Environmental DNA–RNA dynamics provide insights for effective monitoring of marine invasive species
Abstract
Environmental DNA and RNA (eDNA/eRNA) can serve as molecular tools for biodiversity monitoring and biosecurity surveillance. However, uncertainties still exist regarding the persistence and dynamics of marine nucleic acids in the environment and the effects of post-sampling storage on species detectability. To bridge these gaps, an experiment was conducted in an Auckland marina, a known New Zealand entry point for marine non-indigenous species (NIS). We targeted a prominent invader, the Mediterranean fanworm Sabella spallanzanii, for eDNA/eRNA-based detection. Permeable dialysis bags filled with seawater collected near an S. spallanzanii colony were deployed in the marina to simulate environmental conditions, with a subset of bags stored on ice to mimic field storage conditions. Sabella spallanzanii eDNA/eRNA signal was quantified using droplet digital PCR on samples collected over 24 h of dialysis bag deployment. Results challenged traditional first-order decay models, showing inconsistent eDNA/eRNA signal patterns and no significant concentration changes between 0 and 24 h. Consequently, total eDNA fragmentation was assessed using the Agilent 2100 Bioanalyzer® electrophoresis system, which revealed a rise in the number of total eDNA fragments and an unexpected increase in the median fragment size of total eDNA under field conditions over time, likely originating from the ambient microbiome. Additional analysis using long-read sequencing (Oxford Nanopore Technologies, UK) revealed an increase in microbial eDNA reads within the in-field samples, suggesting potential microbial growth within the dialysis bags. In contrast, the ice-stored samples exhibited no significant changes in the number of reads assigned to microbial taxa, implying limited microbial growth in cold storage. These findings provide insights into total eDNA dynamics and its potential impact on targeted eDNA concentration and detection. Further comprehensive research on eDNA/eRNA dynamics, particularly focused on eRNA, is essential, as this understanding is crucial for refining survey interpretation and sampling design for effective environmental management.
1 INTRODUCTION
Revolutionary molecular detection tools incorporating environmental DNA (eDNA) and RNA (eRNA) are effective for detecting and monitoring biodiversity across diverse ecosystems and have opened new avenues for biosecurity applications (Clarke et al., 2023; Collins et al., 2012; Pochon et al., 2017; Trujillo-González et al., 2019, 2022; von Ammon et al., 2019). The benefits of eDNA and eRNA analysis over traditional survey methods are substantial, including minimized ecosystem disturbance, scalability, cost-effectiveness, and the capability to detect elusive species (Bowers et al., 2021; Harrison et al., 2019; Larson et al., 2020; Pearman et al., 2021; Zaiko et al., 2018). The inclusion of eRNA analysis is deemed to improve species presence or absence inference further, enabling better identification of living and viable organisms and providing valuable insights into population dynamics and overall ecosystem health, thus complementing information gathered through eDNA analysis (Bowers et al., 2021; Pochon et al., 2017; Scriver et al., 2023; Tsuri et al., 2021; Wood et al., 2020; Yates et al., 2021).
Despite the growing adoption of eDNA and eRNA in biomonitoring and biosecurity, critical knowledge gaps remain concerning nucleic acid persistence (duration of detectability) and decay processes in marine coastal environments, particularly for eRNA (Barnes et al., 2014; Hinz et al., 2022). These knowledge gaps can impact the interpretation of molecular survey results, leading to incorrect inferences of species presence/absence and distribution (Farrell et al., 2021; Harrison et al., 2019; Yates et al., 2021; Zaiko et al., 2018). Also, limited information is available regarding the decay progression of eDNA and eRNA during post-sample handling, such as storage and transportation (Curtis et al., 2021; Yamanaka et al., 2016). Understanding these dynamics is crucial for optimizing sampling protocols and enhancing the accuracy of eDNA/eRNA-based monitoring. Moreover, comprehending eDNA and eRNA persistence can expand the technology's applications beyond mere presence/absence detection. Quantifying decay rates (the rate at which detectable quantities of eDNA/eRNA decrease in the environment due to degradation) and studying persistence patterns enable the development of hydrodynamic models to predict source populations and refine biosecurity strategies and management plans (Andruszkiewicz et al., 2019; Ellis et al., 2022; Fukaya et al., 2021).
To date, extensive research and reviews have been conducted to investigate the persistence of eDNA/eRNA and to understand the factors influencing the degradation process and decay rate in aquatic environments (Allan et al., 2021; Amarasiri et al., 2021; Barnes et al., 2014; Kagzi et al., 2022; Lamb et al., 2022; Salter, 2018; Scriver et al., 2023; Strickler et al., 2015). However, many of these studies relied mainly on laboratory experiments, possibly leading to enhanced eDNA/eRNA detectability and potentially biased decay estimates (Skinner et al., 2020). Only a limited number of studies have investigated the persistence of eDNA in-situ, with results suggesting that eDNA decays more rapidly and exhibits inconsistent temporal changes in eDNA concentrations challenging the traditional exponential decay model used to determine decay rate constants (Ely et al., 2021; Murakami et al., 2019; Scriver et al., 2023). To our knowledge, no field-based experiments have been conducted to explore eRNA dynamics.
The primary objective of this study was to investigate the in-situ persistence and decay rates of eDNA and eRNA shed from a model coastal marine non-indigenous species (NIS), Sabella spallanzanii (Gmelin, 1791) – a large Mediterranean fanworm. We employed a novel method using solute permeable dialysis bags, that allow water and dissolved constituents to flow through the sample, mimicking realistic environmental conditions, while preventing the passage of larger molecules or particles, including target eDNA and eRNA fragments (Andruszkiewicz et al., 2017). The dialysis bags filled with ambient marine water from a location with an abundant S. spallanzanii population were deployed in the field for 24 h and sampled periodically to study the dynamics of the eDNA and eRNA signal in-situ. In addition, to investigate the effects of sample transportation and storage conditions, a subset of bags was stored on ice for 24 h, simulating conventional field storage conditions. To determine the presence of eDNA and eRNA from S. spallanzanii, we utilized a species-specific droplet digital Polymerase Chain Reaction (ddPCR) assay targeting the mitochondrial Cytochrome c Oxidase subunit 1 (COI) gene. Furthermore, we aimed to employ post-extraction molecular analysis to study the characteristics of total eDNA, such as fragment size, using an Agilent 2100 Bioanalyzer® (Agilent Technologies, USA), an automated electrophoresis system, for better insights into the integrity dynamics of eDNA and its associated signal over time. In addition, to assess the composition of total eDNA, including the identification and analysis of S. spallanzanii-specific eDNA fragments, we performed adaptive sequencing and metagenomic analysis using long-read sequencing with Oxford Nanopore Technology (ONT)'s MinION™ (Oxford Nanopore Technologies, UK).
