Controlled Study of Pre- and Postoperative Headache in Patients with Sellar Masses (HEADs-uP Study)
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Tessa N. A. Slagboom and Tessel M. Boertien contributed equally to this study.
ABSTRACT
Introduction
Sellar masses are common intracranial neoplasms. Their clinical manifestations vary widely and include headache. We aimed to determine whether the prevalence and characteristics of headache in patients with sellar tumours differ from the general population and to investigate the effect of tumour resection on this complaint.
Methods
We performed a prospective, controlled study in a single tertiary centre and included 57 patients that underwent transsphenoidal resection for a sellar mass (53% females, mean age 53.5 ± 16.4) and 29 of their partners (controls; 45% females, mean age 54.8 ± 14.9). Outcome measures were prevalence, characteristics and impact of headache 1 month preoperatively and at neurosurgical follow-up 3 months postoperatively.
Results
Preoperatively, the prevalence of regular headache (≥1 time per month) was higher in patients than in controls (54% vs. 17%, p < 0.001), and patients scored higher on headache impact questionnaires (all p ≤ 0.01). At postoperative follow-up, headache prevalence decreased in both groups, but the decrease in regular headache frequency and impact was larger in patients than in controls, and no between-group differences remained.
Conclusions
More than half of patients with sellar tumours suffer from at least once-monthly headaches, and both regular headache occurrence and impact are higher compared with controls. The more pronounced decrease in headache complaints in patients versus controls at postoperative follow-up suggests an additional effect of tumour resection next to the factor time.
1 Introduction
Sellar masses are common intracranial neoplasms with an estimated population prevalence of 0.1% [1, 2]. Pituitary adenomas account for most of these masses. Clinical manifestations of sellar masses vary widely and depend on hormonal status and mechanical symptoms because of tumour expansion. Although many of these masses are asymptomatic, others can lead to endocrine, ophthalmological or neurological complaints, such as headache.
Headache is a frequently encountered (11%–70%), and sometimes predominant, symptom in patients with sellar masses [3, 4]. The pathophysiology behind this common complaint is not fully understood for most sellar pathology, but might be the net result of combined mechanical, biochemical and/or psychological aspects [3-7]. However, as both headache, with an estimated global prevalence of 52% [8], and (asymptomatic) lesions of the sellar region are common in the general population, it is difficult to establish a causal correlation between the two [9]. The probability that a patient presenting with headache has a concurrent mass in the sellar region as incidental finding on neuroimaging during the diagnostic work-up (i.e., an ‘incidentaloma’) is considerable [10, 11]. It thus remains unclear to which extent the high prevalence of headache is attributable to effects of the sellar mass and should thereby be classified as secondary headache [12]. Secondary headaches can have the characteristics of a primary headache, that is, a headache without underlying cause or condition [12], further complicating the differentiation between primary and secondary headaches in patients with a sellar tumour if based solely on clinical symptoms. Headache in a patient with a sellar mass, especially if this concerns a hormonal inactive tumour that does not threaten the optic nerves, therefore presents a true management dilemma [13]. At present, not much is known about the characteristics and impact of the headache that these patients experience. Large, prospective studies on the effects of tumour resection on headache in patients with sellar masses are scarce. The available literature to date provides conflicting results regarding the improvement in complaints after transsphenoidal surgery [3, 6, 14-16].
Headache can have a major impact on the quality of life [9]. Diagnosis and proper treatment of pituitary tumour-related headache could therefore enhance quality of life to a relevant extent. In order to investigate whether the prevalence and characteristics of headache in patients with sellar tumours differ from the general population and to draw conclusions on the effect of tumour resection versus natural course of this complaint, we performed a prospective, controlled, single-centre study among patients with sellar masses and their partners, to evaluate the presence, characteristics and impact of headache, pre- and postoperatively. Secondly, we investigated determinants of both the presence and impact of, as well as the effect of, tumour resection on headache in patients with sellar masses.
2 Materials and Methods
2.1 Participants
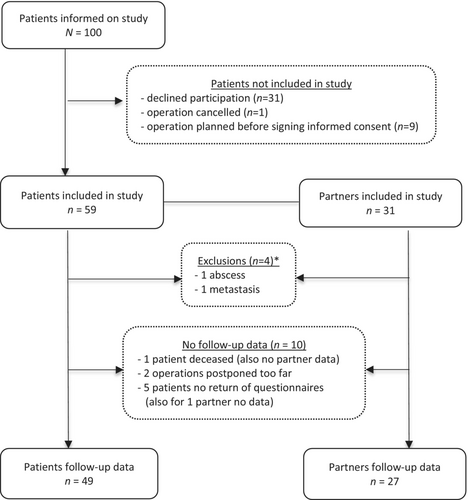
One hundred consecutive, adult patients with a sellar mass or space-occupying cystic lesion planned for elective surgical resection at the Amsterdam University Medical Centre (Amsterdam UMC) between June 2019 and January 2022 were invited to participate in the study, after permission of their treating specialist. If present, their partners were invited to participate in the control group. All patients were recruited through the Pituitary Centre Amsterdam, a multidisciplinary outpatient clinic consisting of a neurosurgeon, otolaryngologist and endocrinologist. Exclusion criteria were inflammatory disease affecting the pituitary (e.g., hypophysitis), other major intracranial affliction <3 months preoperatively (e.g., traumatic brain injury, cerebral infarction or intracranial haemorrhage other than pituitary apoplexy), malignant tumours (e.g., pituitary carcinoma and metastases) and inability to give informed consent. The Medical Ethical Committee of the Amsterdam UMC location AMC approved the study protocol.
