). Nevertheless, the use of two molecular markers (18S and COI) in the metabarcoding analysis allowed a more robust assessment of the diet and provided different levels of taxonomic resolution of prey. In our study, COI detected a higher diversity in the diet (zOTUs = 251) compared to 18S (zOTUs = 223). Several studies have compared the performance of these two universal primers in the taxa detection in metabarcoding studies (e.g., zooplankton: Clarke et al., 2017; marine hard-bottom communities: Wangensteen et al., 2018; reef fish diet: Coker et al., 2023; and ARMS plates: Casey et al., 2021). Therefore, this highlights the importance of including multiple universal markers in gut content metabarcoding studies as they enhance the detection of different prey taxa in fish with a diverse diet such as dottybacks.
However, gut content DNA metabarcoding has methodological biases that may affect the taxonomic diversity detected (e.g., DNA extraction method, amplification bias, sequencing errors, and bioinformatics biases; reviewed in Casey et al., 2021). Likewise, both markers used in this study present limitations that impact the performance of the metabarcoding analysis (Casey et al., 2021). COI has a higher availability of well-curated databases and, due to the high substitution rate, it provides a higher taxonomic precision compared to 18S, which presents a relatively conserved nature and has shown inferior taxonomic resolution in metabarcoding studies (Andújar et al., 2018; Casey et al., 2021). However, the high rates of mutation of COI may cause saturation at high taxonomic levels, which inhibits accurate taxonomic assignments (Deagle et al., 2014). 18S region, which is the more commonly selected universal marker in broad-range biodiversity assessments, underestimates eukaryotic species, especially taxa that are poorly represented in DNA reference databases (e.g., cryptic microbial metazoans; Casey et al., 2021). Another limitation of using universal primers in gut content analysis is the amplification of gut parasites (e.g., nematodes and platyhelminthes) and secondary consumption sequences (e.g., diets of prey, parasites of prey, or material consumed incidentally during feeding), which highlights the importance of the biological knowledge to make good interpretations in dietary analysis (Berry et al., 2015; Pompanon et al., 2012).
Besides the differences found in the prey composition between species, this study also showed differences in the isotopic niche breadth and trophic position of three species of Pseudochromis. There are several possible explanations for these patterns. The isotopic baselines can fluctuate across small spatial scales (Cybulski et al., 2022), which can be reflected in the isotopic values of the fish. While the two color morphs of P. flavivertex were collected in the same coastal habitat during the same sampling period, our sources revealed variation in the isotopic values within the same environment. Pseudochromis flavivertex (M1 and M2) feed predominantly on cryptobenthic invertebrates such as cyclopoid copepods and mollusks, and larvae of coral reef fish, which may exhibit fluctuation in their own isotope values due to potential variation in the available nutrients (Cybulski et al., 2022). Fish with a more generalist diet, such as P. flavivertex (M1 and M2), present a more variable isotopic niche as they likely feed opportunistically on a higher variety of prey with more heterogeneous isotopic values, resulting in larger isotopic niches (Cybulski et al., 2022; Miller et al., 2019; Nagelkerken et al., 2009). On the contrary, small trophic niches are likely characteristic of species having either small foraging ranges or that consume prey with homogenous isotopic signatures and close to the source of the basal resource (e.g., zooplanktivorous) (Miller et al., 2019), as it was found for P. fridmani. Overall, these results complement the dietary information found with the gut content DNA metabarcoding, showing that P. flavivertex, P. fridmani, and P. olivaceus utilize different energy channels to varying degrees. This leads to isotopic niche segregation across species and covaries with the habitat use of these species.
5 CONCLUSIONS
We highlight the role of pseudochromid species as important predators of small cryptic invertebrates and small fishes and illustrate their key role as links in the transfer of energy toward higher trophic levels. In our study, we detected dietary partitioning across congeneric species of pseudochromids in the Red Sea, by using a multimethod approach combining visual and high-resolution molecular techniques (DNA metabarcoding) for gut content analysis and bulk-tissue stable isotope analysis (δ15N, δ13C). We also present the first holistic trophic assessment of three common species of pseudochromids in the Red Sea.
Although visual identification and DNA metabarcoding-based analysis used for stomach content analysis did not show differences in the dietary composition at the color morph level within P. flavivertex, the isotopic signature showed marked differences in the isotopic niche and resource use between morphs. Therefore, this study highlights the importance of using complementary methods and universal markers when assessing species' trophic ecology and delineating their dietary niches as they provide different and complementary information necessary to understand the role that small mesopredators play in coral reefs.
AUTHOR CONTRIBUTIONS
SPN and DJC conceived the study; SPN, DJC, and VNP collected the fish; SPN collected and processed zooplankton and algae samples; SPN and MSJ performed fish dissections; SPN performed the visual analysis of gut content; SPN, MSJ, and VNP carried out DNA extractions; SPN, CB, and EA carried out molecular lab work; CPA conducted bioinformatics processing; MDT processed and contributed to the analysis of the stable isotope samples; SPN performed statistical analysis; SPN wrote the first draft of the manuscript with guidance, feedback, and input from DJC and MLB. All authors contributed to editing and approved the final draft of the manuscript.
ACKNOWLEDGMENTS
We would like to acknowledge the field and labwork support of Irene Salinas, Kaitlyn O'Toole, Alex Kattan, Areen Nasif, and João Duarte, KAUST Coastal and Marine Resources Core Lab, and all the fish that were sampled for this project. We would like to acknowledge the use of the KAUST Bioscience Core Lab (BCL) for DNA extraction, library preparation, and sequencing and the resources at the KAUST Advanced Computing Core Lab for performing the computational analyses. Funding for this work was provided by KAUST (baseline funding to M.L.B.).
CONFLICT OF INTEREST STATEMENT
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data are provided within the manuscript and supporting tables. Final 18S and COI zOTU sequences are available from Dryad Digital Repository. DOI: https://doi.org/10.5061/dryad.fqz612k12. The raw data from the sequencing of COI and 18S rRNA gene-based amplicons are available at the European Nucleotide Archive (ENA) under the study accession numbers PRJEB64896 and PRJEB65108, respectively.