We formulated the following hypotheses for our investigation. First, we hypothesized that eRNA will decay faster than eDNA due to its inherent instability and higher susceptibility to degradation processes (Fontaine & Guillot, 2003; Voet & Voet, 2011). Second, we anticipated a decreased persistence for in-situ samples compared with samples stored on ice, owing to the influence of environmental abiotic and biotic factors, such as temperature and microbial activity (Barnes et al., 2014; Jo et al., 2022; Lamb et al., 2022; Zulkefli et al., 2019). Last, we hypothesized that employing molecular techniques, such as electrophoretic fragment analysis (Agilent Technologies, USA) and long-read sequencing (Oxford Nanopore Technologies, UK), would yield additional information on the characteristics, composition, and state of PCR-amplified DNA versus total DNA, providing further insights into marine-based eDNA decay (Mauvisseau et al., 2022).
2 MATERIALS AND METHODS
2.1 Field setup
Using semi-permeable dialysis bags (Spectrum™ Spectra/Por™ 1 RC Dialysis Membrane Tubing: Spectrum Laboratories Inc., CA, USA), a total of 500 mL per bag of ambient seawater was collected in the close vicinity of a visually observed S. spallanzanii population at the Viaduct marina, Waitematā Harbour (Auckland, New Zealand: 36.81° S and 174.98° E) on June 11, 2021. The dialysis bags had a diameter of 120 × 500 mm with a molecular weight pore size cut-off of 6 ± 8 kilodalton (kDa), equivalent to approximately 9 ± 12 base pairs of double-stranded DNA. This allowed the passage of water and dissolved constituents into the sample but restricted molecules or particles larger than the pore size, such as nucleic acids, from entering or exiting the bag (Andruszkiewicz et al., 2017) (Figure 1).
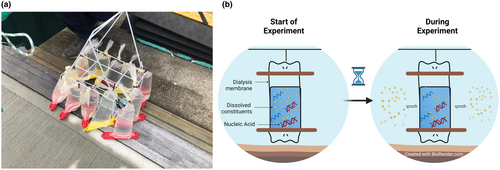
Fifteen dialysis bags containing water samples were tied to a bottle carrier and deployed into the harbour at the collection site. The dialysis bags were placed at a depth of 0.5–1 m, where living S. spallanzanii individuals were observed on the adjacent seawall (Figure 1). An additional 15 dialysis bags were stored on ice to simulate storage conditions typically encountered in the field. The dialysis bags stored on ice were covered with ice inside a cooler, with no light. The cooler was regularly replenished with fresh ice, with the goal of maintaining a consistent temperature and preserving the ice in solid form, minimizing the potential for osmosis between the dialysis bags and ice within the cooler. Three samples were processed for analysis at time point zero; therefore, no experimental treatment was applied to them.
Nine additional dialysis bags, filled with distilled water, were intentionally spiked with naked 100 ng/μL of S. spallanzanii DNA/RNA and 9 bags were spiked with S. spallanzanii tissue (to mimic the heterogenetic complexity of eDNA and eRNA found in natural samples) to serve as positive controls. Three of each positive control sample type were stored on ice to simulate storage conditions (n = 6 positive controls), while another three bags of each type were submerged in the harbour, following the same procedure described for the samples (n = 6 positive controls). Three samples of each positive control were processed for analysis at time point zero. For negative controls, three dialysis bags were filled with 500 mL of distilled water. These negative control bags were suspended in the harbour, following the same procedure as for the samples and positive controls.
Dialysis samples from each treatment group (ice and harbour) were collected in triplicate at specific time intervals: 0, 3, 6, 9, 12, and 24 h (total n = 33). Positive controls were also processed in triplicate at 0 and 24 h (total n = 18), while negative controls were processed at 24 h time points (total n = 3). Immediately after collection, the water from the dialysis bags was filtered onto cellulose acetate 5 μm membrane filters using a manifold vacuum filtration system (Jo et al., 2019; Pochon et al., 2024; Zaiko et al., 2022). Filter membranes were cut in half and stored in microcentrifuge tubes at −80°C until further processing. Between samples, the tweezers and filter holders were soaked in 10% bleach and 0.1% sodium thiosulfate (5 min each), followed by rinsing in Millipore Milli-Q water (MQ Water) and drying. Physico-chemical parameters (temperature and light) were measured at every sampling event using a temperature data logger and photodetector system (Table S1) (HOBO Pendant Temperature/Light Data Logger, Onset, USA).
2.2 DNA and RNA extraction and cDNA synthesis
Each step of the molecular analysis (i.e., DNA and RNA extractions, PCR setup, template addition, ddPCR analysis) was conducted in a separate sterile laboratory dedicated to that step with sequential workflow to ensure no cross-contamination. Each room was equipped with UV sterilization that was switched on for a minimum of 15 min before and after each use. The PCR setup and template addition were undertaken in laminar flow cabinets with HEPA filtration. Aerosol barrier tips (Axygen BioScience, CA, USA) were used throughout.
The filters were transferred into ZR BashingBead Lysis Tubes (2.0 mm; Zymo Research, CA, USA) containing Lysis Buffer (1 mL) from the Quick-DNA/RNA™ Miniprep Kit (Zymo Research, CA, USA). All samples were homogenized via bead beating for 2 min at 1500 RPM (1600 MiniG Spex SamplePrep, NJ, USA) and centrifuged (10,000 g, 5 min, 20°C; Eppendorf Centrifuge 5430R, Hamburg, Germany). Total DNA and RNA were then co-extracted from each sample using the Quick-DNA/RNA™ Miniprep Kit (Zymo Research, CA, USA), following the manufacturer's protocol.
The quantity and quality of the extracted RNA were measured using a NanoPhotometer (Implen, Munich, Germany). Trace DNA molecules carried over in RNA extracts were eliminated by two sequential DNase treatments, as described in Langlet et al. (2013). To confirm the absence of DNA in RNA, eluents were assessed with a universal bacterial marker end-point PCR (16S mitochondrial rRNA primer set) after DNase treatment (Klindworth et al., 2013). The DNase-treated RNA was transcribed into cDNA using the SuperScript™ III reverse transcriptase (Life Technologies, CA, USA). The resulting products, including DNA, RNA, and cDNA, were stored as separate aliquots, and frozen (−20°C for eDNA/cDNA and −80°C for pure RNA and DNase-treated RNA samples) until further analysis. The cDNA products are hereafter referred to as eRNA.