2.2 Study Design
After signing informed consent, participants received baseline questionnaires to assess headache (details below) 1 month in advance of the planned surgery. Participating partners were also asked to fill in a form about their medical history and medication use. Filling in the questionnaires took about 15 min. Three months postoperatively, participants received follow-up questionnaires. Data on demographics and medical history were gathered using medical files (patients) or through the additional questionnaire/form (partners). Routine clinical practice concerning endocrine status and pituitary imaging according to international guidelines was adhered to in all patients [17-19]. Preoperative MRI results were used to determine tumour size, extension (including sinus cavernous invasion), compression of optic nerves or chiasm and most probable diagnosis. Prior to surgery, biochemical testing was performed to determine whether the tumour was hormonally active and to assess pituitary deficiencies, with cut-off scores defined by the Endocrine Society Clinical Practice Guideline [20]. Biochemical testing was performed in the laboratory of the Amsterdam UMC location AMC. All tumour resections were performed via an endoscopic transnasal transsphenoidal approach by a neurosurgeon and otolaryngologist. Three neurosurgeons and four otolaryngologists performed all operations in our centre. Operation details, such as surgical technique and perioperative complications, were documented in an operative report. Resected tumour material underwent pathological and immunohistochemical examination for definitive diagnosis. All patients had a follow-up visit 3 months postoperatively with an assessment of the following aspects: the presence of residual tumour on a postoperative MRI, resolution of hormonal hypersecretion and/or pituitary deficiencies, resolution of preoperative visual field defects or cranial nerve palsies, new pituitary deficiencies, pathology results and postoperative adjuvant therapies.
2.3 Questionnaires
Participants were first asked if they ever experienced headache, and if not, they did not have to fill in the headache questionnaires.
2.3.1 Headache Screening Questionnaire—Dutch Version (HSQ)
This is a validated screening questionnaire for migraine and tension-type headache (TTH) based on criteria of the Internal Classification of Headache Disorders, 3rd edition [21]. It consists of 10 multiple-choice questions about the frequency and clinical manifestations of experienced headache. Outcome measures are total migraine and total TTH score. A total score of ≥6 points corresponds with probable migraine or TTH, and a full score of 8 points with definite migraine or TTH. Sensitivity/specificity for detecting probable migraine, definite migraine, probable TTH and definite TTH are 0.89/0.54, 0.69/0.90, 0.92/0.48 and 0.36/0.86, respectively.
2.3.2 General Headache Questionnaire
Further information regarding headache was gathered with a self-composed questionnaire consisting of 12 items, on the basis of widely used diagnostic tools implemented in headache clinics and designed to complement the Headache Screening Questionnaire. Firstly, the presence of any headache as well as the presence of regular headaches (defined as headache at least once a month) was checked pre- and postoperatively. Postoperative headache within 2 weeks after surgery was excluded. If any headache was present, participants were asked to mark the location(s) of their experienced headache on a picture and to check accompanying symptoms that could suggest TACs (e.g., ptosis or rhinorrhoea). Several following questions checked medication use to treat or prevent headache and the effect as well as side effects of used medication. Although we were not able to report on the number of participants with a definite diagnosis of medication-overuse headache (MOH) based on the International Headache Society (IHS) criteria, we did report on a group that fulfilled two out of three diagnostic criteria for MOH: headache for at least 15 days a month + use of analgesics for at least three times a week. Participants were also asked if they had ever consulted a specialist or other healthcare provider and whether a specific diagnosis or explanation for their headache was given. Additionally, familial occurrence of headache was checked. Finally, the severity of average and worst experienced headache in the last 7 days was quantified using a Visual Analogue Scale (VAS) (100-mm line without sub marks, from ‘no pain’ to ‘worst imaginable pain’) [22]. The VAS is widely used in diverse clinical settings and has good sensitivity of pain rating [23].
2.3.3 Headache Impact Test (HIT-6)
This validated questionnaire quantifies the impact of headache on daily life [24, 25]. It consists of six items on how often headache affects daily activities or mood, rated on a 5-point scale from ‘never’ (6 points) to ‘always’ (13 points). Total score ranges from 36 to 78 points, with higher scores indicating a higher impact on daily life. The HIT-6 has good discriminative validity and test–retest reliability (Cronbach's α = 0.78) [25].
2.3.4 Headache Severity Index (HSI)
Gfrerer et al. constructed a migraine headache severity index (HSI) score on the basis of headache severity, duration and frequency, which was later also used for NFA by Yu et al. [26, 27]. We calculated our own HSI on the basis of the HIT-6 score and two questions from the HSQ, with a score ranging from 3 to 12 points (Table 1).
| Points | Severity (HIT-6 score) | Duration (HSQ Question 4) | Frequency (HSQ Question 3) |
|---|---|---|---|
| 1 | ≤49 | 0–30 min | <1 time a month |
| 2 | 50–55 | 30 min—4 h | 1–15 times a month |
| 3 | 56–59 | 4 h—3 days | >15 times a month |
| 4 | ≥ 60 | 3–7 days | |
| 5 | ≥ 7 days |
2.3.5 Tumour-Related Headache
Schankin et al. proposed three criteria for the definition of tumour-attributable headache in pituitary adenoma: presence of preoperative headache, exclusion of MOH and ≥50% amelioration of headache postoperatively in respect of frequency or severity [28]. The latter criterion was used for current study, in which we defined ≥50% amelioration of headache as ≥50% reduction in HIS, with the inclusion of patients headache resolution postoperatively.