2.3 Droplet digital polymerase chain reaction
Droplet digital PCR (ddPCR) was conducted in an automated droplet generator (QX200 Droplet Digital PCR System™, BioRad, CA, USA). Sabella spallanzanii-specific Cytochrome c Oxidase subunit 1 (COI) copy numbers were quantified using ddPCR for all samples and controls, including all negative extraction controls (DNA and RNA) on a QX200 System™ (BioRad, CA, USA). To calculate target copy number (per μL), S. spallanzanii specific COI primers targeting a 90 base pairs (bp) region (Sab3F: 5′-GCT CTT ATT AGG CTC TGT GTT TG-3′) and (Sab3R: 5′-CCT CTA TGT CCA ACT CCT CTT G-3′) and TaqMan Probe (Sab3-QPCR Probe: 5′-FAM/AAA TAGT TCA TCC CGT CCC TGC CC/BkFQ-3′) were used as described in Wood et al. (2017). The hydrolysis probes were dual-labeled with a 5′-6-carboxyfluorescein (6-FAM) fluorescent tag and a 3′ Black Hole Quencher. Each ddPCR reaction included 450 nM of each primer and probe, 1 × BioRad ddPCR Supermix for probes (No dUTP) (BioRad, CA, USA), 3 μL DNA or eRNA, and sterile water for a total reaction volume of 21 μL. The BioRad QX200 droplet generator partitioned each reaction mixture into approximately 20,000 nanodroplets by combining 20 μL of the reaction mixture with 70 μL of BioRad droplet oil. After processing, this resulted in a total nanodroplet volume of 40 μL, which was transferred to a PCR plate for amplification using the following cycling protocol: hold at 95°C for 10 min, 40 cycles of 94°C for 30 s, 60°C for 1 min, and a final enzyme deactivation step at 98°C for 10 min. Each well of the plate was then individually analyzed on the QX200 instrument to establish the threshold value separating negative and positive droplets and perform absolute quantification of target DNA or RNA. A positive control of extracted DNA from S. spallanzanii tissue and a negative (MQ-water) control was included on each plate. The results were converted to copies per mL using the following formula: number of copies per μL × 21 [the initial volume of the PCR reaction (μL)] × 50 or 25 [the volume used to elute the DNA/RNA during extraction (μL)] and assuming 100% extraction efficiency which we acknowledge is unlikely, divided by 500 or 300 (the amount of water filtered). Based on dilution experiments undertaken with serial dilutions of S. spallanzanii by Wood et al. (2020) and our experience and observations of noise (e.g., proportions of fluorescing droplets in water blanks), the detection of the assay was set to 0.1 copies/μL, with any concentrations below 0.1 copies/μL recorded as not detected and transformed to the lowest level of detection in copies/mL (Table S2).
2.4 Agilent 2100 bioanalyzer® electrophoresis
DNA concentrations were quantified using Qubit 1× dsDNA HS Assay (Life Technologies, USA), and fragment analysis of 1 ng DNA samples was performed using the Agilent 2100 Bioanalyzer® (Agilent Technologies, USA) with the Agilent DNA 1000 Reagents (Agilent Technologies, USA) and High Sensitivity DNA Chips® (Agilent Technologies, USA). The data were analyzed using the 2100 Expert software.
2.5 Oxford nanopore technologies minion™ sequencing library preparation
Libraries were prepared using the Native Barcoding Kit 96 V14 SQK-NBD114.96 (Oxford Nanopore Technologies, UK) following the “Ligation sequencing gDNA – Native Barcoding Kit 96 V14 (SQK-NBD114.96)” (version NBE_9171_v114_revI_15Sep2022) (Oxford Nanopore Technologies, 2023b). Prior to library preparation, the concentrations of the samples (extracted DNA from Section 2.2) were measured using the Qubit fluorometer with the 1× dsDNA HS Assay Kit (Thermo Fisher Scientific, USA). For the end-prep, 11.5 μL of samples with concentrations between 5 and 200 fmol (2–77 ng) were used, and the NEBNext FFPE DNA Repair Kit (New England Biolab, USA) was employed to improve the sequencing of samples and increase yield. During native barcoding ligation, 5 μL of each barcoded sample was pooled and purified using Agencourt AMPure XP beads (Beckman Coulter, USA) at a 0.4× ratio. Following native barcode ligation, adapter ligation and clean-up were performed as suggested in the protocol, using the short fragment buffer (SFB) to purify all fragments equally. The pooled library was purified again using Agencourt AMPure XP beads (Beckman Coulter, USA) at a 0.4× ratio. The final pool was diluted to 20 fmol and sequenced on a MinION™ sequencer (Mk1B; Oxford Nanopore Technologies, UK) using an R10.4.1 flow cell (FLO-MIN114; Oxford Nanopore Technologies, UK), targeting an average coverage of 35,000 reads per barcoded sample (Egeter et al., 2022). The sequencing run lasted approximately 65 h, and at 54.8 h, the run was paused to allow for the washing of the cell to recover pores. To restart the run, a total of 2 μL of wash solution (Oxford Nanopore Technologies, UK) was mixed with 398 μL of diluent to make a wash mix. The liquid was withdrawn from the waste port and discarded before 400 μL of the wash mix was loaded into the priming port and left for 60 min. This was then removed from the waste port, and more priming mix was loaded into the priming port, followed by more library loaded into the SpotON port. The sequencing run was then allowed to continue for another approximately 10.2 h. The unprocessed MinION™ files used in this study have been deposited into the National Centre for Biotechnology Information (NCBI) Sequence Read Archive under the accession number: PRJNA1033365.
2.6 Minion™ data processing
To enrich the potentially low-abundant targeted S. spallanzanii reads, adaptive sequencing (real-time selective sequencing) (Loose et al., 2016; Oxford Nanopore Technologies, 2023a; Urban et al., 2023) with the MinION™ platform was initiated, employing the entire mitochondrial genome (NCBI Genbank: MW002660.1) as a reference. All output sequencing data (across all adaptive sampling decisions, including ‘unblock’ for rejected reads, ‘no_decision’ for reads that were too short for a decision to be taken, and ‘stop_receiving’ for accepted reads) underwent base calling, demultiplexing, and barcode trimming through Guppy (v6.3.8; Oxford Nanopore Technologies, UK) with high accuracy settings, including parameters such as -qscore_filtering (default q-score 7), --trim_barcodes, --detect_mid_strand_barcodes, specified as -c dna_r10.4.1_400bps_hac.cfg. MinIONQC (v1.4.1, https://github.com/roblanf/MinION_qc) (Lanfear et al., 2019) was utilized to generate various diagnostic plots and data for quality control of the sequencing data from the MinION™. To recover some additional reads, manual demultiplexing and trimming of unclassified and unused barcodes were performed using Porechop (v0.2.4, https://github.com/rrwick/Porechop) (Wick et al., 2017). To quantify the enhancement in recovered sequences per barcode, we calculated the percent increase by comparing the number of sequences before and after applying Porechop (Becerra-Wong et al., 2023); notably, this resulted in a 55–82% increase in the number of reads per barcode in this dataset. The resultant demultiplexed and trimmed reads were consolidated into a single directory. For sequence alignment, Minimap2 (v2.24-r112, https://github.com/lh3/minimap2) (Li, 2018, 2021) was used to map FASTQ files to the S. spallanzanii genome (GenBank: MW002660.1) and Samtools (v1.9, https://github.com/samtools/samtools) (Danecek et al., 2021) was used to process the alignment data. The reads were then categorized into mapped (aligned to the S. spallanzanii genome) and unmapped FASTA files. R version 4.2.1 software (R Core Team, 2019) was used for subsequent analyses, including the evaluation of sequence length, sequence count, and fragment frequency for both S. spallanzanii sequences (mapped) and metagenomics data (unmapped). The EPISME labs Nextflow (v23.04.2) (Di Tommaso et al., 2017) bioinformatics wf-metagenomics workflow was used to perform shotgun metagenomics and identify the origin of single reads from the unmapped data. The kraken2 (v2.2.1) (Wood et al., 2019) pipeline, using the kraken2 PluPFP8 database from 6/5/2023 (Langmead, 2023), encompassing references for Archaea, Bacteria, viruses, plasmids, humans, UniVec_Core, protozoa, fungi, and plants, was employed for classification. The Pavian interactive application (Breitwieser & Salzberg, 2020) was utilized to visualize classification outcomes.
2.7 Data analysis
All statistical analyses were conducted in R version 4.2.1 software using “tidyverse” (v1.3.0) and “dplyr” (v1.4.0) packages (R Core Team, 2019; Wickham et al., 2019, 2022). All data visualization was carried out in R using “ggplot2” (v3.4.0) (R Core Team, 2019; Wickham, 2016). A Kruskal-Wallis test was performed to determine whether the eDNA/eRNA signal strengths (copies/mL) were significantly different among treatments (harbour and ice storage conditions), and Wilcoxon rank sum test and Wilcoxon signed rank test for paired samples were used to determine whether eDNA/eRNA signal strengths (copies/mL) were significantly different between eDNA/eRNA and timepoints. Total eDNA fragment data was divided into four groups: >10,000 bp, between 5000 and 10,000 bp, between 1000 and 5000 bp, and <1000 bp, and fragments <100 bp were removed.
3 RESULTS
3.1 Sabella spallanzanii environmental DNA and RNA signal strength
No amplification was observed from the negative controls throughout the entire experiment. In addition, no notable volume loss was observed in the environmental water samples at the time the bags were filtered.
The positive controls showed successful detection in both the harbour and ice samples, for both naked S. spallanzanii DNA/RNA and S. spallanzanii tissue. Notably, the eDNA signal from positive controls with naked S. spallanzanii DNA exhibited a substantial decrease in signal strength (copies/mL) for the ice storage treatment (72.4–0.740 copies/mL) and a slight decrease in harbour samples after 24 h (72.4–46.8 copies/mL) (Figure 2a,b). On the other hand, in the case of naked S. spallanzanii eRNA controls, a smaller difference was observed in S. spallanzanii COI eRNA signal strength after 24 h. In ice-stored samples, the signal strength decreased from 5.23 to 2.72 copies/mL, while in harbour-stored samples, a significant decrease was observed, with signal strength dropping from 5.23 to 0.320 copies/mL (Figure 2c,d). In positive controls spiked with S. spallanzanii tissue, the eDNA signal in the ice and harbour samples remained relatively stable, slightly increasing (from 109,000 to 186,000 copies/mL) and decreasing (from 109,000 to 38,000 copies/mL), respectively (Figure 2e,f). However, there was a notable increase in eRNA signal strength in both storage conditions (ice: 809–754,000 copies/mL and harbour: 809–9850 copies/mL) for tissue-base positive controls (Figure 2g,h).
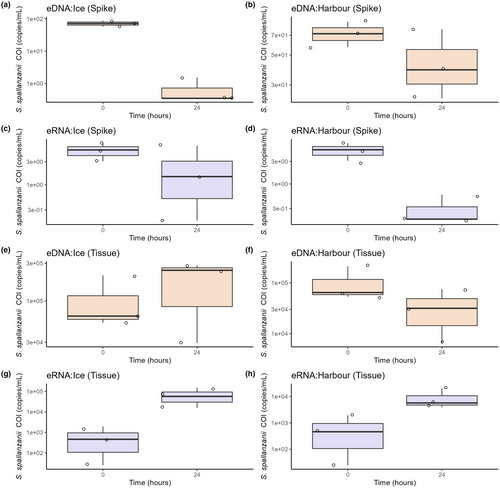
Statistical analysis using a Kruskal-Wallis chi-square test revealed an overall significant difference in S. spallanzanii eDNA and eRNA signal among the control samples (Kruskal-Wallis chi-squared = 5.8303, df = 1, p-value = 0.01575), with eDNA generally having higher detected signal compared to eRNA (Figure 2). Furthermore, there was a significant difference in eDNA and eRNA signal when comparing controls based on the material used (naked S. spallanzanii DNA/RNA or S. spallanzanii tissue) (Kruskal-Wallis chi-square, p < 0.0001 for both), with S. spallanzanii tissue showing significantly higher eDNA/eRNA (copies per mL) (Figure 2). However, there was no significant difference when comparing eDNA and eRNA signal between the ice and harbour storage conditions (the Kruskal–Walli's chi-square, p = 0.628 and p = 0.7285, respectively).
Analysis of the environmental water samples showed both S. spallanzanii eDNA and eRNA were detected in at least one replicate in both the harbour and ice samples at all designated time points, except for eRNA in the harbour samples at 6 h. Surprisingly, the detected eDNA and eRNA signals did not consistently decrease, with signal strength oscillating between time points (Figure 3). In the harbour samples, there was a decrease in S. spallanzanii COI eDNA and eRNA signals between 0 and 6 h, followed by a substantial increase at the 12 h time point, with the average signal strength rising from 1.02 to 7.72 copies/mL and 0.465 to 46.0 copies/mL, respectively (Figure 3a,b). This increase was then followed by a subsequent decrease in signal. Average S. spallanzanii COI eDNA signals for the samples stored on ice increased at the 6 h timepoint (1.86–4.86 copies/mL), while the average eRNA signal increased at the 3 h timepoint (1.31–21.4 copies/mL); both increases were followed by signal decreases (Figure 3c,d). Notably, time points associated with increases (12 h for both eDNA and eRNA in the harbour samples, and 6 h and 3 h for eDNA and eRNA ice samples) displayed higher data variability, with standard deviations (SDs) of 6.51, 42.5, 6.32, 12.3, and 6.41, in contrast to the average SD observed at other time points of 0.640 (STDEV ±0.543).