2.4 Statistical Analysis
SPSS Statistics version 28.0.1.1 was used for statistical analysis. Numerical data are summarised as mean ± SD or as median (IQR), depending on distribution. Categorical and ordinal data are presented as counts (%). Between-group differences in numerical variables were analysed with independent t-tests (normal distributed variables) or the Mann–Whitney U (non-normal distributed variables), and differences in categorical variables with the chi-squared or Fisher exact (if expected cell count <5) test. Comparisons of pre- and postoperative data within the patient group were evaluated by paired t-tests, the Mann–Whitney U or chi-squared test, as appropriate. Correlations were calculated using Pearson correlation coefficient r (normal distributed variables) or Spearman's correlation coefficient ρ (non-normal distributed variables). Within the patient group, the following determinants of (presence and impact of) headache were analysed: age, tumour size (in mm), tumour expansion (based on sinus cavernous invasion and/or visual field defects), apoplexy, functioning adenoma (any, and separately for Cushing's disease, acromegaly and prolactinoma), hypopituitarism and complete resection of tumour (based on postoperative MRI results). We also performed subanalyses on pituitary neuroendocrine tumours (PitNET) transcription factors in those patients with a pituitary adenoma (Appendix B). When data were missing or invalid (e.g., in case multiple answers were given), the participant was excluded from the analysis for that variable. Outcomes were considered statistically significant at p ≤ 0.05.
3 Results
3.1 Study Population
Of the 100 patients who received information on the study, 59 (59%) agreed to participate. Of these, 31 (53%) had a partner willing to participate. Two patients and accompanying partners were excluded. Follow-up data were gathered from 49 patients (83%) and 27 partners (87%). Figure 1 shows the inclusion flow chart with reasons for exclusions and missing follow-up data. No differences were found between patients with and without follow-up data with respect to age, gender, adenoma size, percentage of hormonally active tumours and percentage of hypopituitarism (data not shown). Also, no statistical differences in sex or mean age at inclusion were found between patients (53% females, age 53.5 ± 16.4) and controls (45% females, age 54.8 ± 14.9), p = 0.49 and 0.72, respectively. Tumour characteristics of patients are summarised in Table 2.
| Tumour characteristics | Sellar lesion (n = 57) | Pituitary adenoma | Total | 95% | ||
| Size | In mm (median [IQR]) | 21.0 [10.0 – 31.8] | ||||
| Micro (<1 cm) | 22% | |||||
| Macro (≥ 1cm) | 78% | |||||
| Hormonally active | No | 65% | ||||
| Yes | GH | 13% | ||||
| ACTH | 15% | |||||
| TSH | 2% | |||||
| Prolactin | 6% | |||||
| Rathke's cleft cyst | 4% | |||||
| Craniopharyngeoma | 2% | |||||
| Hypopituitarism (n = 57) | No | 46% | ||||
| Yes | GH | 5% | ||||
| ACTH | 33% | |||||
| TSH | 32% | |||||
| LH/FSH | 35% | |||||
| ADH | 0 | |||||
| Local tumour effects (n = 57) | Chiasm compression | 68% | ||||
| Visual defect | Any | 47% | ||||
| Quadrant hemianopsia | 4% | |||||
| Bitemporal hemianopsia | 26% | |||||
| Other | 18% | |||||
| Apoplexy | 5% | |||||
| Cranial nerve deficit | 0 | |||||
| Sinus cavernous invasion | 51% | |||||
| Surgical outcomes | Time between indication and surgery (n = 55a) | Average in days [range] | 75.2 ± 66.9 [6 – 280] | |||
| % of patients operated <30 days | 30% | |||||
| Postoperative follow-up (n = 54b) | Average (in days) | 96.9 ± 39.7 | ||||
| Postoperative MRI (n = 54b) | Complete resection of tumour | 17% | ||||
| Residual tumour | 74% | |||||
| Uncertain | 9% | |||||
| Resolution of hormonal hypersecretion (n = 19) | 58% | |||||
| Resolution of preoperative pituitary deficiencies (n = 31) | Complete | 16% | ||||
| Partial | 23% | |||||
| Visual field defects preoperative (n = 27) | Improvement | 56% | ||||
| Stable | 18% | |||||
| Requiring further follow-up | 26% | |||||
| Adjuvant therapy (n = 54b) | Additional surgery | 17% | ||||
| Radiotherapy | 4% | |||||
| Medication | 4% |
- a Data missing for two patients with postponed surgery.
- b Data missing for two patients with postponed surgery and one deceased patient.
3.2 Surgery and Follow-Up
Table 2 shows the surgical outcomes in the patient group. Indications for resection were objectified visual disturbances (46%), hormonal hypersecretion (26%), prevention of visual disturbances (23%) and side effects or contra-indication for dopamine agonists in case of prolactinoma (5%). No resections were performed with headache as the sole indication. Pathology results confirmed a pituitary adenoma in 48 cases (84%), two Rathke's cleft cysts and one craniopharyngioma. For pituitary adenoma, the clinicopathological classification can be found in Appendix B.
3.3 Between-Group Comparisons
3.3.1 Occurrence
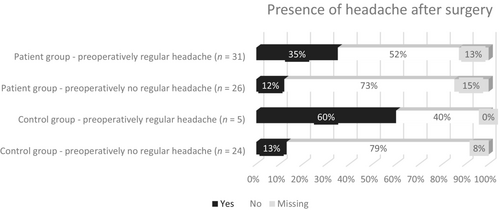
Preoperatively, a similar proportion of patients (47/57 [82%]) and controls (23/29 [79%]) had ever experienced headache (χ2(1, N = 86) = 0.13, p = 0.72). Postoperatively, headache was reported in 14/49 (29%) patients and 6/27 (22%) controls (χ2(1, N = 76) = 0.36, p = 0.55) (Table 3). Before surgical treatment, regular headache (≥1 time per month) was present more often in patients than in controls (54% vs. 17%, χ2(1, N = 86) = 10.9, p < 0.001), whereas this difference was not found after surgery (27% vs. 15%, χ2(1, N = 76) = 1.38, p = 0.55). No new cases of headache were reported in either group, but headache frequency worsened from <1 time a month to 1–15 times a month for two patients and two controls. Of the participants with missing follow-up data, seven out of the eight patients reported to ever having experienced headache preoperatively (of which three regular headaches), and none of the two controls. Figure 2 shows the course of headache in four groups of participants, based on the presence of regular (≥1 per month) preoperative headache (yes/no) and group (patient/controls). McNemar test in patients showed that the chance of regular headache resolution after transsphenoidal resection (16/27) was greater than the chance of experiencing headache postoperatively in patients who did not report regular headache preoperatively (3/22—of which 2 worsened from <1 a month to 1–15 times a month and one remained the same with frequency < 1 per month), p = 0.004; versus p = 1.00 in controls.