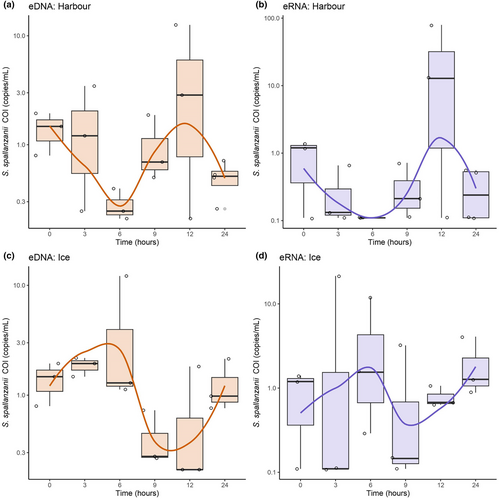
In addition to monitoring patterns of eDNA/eRNA dynamics and signals over time, we investigated changes in final signal strength (copies/mL) at the end of the 24 h. Remarkably, only the eDNA signal strength in the harbour treatment exhibited a marginally significant change in signal strength (Wilcoxon signed rank exact test, p-value = 0.05714) after 24 h. No significant differences were observed in eDNA signal strength following ice treatment or eRNA signal from either the ice or harbour treatments (Wilcoxon rank sum exact test, p-value = 0.75, p-value = 0.5, and p-value = 0.359, respectively).
We subsequently analyzed the end-point data for significant differences between the molecule type (eDNA/eRNA) and treatment conditions (harbour vs. ice) using the Kruskal-Wallis chi-squared test. When analyzing eDNA and eRNA signal (copies per mL) in relation to treatment (ice vs. harbour), no significant differences were observed between treatments for eDNA (Kruskal-Wallis chi-squared = 0.97692, df = 1, p-value = 0.323). A marginally significant difference was evident between treatments for eRNA (Kruskal-Wallis chi-squared = 3.36, df = 1, p-value = 0.0668). Similarly, we noted a marginally significant difference in signal between S. spallanzanii COI eDNA and eRNA (Kruskal-Wallis chi-squared = 3.3592, df = 1, p-value = 0.06683). Moreover, we observed a marginally significant difference in eDNA and eRNA signals when comparing environmental nucleic acid within the harbour treatment (Kruskal-Wallis chi-square p = 0.04605). Conversely, such a significant difference was not observed for samples subjected to the ice treatment (Kruskal–Wallis chi-square p = 0.529).
Finally, both treatments showed that eDNA and eRNA did not follow an exponential decay pattern (Figure 3). Attempts to fit a first-order decay rate model were unsuccessful, as indicated by the poor fit (R2 < 0.1 for all, data not shown).
3.2 Total environmental DNA fragment size distribution patterns
The Agilent 2100 Bioanalyzer® was used to investigate total eDNA fragment patterns both over time and by treatment in all samples. Positive control samples spiked with S. spallanzanii tissue were included. However, positive control samples spiked with naked S. spallanzanii DNA did not generate sufficient signal to be included in this analysis.
For the positive tissue control samples, an increased percentage of total eDNA fragments less than 1000 bps for both the ice and harbour treatments was detected at the 24 h timepoint (Figure 4). This trend aligns with the observed decrease in eDNA median fragment length over time, with a more significant decrease observed in the harbour samples compared to the ice samples (Figure S1). Additionally, both treatments showed an increase in the overall number of fragments; again, this shift was more pronounced in the harbour samples (harbour treatment; 0 h = 42 fragments, 24 h = 188 fragments and ice treatment; 0 h = 42 fragments, 24 h =89 fragments) (Figure S2).
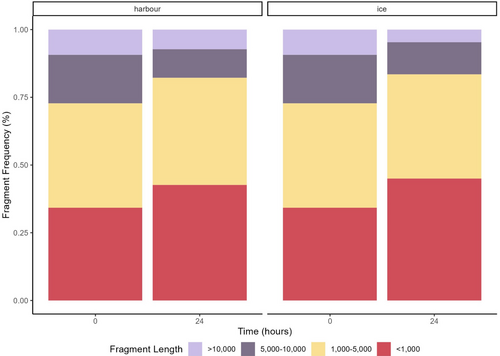
Total eDNA fragment size patterns for the environmental samples varied across the time points for both treatments (Figure 5). There was a general decrease in the percentage of smaller fragments (<1000 bp) for the harbour samples and an increase in larger fragments (>10,000 bp), consistent with the observed increase in the median total eDNA fragment length over time (Figure S3). In contrast, the ice samples displayed a relatively stable fragment pattern, with a slight increase in the number of smaller fragments at the 6 h time point (Figure 5). Similarly, the median eDNA fragment length stored on ice showed a relatively consistent pattern throughout the time series, with notable variability observed at 3 and 6 h time points (Figure S3).
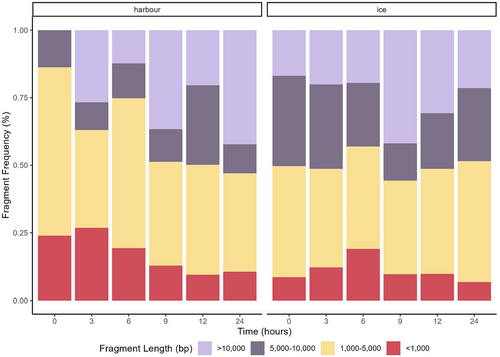
The samples stored in the harbour showed an increase in the number of fragments (0 h = 33 fragments and 24 h = 68 fragments) over time, while the number of fragments in the ice treatment remained relatively stable (0 h = 45 fragments and 24 h =46 fragments), with the in-harbour samples showing an increase at the 6 h time point (Figure S4).