| Patients | Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preoperatively | Postoperatively | Preoperatively | Postoperatively | |||||||
| Does headache occur in your family? (Yes) | 25/57 (44%) | N/A | 11/29 (38%) | N/A | ||||||
| Have you ever experienced a headache? (Yes) | 47/57 (82%) | N/A | 23/29 (79%) | N/A | ||||||
| Did you experience a headache, ≥2 weeks after surgery? (Yes) | N/A | 14/49 (29%) | N/A | 6/27 (22%) | ||||||
| Do you experience regular headache (≥1 per month)? (Yes) | 31/57 (54%)*& | 13/49 (27%) & | 5/29 (17%)* | 4/27 (15%) | ||||||
| How often in your life have you had a headache? | 1–4 times | n = 47 | 6% | n = 14 | 0% | n = 23 | 9% | n = 6 | 0% | |
| 5–9 times | 9% | 14% | 13% | 0% | ||||||
| ≥ 10 times | 85% | 86% | 78% | 100% | ||||||
| How often per month do you experience a headache? | <1 time | n = 47 | 34% | n = 14 | 7% | n = 23 | 78% | n = 6 | 33% | |
| 1 to 15 times | 43% | 86% | 22% | 67% | ||||||
| ≥ 15 times | 23%* | 7% | 0%* | 0% | ||||||
| How often did you have a headache attack? | 0–4 times | n = 46 | 41% | n = 14 | 57% | n = 23 | 52% | n = 6 | 50% | |
| 5–9 times | 17% | 14% | 17% | 0% | ||||||
| ≥ 10 times | 41% | 29% | 30% | 50% | ||||||
| How long does your headache last, without medication? | 0–30 min | n = 45 | 22% | n = 14 | 14% | n = 23 | 13% | n = 6 | 0% | |
| 30 min. – 4 h | 42% | 64% | 70% | 67% | ||||||
| 4 h—3 days | 24% | 21% | 17% | 33% | ||||||
| 3–7 days | 4% | 0% | 0% | 0% | ||||||
| >7 days | 7% | 0% | 0% | 0% | ||||||
| Is your headache one- or two-sided | One-sided | n = 37 | 46% | n = 14 | 50% | n = 21 | 57% | n = 6 | 83% | |
| Two-sided | 54% | 50% | 43% | 17% | ||||||
| Which word would you use to describe your headache | Pulsing | n = 43 | 12% | n = 12 | 0% | n = 22 | 14% | n = 6 | 0% | |
| Tight or pressing | 58% | 83% | 68% | 83% | ||||||
| Burning or stinging | 16% | 17% | 5% | 17% | ||||||
| Other | 14% | 0% | 14% | 0% | ||||||
| Headache accompanying symptoms | Sensitivity to light | n = 47 | 40% | n = 14 | 36% | n = 23 | 48% | n = 6 | 50% | |
| Sensitivity to noise | 19%* | 21% | 48%* | 50% | ||||||
| Nausea/vomiting | 17% | 14% | 17% | 0% | ||||||
| Other | 38%** | 7% | 13%** | 17% | ||||||
| Cranial autonomic symptoms | Facial transpiration | 11% | 14% | 22% | 0% | |||||
| Swollen eyelid | 11% | 0% | 0% | 0% | ||||||
| Ptosis | 11% | 14% | 13% | 17% | ||||||
| Conjunctival hyp. and lacr. | 21% | 7% | 9% | 33% | ||||||
| Myosis | 2% | 0% | 4% | 0% | ||||||
| Rhinorrhoea and nasal cong. | 19%** | 21% | 0%** | 0% | ||||||
| Describe the seriousness of your headache | Mild | n = 44 | 30% | n = 14 | 29% | n = 23 | 30% | n = 6 | 0% | |
| Moderate | 39% | 57% | 52% | 83% | ||||||
| Serious | 25% | 7% | 9% | 17% | ||||||
| Very serious | 7% | 7% | 9% | 0% | ||||||
| Do you use any medication to stop your headache? | No | n = 44 | 34% | n = 14 | 36% | n = 22 | 32% | n = 6 | 17% | |
| Per attack | 11% | 7% | 14% | 50% | ||||||
| <1 time a month | 16% | 0% | 28% | 0% | ||||||
| 1–8 times a month | 11% | 21% | 18% | 33% | ||||||
| 3–5 times a week | 11% | 21% | 5% | 0% | ||||||
| Daily | 18% | 21% | 5% | 0% | ||||||
| Total score on the HSQ | Possible migraine | n = 36 | 19% | n = 13 | 31% | n = 22 | 32% | n = 6 | 33% | |
| Migraine | 5% | 8% | 9% | 17% | ||||||
| Possible tension-type headache | n = 38 | 82% | n = 12 | 75% | n = 22 | 73% | n = 6 | 100% | ||
| Tension-type headache | 37% | 42% | 50% | 67% | ||||||
| Both possible migraine + tension-type headache | n = 36 | 17% | n = 12 | 14% | n = 22 | 23% | n = 6 | 33% | ||
- Note: Comparisons between groups were conducted, and if significant indicated by *p ≤ 0.01, **p ≤ 0.05; within groups by &p ≤ 0.01.
- Abbreviations: con., congestion; conjunctival hyp. and lacr., conjunctival hyperaemia and lacrimation; n, number of responders per item.