3.3 Pilot study using long-read sequencing for environmental DNA dynamics
A total of 13,332,840 reads were generated from 59 libraries sequenced on a single Nanopore flowcell, with an average quality score (q-score) of 8.2. Oxford Nanopore sets a q-score cut-off value of 7 for quality filtering. After applying this 7 q-score cut-off across the run, we obtained 8,515,112 high-quality reads, with an average q-score of 9.33.
eDNA fragments from environmental harbour samples <1000 bp aligned sparsely with the S. spallanzanii mitochondrial genome. Larger eDNA fragments (>1000 bps) aligning to S. spallanzanii were absent in these samples, suggesting only smaller fragments (200–550 bps and 500–1000 bps, median length 497 bps SDTEV = 112) of S. spallanzanii eDNA were present across both treatment groups and time points (Figure S5).
Again, the positive control samples spiked with naked S. spallanzanii genomic DNA provided limited data, with these samples only yielding approximately 2500 reads, just above negative controls (average of 2215 reads); therefore, our primary focus shifted to tissue control samples with an average of 232,670 reads per barcode (ranging from 16,600 to 798,000). These exhibited a decline in larger S. spallanzanii sequence-aligned eDNA fragments (>10,000 bps and 5000–10,000 bps) across both treatment groups over time. Notably, ice samples exhibited an increased proportion of S. spallanzanii-aligned fragments in the 200–500 bps range (Figure S6).
The shotgun metagenomics analysis of the data revealed a large presence of unclassified reads in the environmental samples, constituting an average of 77.24% (STDEV = 18.86%) of the total reads. However, Oxford Nanopore sequencing revealed an increasing trend in read counts over time for both samples and controls in the harbour treatment, whereas the ice treatment displayed minimal fluctuations (Figures S7 and S8). Of note, the control tissue sample with the highest read count (797,624 reads) exhibited a larger proportion of reads associated with microbial (46.10%) and bacterial (45.90%) domains. This taxonomic classification pattern was only observed in the harbour samples (Figure S9).
4 DISCUSSION
While our experiment does not provide a determination of the decay rate, defined here as the rate at which detectable quantities of eDNA/eRNA decrease in coastal marine habitats due to degradation (Scriver et al., 2023), we instead focus on discussing the detection dynamics of eDNA/eRNA within a marine environment. This work encompasses both in-situ and post-sampling storage conditions, utilizing data from Agilent 2100 Bioanalyzer® electrophoresis and supplementary insights from the ONT's MinION™ to enhance our understanding of changes in the ecology of total eDNA.
4.1 Sabella spallanzanii environmental DNA and RNA signal dynamics
Detecting and quantifying eDNA and eRNA are challenging due to their susceptibility to various ecological and environmental factors (Barnes & Turner, 2015; Scriver et al., 2023). Studying species-specific eDNA and eRNA temporal changes under real-world conditions is crucial for accurate assessments, as controlled lab setups may distort findings and misrepresent natural conditions (Skinner et al., 2020).
Our study observed unexpected oscillations in S. spallanzanii eDNA and eRNA detected signal in the in-situ or harbour treatment over 24 h, including unexpected increases in eRNA and eDNA signal in control tissue samples. Variation in the eDNA concentration has been noted in many other eDNA studies (Allan et al., 2021; Klymus et al., 2015; Minamoto et al., 2017; Pilliod et al., 2013; Turner et al., 2014), with similar inconsistent detection patterns being reported by Ely et al. (2021) in in-situ coastal environments for grass carp (Ctenopharyngodon idella) using DNA sequencing and by Allan et al. (2021) for scyphomedusae (moon jelly, Aurelia aurita, and nettle, Chrysaora spp.) in laboratory settings using qPCR. Ely et al. (2021) attributed the patterns to eDNA transport, while Allan et al. (2021) hypothesized that scyphomedusae shed larger eDNA particles than other organisms, and as these larger eDNA fragments break down over time, it leads to an increase in extracellular DNA captured via sampling. These increases in extracellular DNA may lead to increases in eDNA signal, which could account for the sporadic spikes we observed in our in-situ samples and tissue control samples. Though we began to investigate this pattern using an electrophoretic assessment (Agilent 2100 Bioanalyzer®) for eDNA, the need to delve into the ecological properties and fate of eDNA and eRNA remains evident, particularly for eRNA in marine settings, where essential factors such as common eRNA forms (particulate, cellular, or extracellular), persistence, and degradation mechanisms are still unclear (Jo et al., 2022; Scriver et al., 2023; Yates et al., 2021).
This phenomenon might not be restricted to in-situ samples alone; it could also explain the inconsistent eDNA/eRNA signal patterns in field-stored (ice) samples. While similar oscillating patterns were observed in the ice samples, the pattern may indicate a delayed but similar detection trend. For instance, the spike-in harbour treatment signal at 12 h could align with the 24 h spike in ice-stored samples, suggesting that lower temperatures might slow down degradation mechanisms (Barnes et al., 2014; Jo et al., 2019, 2022; Kasai et al., 2020). Moreover, the lack of significant changes in copy numbers (copies/mL) over 24 h suggests that short-term storage on ice in the field does not hinder eDNA and eRNA signal detection. Therefore, complex preservation measures might not be necessary when samples can be processed within 24 h after collection. While this finding aligns with Curtis et al. (2021), who reported that eDNA samples can be filtered up to 2 days after collection without a significant loss in concentration or detectability when stored in dark and chilled conditions, our results offer valuable insights into the simplified handling of eRNA samples. Currently, there is limited data on the effects of in-field storage conditions on eRNA signal detection, with current methods suggesting the immediate storage of eRNA samples at −80°C until extraction (Bowers et al., 2021; Marshall et al., 2021; Wood et al., 2020). Therefore, our findings may also contribute to optimizing eRNA sample handling practices in the field.
When analyzing detection dynamics in the in-situ treatment, it is essential to consider environmental factors, particularly temperature, which numerous studies have identified as a significant determinant of eDNA/eRNA persistence and detection (Barnes et al., 2014; Cowart et al., 2018; Eichmiller et al., 2016; Jo et al., 2022; Kasai et al., 2020; Qian et al., 2022; Scriver et al., 2023). Light levels are also considered an important factor to monitor, but the existing evidence does not firmly support a direct impact of light on eDNA persistence (Andruszkiewicz et al., 2017; Machler et al., 2018; Strickler et al., 2015). In our study, the temperature, recorded by data loggers, remained relatively stable in the harbour treatment, averaging 16.27°C with minor fluctuations (STDEV = 3.21°C) (Table S1); therefore, this suggests temperature alone does not explain the observed oscillations in eDNA and eRNA detection. Similarly, while light displayed distinct day and night patterns, it also did not appear to correspond to the observed oscillations in eDNA and eRNA detection (Tables S1, S2). Correlation analysis conducted in R (R Core Team, 2019), using data from Tables S1 and S2, revealed no significant relationships between temperature (in °C) (correlation = −0.0791, p = 0.6369) or light levels (in LUX) (correlation −0.1629, p = 0.384) and eDNA/eRNA signal strength (copies/mL) in the harbour treatment. Unmeasured variables, such as microbial activity, pH, salinity, and dissolved oxygen, may have contributed to these dynamics (Lamb et al., 2022; Mauvisseau et al., 2022; Scriver et al., 2023). Furthermore, the ice treatment, which maintained consistent light and temperature conditions, exhibited similar oscillating detection patterns when compared to the harbour samples, emphasizing the complex interplay of factors influencing eDNA/eRNA signal detectability.