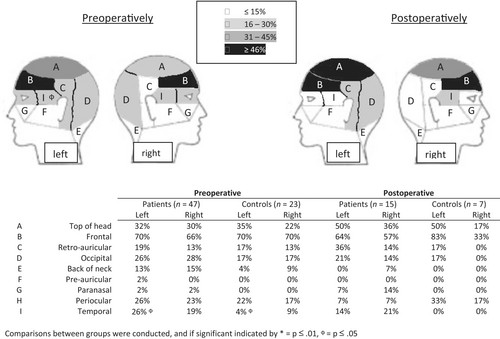
3.3.2 Characteristics
Frontal headache was reported most frequently in both groups, and besides a higher frequency of left-sided temporal pain for patients preoperatively (p = 0.05), no differences between the two groups were found (Figure 3). Preoperatively, controls experienced significantly more sensitivity to noise (p = 0.01), whereas patients experienced more rhinorrhoea and nasal congestion (p = 0.03), and other symptoms (p = 0.03) including fatigue, dizziness, pressure and loss of concentration. Symptoms suggestive of a form of trigeminal autonomic cephalalgias (TAC) (i.e., one-sided headache with a usual duration less than 30 min) were present in five patients before surgery, of which three also in combination with accompanying symptoms of facial transpiration, swollen eyelid or conjunctival hyperaemia and lacrimation, versus no controls. These headaches where predominantly frontal (3/5) and occipital (2/5) and did not meet the HSQ criteria for migraine or tension-type headache (three possible tension-type headache and one possible migraine), whereas one patient previously received a diagnosis of migraine and another of cluster headache. HIT scores were 0 in two patients (no or little impact on daily life), 1 in two patients (some impact on daily life) and 3 in one patient (considerable impact on daily life). None of these patients used medication to stop their headaches. The diagnoses included one patient with acromegaly (microadenoma without expansion), one patient with Cushing's disease (microadenoma without expansion) and three nonfunctional pituitary adenomas (macroadenoma with parasellar and sinus cavernous extension, also one with suprasellar expansion). Postoperatively, headache resolution occurred in 3/5 patients, persisted in 1/5 (with same HIT score: 3) and was unknown in one patient because the surgery was postponed.
Because of missing or double/ambiguous answers on the HSQ questions (especially the question about one- or two-sided headache), total score for (possible) migraine or TTH could not be calculated for, respectively, 11 and 9 patients, and 7 controls. Preoperatively, six patients (13%) fulfilled two of three MOH criteria versus none of the controls (p = 0.17), whereas no participants fulfilled two of three MOH criteria postoperatively.
3.3.3 Impact
Regarding headache impact and severity, patients had statistically significant higher average scores on the HIT-6, VAS and HSI preoperatively, indicating more severe headache with higher impact on daily life (Table 4). Although postoperative differences in HIT-6 and VAS still seem apparent, these differences could not be demonstrated in a statically significant manner, possibly due to top low group numbers. Tumour-related headache, defined as an improvement of HSI of ≥50% (including participants with headache resolution postoperatively) was found in 76% of both patients as controls.
| Patients | Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Preoperatively | Postoperatively | Preoperatively | Postoperatively | ||||||
| Daily activities worsen my headache (Yes) | n = 45 | 24% | n = 14 | 29% | n = 23 | 17% | n = 6 | 33% | |
| I avoid daily activities during my headache (Yes) | n = 46 | 22% | n = 14 | 21% | n = 23 | 43% | n = 6 | 33% | |
| HIT-6 | Total | 52.2 ± 9.8* | 53.6 ± 12.1 | 46.1 ± 6.5* | 48.5 ± 6.0 | ||||
| <49 (no or little effect on daily life) | n = 46 | 41% | n = 14 | 50% | n = 25 | 68% | n = 6 | 67% | |
| 50–55 (some effect on daily life) | 22% | 14% | 24% | 17% | |||||
| 56–59 (considerable effect on daily life) | 7% | 7% | 4% | 17% | |||||
| ≥ 60 (severe effect on daily life) | 31%** | 29% | 4%** | 0% | |||||
| VAS | Average headache score past week | 20.2 [4.5–58.3]* | 22.6 [9.7–36.6] | 1.5 [0.0–7.5]* | 3.5 [1.5–24.4] | ||||
| Worst headache score past week | 20.9 [4.0–67.0]* | 30.0 [16.8–55.4] | 1.5 [0.0–15.9]* | 3.9 [1.8–30.9] | |||||
| HSI | Total | 8.0 [3.0–18.0]** | 6.0 [4.0–18.0] | 2.0 [2.0–4.0]** | 4.0 [3.5–9.8] | ||||
| Improvement in HSI score ≥ 50% | n = 37 | 76% | n = 16 | 76% | |||||
- Note: n, number of responders per item/participants of which a sum of score could be calculated. Comparisons between groups were conducted and, if significant, indicated by *p ≤ 0.01, **p ≤ 0.05.
3.3.4 Diagnosis Other than Pituitary Adenoma
We performed sensitivity analyses excluding patients with a diagnosis other than pituitary adenoma (Rathke's cleft cyst [n = 2] and craniopharyngeoma [n = 1]), but this did not significantly change the results (data not shown).
3.3.4.1 Within Patient Comparisons
Although the prevalence of regular headache significantly lowered after surgery in the patient group (Table 4), the impact of headache (measured by mean HIT-6, median average and worst VAS and median HSI score) did not significantly change from preoperative status to postoperative follow-up (all p > 0.05). Preoperatively, the only significant headache determinants were age on presence of headache (p = 0.01) and presence of hormonal hypersecretion on headache impact (p = 0.02) (Appendix A). A greater impact of headache postoperatively was found in patients with hormonal hypersecretion (p = 0.006, especially GH secretion p = 0.03) and lack of tumour expansion (no sinus cavernous invasion: p = 0.008, no preoperative visual defect: p = 0.008). A strong negative association between postoperative headache impact and tumour size was found (ρ = −0.88). Within patients with a pituitary adenoma, the influence of transcription factors (T-PIT, SF-1 and PIT-1) and Ki-67 index on presence and impact of pre- and postoperative headache was determined (Appendix B). The absence of SF-1 (p = 0.002) and presence of PIT-1 (p = 0.006) were determinants of headache impact postoperatively.
4 Discussion
In this prospective study, we found that although the preoperative prevalence of any headache was similar, patients with a sellar mass suffered from more frequent and more intense headaches with a higher impact on daily life when compared to controls. Furthermore, these differences were nonsignificant at postoperative follow-up, possibly indicating a positive effect from transsphenoidal surgery on severity and impact of headache in these patients. Since the presence of headache lowered in both groups, we suggest that the factor of time also plays a role in resolution of this complaint. Finally, we identified multiple determinants (age, hormonal hypersecretion, tumour size and expansion, and transcription factors) of both presence and impact of headache in patients with sellar masses.
Preoperative prevalence of regular monthly headache in patients with sellar masses was 54% in the current study, which corroborates with previously reported prevalence rates ranging between 37% and 70% [15, 16, 27-32]. Concerning headache characteristics, we found that most patients experienced a tension-type-like headache (61%), followed by undefined headache (21%), mixed tension-type- and migraine-like headache (16%) and migraine-like headache (3%). This is contradictive to other studies finding a higher prevalence of migraine-like than tension-type-like headaches in these patients [15, 27, 33]. With respect to headache location, we found more temporally located headache in patients; however, this is nondistinctive for a specific headache diagnosis. Although no official diagnosis of TACs could be established by our questionnaires, five patients reported short (<30 min), unilateral headache attacks preoperatively, with 3/5 patients also experiencing accompanying symptoms of facial transpiration, swollen eyelid or conjunctival hyperaemia with lacrimation; this combination was not found in any of the controls and postoperative headache resolution occurred in three of them. Some previous studies have described cases of TACs in relatively small sample sizes of patients with pituitary tumours, indicating a higher prevalence of these headaches than what could be expected from numbers in the general population [15, 28, 33, 34], but this might also be due to selection bias (e.g., more often actively asked upon). Next to a higher monthly prevalence of preoperative headache, impact and severity of headache were also higher in patients than controls. Our data show that headache had a severe effect on daily life in 31% of patients versus in 4% of controls. This is comparable to previous studies using the Migraine Disability Assessment (MIDAS) questionnaire as instrumental tool for headache disability in pituitary tumours, with headache affecting daily life severely in 21% to 48% of patients [15, 16, 33]. We found a higher impact of preoperative headache in patients with hormonal hypersecretion (HIT-6). This is in line with previous studies showing the highest impact (disability) of headache in acromegaly and prolactinoma [6, 16, 33]. Of the 31/57 patients who were experiencing regular headache before surgery, more than half did not report this at postoperative follow-up. This is in concurrence with previous studies demonstrating resolution of headache in 40%–63% of patients [14-16, 27, 35]. However, these studies did not include a control group to account for the factor of time in resolution of complaints. We found that the decrease in regular headache prevalence at follow-up was much more prominent in the patient group than in the control group, lowering the between-group difference to nonsignificance. This clearly suggests a positive effect of transsphenoidal surgery additional to the factor time.
As in other studies, no predictors on the resolution of headache could be identified [3, 14]. Concerning headache severity based on the HSI, we found improvement in 76%, worsening in 11% and equal scores in 11% of patients (data partly shown), which is in line with previous studies (improvement 49%–83%; worsening 6%–17%, equal 12%–36%) [3, 14, 16, 27, 28, 32, 33]. In contrast to three other studies, we found no new cases of headache after surgery [14-16]. Two of these studies were prospective and showed 10/34 (30%) and 4/85 (5%) new headache cases, the other was retrospective and identified 5/56 (9%) new cases. The discrepancy between current and previously mentioned studies could be due to timing of follow-up: on average 3 months in our study versus 6 months or more in the other studies. Contrary to Jahangiri et al., we found no effect on complete resection versus residual tumour on headache presence or impact postoperatively [3].
Various determinants of headache impact in current study share a common factor: functional adenoma. These adenomas were more often diagnosed at a younger age, microadenomas without tumour expansion and SF-1 negative/PIT-1 positive (data not shown). Together, this suggests that the presence, severity and impact of headache depend on biochemical factors more than on mechanical factors. PIT-1 positive tumours and GH overproduction alone were associated with more impact of headache during postoperative follow-up. Headache is a commonly described complaint in both acromegaly and prolactinoma, and while aetiology is not yet unravelled, it is speculated that these hormones inhibit nociceptive peptide(s) [13]. Medical therapy aimed at reducing hormonal hypersecretion generally has positive effect on headaches, although for somatostatin analogues that result might also be due to antinociceptive effects rather than normalisation of hormonal levels. Moreover, (severe) headache exacerbations have been reported in dopamine agonists [33, 36-41]. To clarify a possible relationship between tumour size and headache frequency and impact in nonfunctioning sellar masses, we performed sensitivity analyses excluding secretory adenomas, but found no changes (no relationship between tumour size and headache occurrence, negative relationship between tumour size and postoperative headache impact, data not shown). A different hypothesis on headache and sellar masses, is that tumour volume and extension or invasion in nearby structures play a less important role than an increase in intrasellar pressure, which can especially be present in microadenomas without destruction of surrounding bone tissue [5, 42, 43]. Hayashi et al. evaluated headache impact according to the HIT-6 and intrasellar pressure during transsphenoidal surgery in 108 patients with pituitary adenoma and hypothesised that headache as a result of increased intrasellar pressure was due to dura mater stretch in the sella turcica [43]. However, a study by Pereira-Neto et al. in 25 pituitary adenoma patients failed to show a relationship between intrasellar pressure and headache impact, also based on HIT-6 scores. The fact that in our population impact of headache after surgery negatively correlated with tumour size also argues against this hypothesis, since one would expect intrasellar pressure to normalise after transsphenoidal removal of the tumour.