Although both eDNA and eRNA displayed similar detection patterns, especially in the harbour samples, it is essential to note that eRNA starting signal strength (copies/mL) was consistently lower for most time points compared to eDNA, though this was only marginally significant. This could be attributed to eRNA loss during sample processing steps (e.g., extraction, cDNA synthesis, and quantification protocols) and variations in eRNA production rate (e.g., RNA copy by number per cell or organelle, the number of tandem repeats) or eRNA transcription rate; all areas that require further study (Jo et al., 2022; Laroche et al., 2017; Marshall et al., 2021; Wood et al., 2020; Yates et al., 2021).
We acknowledge that including additional sampling time points, ideally until all replicates had no detectable signal, could have provided deeper insights into the oscillating eDNA/eRNA signal patterns. These extended time points could also potentially assist in modeling the intricate decay process using advanced models such as two-phase decay or Weibull decay models, which might better mirror the complex eDNA/eRNA decay mechanisms occurring in nature (Allan et al., 2021; Bylemans et al., 2018; Lance et al., 2017; Saito & Doi, 2021).
4.2 Total environmental DNA fragmentation patterns
Further exploration of the molecular integrity dynamics was initiated to understand the oscillating patterns observed in eDNA and eRNA signal strength. Although eRNA integrity assessment can be performed on the bioanalyzer, measuring RNA integrity relies on assessments such as the RIN (RNA Integrity Number) (Schroeder et al., 2006). Due to the overall poor quality of eRNA in this experiment, we observed sporadic RINs and undefined peaks, making the results less clear. We did find that DV200, which evaluates the percentage of fragments >200 nucleotides (Matsubara et al., 2020), could be a more appropriate measurement for analyzing eRNA integrity, especially as it is commonly used for formalin-fixed paraffin-embedded (FFPE) samples, which are often degraded and highly fragmented (Levin et al., 2020). However, for the purpose of this experiment and considering the need for further exploration and validation of the eRNA results, potentially exploring other platforms such as the ScreenTape® platform and alternative assessment measurements (Levin et al., 2020; Wilkes et al., 2010), we chose to focus only on the further investigation of eDNA fragment and integrity.
We explored how total eDNA fragmentation changes over time and its potential contribution to the detection of species-specific eDNA signals (Suter et al., 2023). While DNA fragmentation contributes to eDNA degradation, other factors like intracellular-to-extracellular conversion and large-to-small fraction breakdown also play important roles in releasing and creating detectable eDNA fragments (Allan et al., 2021; Mauvisseau et al., 2022). Shorter fragments can accumulate due to enzymatic and mechanical microbial processes (Bylemans et al., 2018). We hypothesized that fragmentation could enhance the accessibility of DNA molecules to downstream processing, potentially boosting detected signal strength. Therefore, we employed Bioanalyzer® electrophoretic analysis to observe total eDNA fragment changes over time, possibly linking them to eDNA signal dynamics.
Based on the data obtained from our Agilent 2100 Bioanalyzer® fragment analysis, we found that over time, total eDNA became more fragmented (as indicated by the total number of fragments detected). Surprisingly, when examining the environmental harbour samples, we observed an intriguing increase in the median fragment size over time and an increase in larger fragments (>10,000 bp). This finding prompted us to consider the possibility that the larger fragments detected could potentially be DNA from other organisms accumulating throughout the experiment, such as bacteria or microscopic eukaryotes, which are known to play a significant role in the degradation of eDNA (Brasell et al., 2022; Scriver et al., 2023; Zulkefli et al., 2019). The samples stored on ice may experience some protection against this effect due to the inhibition of microbial activity at lower temperatures (Brasell et al., 2022; Eichmiller et al., 2016; Pilliod et al., 2014; Strickler et al., 2015; Zulkefli et al., 2019). This is supported by the stability in the number of total DNA fragments and median fragment size observed across ice treatment sample time points.
While our findings shed light on the role of DNA fragmentation in eDNA dynamics, further research is needed to understand the total composition of eDNA, including shifts in its overall composition and how DNA from specific organisms, such as microbes and microorganisms, contributes to changes in eDNA composition and fragmentation. In addition, it is important to investigate how these factors affect eDNA detection and dynamics within different environments. Despite some progress in understanding the role of bacteria in eDNA detection and persistence, there is still much to be explored in this area (Jo, 2023; Salter, 2018; Zulkefli et al., 2019).
4.3 Nanopore sequencing potential for comprehensive environmental DNA insights
In our final experiments to comprehend eDNA and eRNA compositions and fragmentation, we utilized next-generation sequencing. Unfortunately, due to the lack of barcodes available for direct RNA analysis on the MinION™ Oxford Nanopore sequencing platform, at the time of this experiment, we opted to narrow our further investigation to eDNA only.
Our initial exploration with MinION™ yielded valuable insights and highlighted areas for improvement if this technology is harnessed for comprehensive metagenomics and ecological eDNA elucidation. A notable limitation in this analysis, likely attributed to adaptive sequencing (Loose et al., 2016; Oxford Nanopore Technologies, 2023a; Urban et al., 2023) and low sample input, was the relatively low number of detected reads per sample (an average of 26,705 reads per barcoded sample), which impeded the ability to draw robust conclusions for both the environmental samples and the naked S. spallanzanii DNA control samples (Egeter et al., 2022; Truelove et al., 2019).
However, for tissue control samples that had a higher read count (average of 232,670 total reads per barcoded sample), the output of S. spallanzanii-specific fragments from the tissue control samples aligned with the Bioanalyzer® fragment analysis, revealing a decline in larger fragments (>10,000 bps and 5000–10,000 bps) across both treatment groups. This shift in fragment sizes may explain the variations in eDNA signal determined by S. spallanzanii-specific ddPCR. For instance, control tissue ice samples exhibited a higher proportion of fragments within the 200–500 bps range, possibly correlating with higher ddPCR copy numbers (Figure 2e). Nevertheless, it is essential to exercise caution when interpreting these results in terms of length, as adaptive sequencing runs tend to produce many reads of approximately 200–400 bp, representing the average sequencing length at which reads are processed and either accepted or rejected (Oxford Nanopore Technologies, 2023a).
In this study, we utilized adaptive sequencing to enrich the S. spallanzanii eDNA reads. This process involves real-time base calling and targets a specific region of interest, with the system making dynamic decisions about the sequencing process (Loose et al., 2016; Weilguny et al., 2023). For example, if a sequence aligns with the gene of interest, sequencing continues, but if not, the strand is rejected, making the pore available for the next strand (Loose et al., 2016; Weilguny et al., 2023). While this method is designed to enhance the detection of our targeted species, a limitation in our study is that only 0.01% of total reads, in both control and environmental samples, were accepted, meaning the reads aligned to the region of interest, the S. spallanzanii mitochondrial genome, and sequencing was permitted to proceed. This acceptance rate falls well below ONT's recommended threshold of 1% for reads aligned to regions of interest, indicating that S. spallanzanii mitochondrial DNA constitutes only a small portion of the total eDNA (Oxford Nanopore Technologies, 2023a).
To enhance our understanding of the dynamics of target species-specific eDNA, we suggest that future investigations refine nanopore sequencing protocols for eDNA samples, particularly those that are degraded or have low input. Optimization can be achieved through adjustments in individual library and pooled library concentrations, PCR enrichment techniques, cleanup strategies, and careful consideration of barcode balance. For instance, in an experiment focusing on sequencing short DNA fragments on the MinION, the authors discovered that while a one-fold AMPure XP bead did not eliminate most of the 200 bp DNA fragments, using less than 0.65-fold AMPure XP beads reduced 200 bp DNA fragments to 5% of the original input (Wei et al., 2018). While dynamic sequencing methods like adaptive sequencing hold promise for amplification-free target enrichment, which can lead to a more even distribution of sequencing reads, it is crucial to consider that if the region of interest is lower than expected (<0.1%), more time may be allocated to reading and unblocking, potentially resulting in less efficient sequencing and reduced data output (Martin et al., 2022; Oxford Nanopore Technologies, 2023a; Weilguny et al., 2023). In addition, frequent pore flipping during adaptive sequencing could impact pore stability (Martin et al., 2022). Hence, prior knowledge of the target region's proportion is valuable for selecting the appropriate sequencing strategy, cleaning method and improving the workflow.
In addition to investigating Sabella-specific fragments, we conducted a metagenomic analysis to gain insights into the composition of total eDNA. However, the limited sequencing depth of the environmental samples, likely due to the choice of using adaptive sequencing, once again constrained our conclusions. Despite these limitations, the metagenomic analysis unveiled a noteworthy trend within the harbour treatment, with an increase in total reads over time. While only a modest number of reads (22.76%) could be taxonomically classified in the environmental samples, a significant portion of control samples exposed to the harbour treatment could be attributed to microbes and bacteria. Notably, for prevalent marine ubiquitous microbes, we observed a substantial surge in the number of classified reads (Figure S5; S10), signifying a remarkable shift in the quantity of microbe DNA present in the harbour samples. This pattern was exclusive to the harbour samples, as the ice tissue samples exhibited no changes in the number of classified reads for these prevalent marine microbes (Figure S10). These observations support our hypothesis that an expanding portion of the total eDNA composition, as detected by the Bioanalyzer®, may be linked to the growth of microbial and bacterial sources within the dialysis bag. This, in turn, could potentially influence the detection dynamics of target eDNA concentration.
Investigating changes in microorganism composition within harbour samples over time may unveil variations in relative abundance and their impact on detection dynamics. Future investigations might consider targeting specific metabarcoding regions like the V3-V4 segments of the prokaryote 16S rRNA gene and the V4 region of the eukaryote 18S rRNA gene to gain comprehensive insights into shifts within bacterial and eukaryotic communities (Brasell et al., 2022).
5 FINAL CONCLUSION
Our pioneering study delved into the intricate dynamics of marine NIS-specific eDNA/eRNA COI signals, both in-situ and post-sampling storage. These investigations revealed unexpected deviations from lab-based first-order decay rates and persistence experiments. We observed patterns of fluctuations within the harbour and ice treatments, that impacted concentration measurements at specific time points. Despite these fluctuations, the detectability of specific eDNA/eRNA signals remained relatively stable over the 24 h period.
Bioanalyzer® and Minion analyses of total eDNA have indicated an increase in fragments, and larger fragment sizes in the in-situ samples, suggesting changes within the composition of total eDNA. Initial data from the Minion has suggested an increase in fragments assigned to microbes and bacteria, that could potentially influence species-specific eDNA detection. Interestingly, ice storage appeared to be efficient at suppressing substantial shifts in total eDNA composition and inhibit the growth of microbes, as evidenced by the absence of significant changes in fragmentation and species-specific eDNA signal in control samples, along with the relative stability in the number of microbial reads (indicated by MinION™ analysis). As we uncover these nuances of eDNA/eRNA dynamics, the potential of innovative shotgun sequencing technologies, such as Oxford Nanopore, becomes evident in capturing the nature of observed fragmentation and understanding the composition of total eDNA over time.
Moreover, our results demonstrate remarkably similar detection patterns between eDNA and eRNA in the field. Even after 24 h within the harbour sample, we continued to detect species-specific eRNA, potentially indicating a longer eRNA persistence in-situ than previously reported. Additionally, the lack of significant changes in detection after 24 h in the ice treatment implies that preserving samples under field storage conditions for up to 24 h may be acceptable for both eDNA and eRNA samples. These findings deepen our understanding of eDNA and eRNA detection and ecology, enhancing our capacity to employ molecular detection techniques for sustainable resource management and preserving biodiversity for future generations.
AUTHOR CONTRIBUTIONS
UA, MS, and AZ contributed to the study's design and conception. UA, VA, XP, and AZ conducted the fieldwork, while MS performed all the laboratory work. MS initiated the review and prepared the initial draft. AZ, XP, JS, NG, VA, and UA provided extensive edits and revisions to the manuscript. MS created the figures with input from all the authors. All authors have reviewed and approved the final manuscript for publication.
ACKNOWLEDGMENTS
This research was supported by the New Zealand Ministry of Business, Innovation and Employment funding (CAWX1904—A toolbox to underpin and enable tomorrow's marine biosecurity system). Open access publishing facilitated by The University of Auckland, as part of the Wiley - The University of Auckland agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Raw sequence reads were deposited in the NCBI Sequence Read Archive under the accession number: PRJNA1033365. Additional tables and figures are uploaded as supplementary material.