Besides mechanical and biochemical aspects, psychosocial factors also seem to play an important role in the pathophysiology, experiencing and suffering of headache in patients with sellar tumours [4]. Two recent studies by Siegel et al. found that in a group of 112 patients with sellar masses, just like in primary headache, the presence of and disability due to headache were associated with depression, neuroticism and pain catastrophising [7, 15]. However, our study did not assess psychological factors.
A major strength of the current study is the prospective assessment of headache; an important feature in patient reported outcomes to minimise recall bias. Furthermore, by including a control group consisting of patients' partners with similar sociodemographic and -economic status, we minimised environmental bias. Unfortunately, not all patients provided a participating partner. We are also the first to include the new PitNET classification in relation to headache in our pituitary adenoma patients. One limitation of the current study is that not all participants fully completed the questionnaire, or gave ambiguous (e.g., multiple) answers. In order to lose as little data as possible, we have chosen to exclude missing data per variable. Since we only included patients that were scheduled for elective transsphenoidal surgery, a selection bias was raised, excluding patients with sellar masses requiring no surgery (e.g., small NFA or medically treated prolactinoma) or emergency surgery (e.g., apoplexy involving fast visual deterioration). Also, the COVID-19 pandemic changed usual practice temporarily, with expedited surgery for patients with an absolute indication (e.g., clear visual field defects). This shortened the time window for inclusion in the study, whereas surgery was postponed for patients with smaller (nonfunctioning) tumours. Besides, it is difficult to distinguish a new headache due to the sellar mass in patients, as the diagnostic delay of sellar masses is usually long or because of previous therapy such as transsphenoidal surgery, thereby lacking timeline information [44]. Another limitation is that participants were not individually interviewed in order to gain more detailed information. Since, therefore, the exact time course of the complaints is unknown, we could not diagnose ‘headache attributed to intracranial neoplasm’ or TACs according to the ICHD-3 [12]. Lastly, the duration of follow-up was quite short in this study. Although a longer follow-up is desirable to better understand the further course of headache, the 3 month period was chosen to coincide with the regular postoperative follow-up in these patients.
To our best knowledge, this is the first prospective study on headache in patients with sellar tumours that included a control group. Our results contribute to improvement of management of this complaint, by showing the effect of surgical treatment and determining predictors of headache occurrence and impact. In order to manage expectations and to ameliorate quality of life in these patients, we suggest that more attention to and consultation on headache is given.
In conclusion, we demonstrate that about half of patients with sellar tumours suffer from monthly, mostly tension-type-like, headache and that both regular headache occurrence and impact are higher compared with controls. At postoperative follow-up, the decrease in regular headache frequency and impact was more pronounced in patients than in controls. We also found that the impact of headache was dependent on secretory status, with no clear association with mechanical factors. In order to further evaluate whether the chance of headache improvement should be taken into account during counselling for transsphenoidal surgery of a sellar mass, we suggest future research to explore this complaint in larger studies that include structured patient interviews, and possibly add an additional group consisting of patients with sellar masses without an operation indication to provide more data on specific headache diagnoses and the natural course of these complaints.
Author Contributions
Tessa N. A. Slagboom: conceptualization (lead), data curation (lead), formal analysis (lead), investigation (lead), methodology (equal), project administration (equal), writing–original draft (lead). Tessel M. Boertien: conceptualization (lead), investigation (lead), methodology (equal), project administration (equal), supervision (lead), writing–original draft (lead), writing–review and editing (equal). Peter H. Bisschop: conceptualization (equal), writing–review and editing (equal). Eric Fliers: conceptualization (equal), writing–review and editing (equal). Johannes C. Baaijen: conceptualization (equal), writing–review and editing (equal). Jantien Hoogmoed: conceptualization (equal), writing–review and editing (equal). Madeleine L. Drent: conceptualization (lead), supervision (lead), writing–review and editing (lead).
Acknowledgements
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A: Subanalyses on the influence of multiple determinants on presence and impact of headache and tinnitus, before and after operation within the patient group.
| Headache | ||||
|---|---|---|---|---|
| Presence of headachea | Impact (HIT-6 score) | |||
| Preoperatively | Postoperatively | Preoperatively | Postoperatively | |
| Age (in years) | ||||
| With | 46.9 ± 15.1 | 50.7 ± 16.4 | ρ = −0.29 | ρ = −0.25 |
| Without | 58.7 ± 15.5 | 53.1 ± 16.4 | ||
| p | 0.01* | 0.64 | ||
| Tumour size (in mm) | ||||
| With | 20.0 ± 12.2 | 24.1 ± 13.8 | ρ = −0.26 | ρ = −0.88* |
| Without | 25.0 ± 15.0 | 22.1 ± 14.0 | ||
| p | 0.20 | 0.65 | ||
| Sex | ||||
| Male | 12/27 (44%) | 7/23 (30%) | 50.7 ± 8.7 | 55.8 ± 15.4 |
| Female | 19/30 (63%) | 7/26 (27%) | 53.5 ± 10.7 | 52.0 ± 10.3 |
| p | 0.15 | 0.79 | 0.35 | 0.62 |
| Sinus cavernous invasion | ||||
| Yes | 16/29 (55%) | 8/25 (32%) | 51.1 ± 10.0 | 45.2 ± 8.8 |
| No | 15/28 (54%) | 6/24 (25%) | 53.5 ± 9.8 | 62.0 ± 8.7 |
| p | 0.90 | 0.59 | 0.40 | 0.008* |
| Visual defect preoperative | ||||
| Yes | 15/27 (56%) | 6/24 (25%) | 51.6 ± 10.2 | 43.6 ± 7.1 |
| No | 16/30 (53%) | 8/25 (32%) | 52.8 ± 9.8 | 60.7 ± 9.7 |
| p | 0.87 | 0.59 | 0.70 | 0.008* |
| Apoplexy | ||||
| Yes | 2/3 (67%) | 2/3 (67%) | 53.0 ± 15.6 | 53.5 ± 10.6 |
| No | 29/54 (54%) | 12/46 (26%) | 52.3 ± 9.8 | 53.6 ± 13.0 |
| p | 1.00 | 0.19 | 0.92 | 0.99 |
| Complete resection | ||||
| Yes | 4/9 (44%) | 2/2 (100%) | 50.9 ± 10.4 | 59.0 ± 7.1 |
| No | 27/46 (59%) | 11/12 (92%) | 53.5 ± 9.4 | 52.5 ± 12.9 |
| p | 0.47 | 1.00 | 0.52 | 0.39 |
| Hormonal hypersecretion | ||||
| Yes | 12/19 (63%) | 4/15 (27%) | 56.8 ± 9.4 | 65.8 ± 8.4 |
| No | 19/38 (50%) | 10/34 (29%) | 49.8 ± 9.3 | 47.5 ± 8.6 |
| p | 0.35 | 1.00 | 0.02** | 0.006* |
| Adrenocorticotrophic hormone producing | ||||
| Yes | 5/9 (56%) | 2/7 (29%) | 53.9 ± 9.4 | 60.5 ± 2.1 |
| No | 24/45 (53%) | 12/39 (31%) | 52.4 ± 10.1 | 52.2 ± 12.9 |
| p | 1.00 | 1.00 | 0.72 | 0.40 |
| Growth hormone producing | ||||
| Yes | 4/7 (57%) | 1/6 (17%) | 60.0 ± 11.2 | 78.0 (n = 1) |
| No | 25/47 (53%) | 13/40 (33%) | 51.7 ± 9.4 | 51.4 ± 9.9 |
| p | 1.00 | 0.65 | 0.08 | 0.03** |
| Prolactinoma | ||||
| Yes | 3/3 (100%) | 1/2 (50%) | 59.0 ± 6.1 | 64.0 (n = 1) |
| No | 26/51 (51%) | 13/44 (30%) | 52.2 ± 10.0 | 52.6 ± 12.3 |
| p | 0.24 | 0.52 | 0.18 | 0.40 |
| Hypopituitarism | ||||
| Yes | 19/31 (61%) | 8/27 (30%) | 50.44 ± 10.35 | 51.3 ± 13.4 |
| No | 12/26 (46%) | 6/22 (27%) | 54.74 ± 8.87 | 56.8 ± 10.7 |
| p | 0.25 | 0.86 | 0.16 | 0.46 |
- a preoperatively defined as regular headache (≥1 a month), postoperatively defined as headache >2 weeks surgery; Pre- and postoperative comparisons within patients were conducted and, if significant, indicated by *p ≤ 0.01; **p ≤ 0.05.
Appendix B: Pathology outcomes for n = 54 pituitary adenoma.
| Transcription factor | Immunophenotypes | Tumour type | |||
|---|---|---|---|---|---|
| T-PIT + | 9 (17%) | ACTH + | 8 (15%) | Corticotroph adenoma | 9 (17%) |
| SF-1 + | 28 (52%) | FSH + | 24 (44%) | Gonadotroph adenoma | 28 (52%) |
| LH + | 20 (37%) | ||||
| ɑ-subunit + | 12 (22%) | ||||
| PIT-1 + | 11 (20%) | GH + | 6 (11%) | Somatotroph adenoma | 2 (4%) |
| PRL + | 7 (13%) | Lactotroph adenoma | 4 (7%) | ||
| ɑ-subunit + | 4 (7%) | Mammosomatotroph adenoma | 4 (7%) | ||
| TSH + | 1 (2%) | Plurihormonal (GH, PRL, TSH, ɑ-subunit) | 1 (2%) | ||
| Unknown | 6 (11%) |
Reasons: - surgery postponed (2 nonfunctioning adenoma) - too little material for pathology (1 acromegaly, 1 Cushing's disease) - normal pituitary tissue (1 Cushing's disease) or variable pathology outcome (1 Cushing's disease) |
|||
| Headache | |||||
|---|---|---|---|---|---|
| Presence of regular headache | Impact (HIT-6 score) | ||||
| Preoperatively | Postoperatively | Preoperatively | Postoperatively | ||
| SF-1 | Yes | 13/28 (46%) | 8/26 (31%) | 50.3 ± 9.4 | 44.0 ± 6.5 |
| No | 13/20 (65%) | 5/16 (31%) | 55.1 ± 9.5 | 63.4 ± 8.8 | |
| p | 0.20 | 1.00 | 0.14 | 0.002* | |
| PIT-1 | Yes | 7/11 (64%) | 3/9 (33%) | 57.6 ± 10.0 | 67.7 ± 9.1 |
| No | 19/37 (51%) | 10/33 (30%) | 50.9 ± 9.1 | 47.3 ± 8.2 | |
| p | 0.47 | 1.00 | 0.07 | 0.006* | |
| T-PIT | Yes | 6/9 (67%) | 2/7 (29%) | 52.4 ± 8.7 | 57.0 ± 2.8 |
| No | 20/39 (51%) | 11/35 (31%) | 52.6 ± 10.0 | 51.9 ± 13.7 | |
| p | 0.48 | 1.00 | 0.96 | 0.63 | |
| Ki-67 | ≤1% | 7/19 (37%) | 6/18 (33%) | 52.4 ± 10.6 | 58.0 ± 16.7 |
| 2% | 17/27 (63%) | 5/22 (23%) | 52.1 ± 9.6 | 49.0 ± 10.8 | |
| ≥ 3% | 2/2 (100%) | 2/2 (100%) | 62.0 ± 2.8 | 60.0 ± 1.4 | |
| p | 0.09 | 0.13 | 0.59 | 0.48 | |
Note: Pre- and postoperative comparisons within patients were conducted and, if significant, indicated by *p ≤ 0.01, **p ≤ 0.05.
Open Research
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.




