Effector memory and late memory T cells accumulate in the blood of CMV-carrying individuals but not in their cerebrospinal fluid
Abstract
Cytomegalovirus (CMV)-carrying individuals have significantly higher levels of effector memory and late memory T lymphocytes in their blood than non-carriers. To date, it is well recognized that the central nervous system is subjected to active immunosurveillance, as evidenced by the presence of central memory T cells in cerebrospinal fluid (CSF) of healthy individuals. In order to investigate whether levels of effector memory and late memory T cells were also increased in the CSF of CMV-carrying individuals, we characterized CD4+ and CD8+ T-cell subsets in CSF and blood of both groups. Effector memory and late memory T cells were only rarely seen in CSF, which was similar in CMV carriers and non-carriers. In conclusion, there was no demonstrable difference in the numbers of CSF effector memory and late memory T cells between CMV seronegative and CMV seropositive individuals. © 2013 International Clinical Cytometry Society
Cytomegalovirus (CMV)-carrying individuals are known to have higher absolute numbers of effector memory and late memory T cells in their blood than non-carriers 1, 2. These T cells express CD57, do not express CD27 or CD28 and are CD45RA− (effector memory) or CD45RA+ (late memory) 3. In the blood, CMV-specific T cells targeted against a single epitope can make up to 42% of CD8+ T cells, and most CMV-specific T cells are effector memory or late memory T cells 3. In contrast to earlier paradigms, it has become clear that the central nervous system (CNS) is also subjected to active immunosurveillance 4. Central memory T cells are found in cerebrospinal fluid (CSF) of healthy individuals, and mice models showed trafficking of these cells between the CNS and cervical lymph nodes 5-7. Recently, it has been shown that, in contrast to the blood, lymph nodes of CMV-carrying individuals have only low numbers of effector memory and late memory T cells 3. A possible explanation for this finding is that effector memory and late memory T cells do not express the chemokine receptor CCR7 needed for T cell-homing to the lymph nodes 8. Other chemokine receptors such as CCR5 and CCR6 are thought to promote T cell homing to the CNS 9, 10. Similar to CCR7, the level of expression of these chemokine receptors is higher in central memory T cells than in effector memory or late memory T cells 8. Therefore, we hypothesized that the effector memory or late memory T cells in the blood of CMV-carrying individuals do not traffic to the CNS. To test this hypothesis, we studied absolute numbers of naïve, central memory, effector memory, and late memory T cells in CSF and blood of CMV-carrying individuals and non-carriers.
METHODS
Twenty-five patients were included who underwent lumbar puncture for spinal anesthesia (n = 15), or for diagnostic purposes (n = 10; Table 1). The study was approved by the local ethical review committee, and written informed consent was obtained. About 2–5 mL CSF was collected in 2 mL serum-containing medium, and analyzed by 6-color flow cytometry as previously described 5. For enumeration of leukocyte and lymphocyte subsets in CSF, we used a single 6-color mixture of monoclonal antibodies (mAb): CD3 conjugated with fluorescein isothiocyanate (FITC; clone SK7), CD56 conjugated with phycoerythrin (PE; clone C5.9), CD45 conjugated with peridinyl cholorophyllin (PerCP; clone 2D1), CD4 conjugated with PE-Cy7 (clone SK3), CD19 conjugated with allophycocyanin (APC; clone HIB19), and CD8 conjugated with APC-Cy7 (clone SK1). For blood, two 4-color mAb mixtures were used: CD3-FITC, CD56-PE, CD45-PerCP and CD19-APC and CD4-FITC (clone HP2/6), CD8-PE (clone SK1), CD45-PerCP and CD3-APC (clone SK7). T cell subsets in both compartments were assessed with a 6-colorpanel: CD45RA-FITC (clone L48), CD127-PE (clone hIL-7R-M21), CD4-PerCP (clone SK3), CD25-PE-Cy7 (clone 2A3), CD27/28-APC (clone L128/CD28.2), and CD3-APCeFluor780 (clone UCHT1). All mAb were obtained from BD Biosciences (San Jose, CA) with the exception of CD56-PE (Dako, Glostrup, Denmark), and CD3-APC-eFluor780 and CD19-APC (eBioscience, San Diego, CA). CD4+ and CD8+ T cells were further classified according to CD45RA expression and CD27 and CD28 expression in naïve T cells (CD45RA+, 27/28+), central memory T cells (CD45RA−, CD27/28+), effector memory T cells (CD45RA−, CD27/28−), and late memory T cells (CD45RA+, CD27/28−) as previously described 5. Samples were acquired on a FACSCanto flow cytometer (BD Biosciences) and analyzed using FCS Express software (De Novo Software, Los Angeles, CA). For absolute numbers of total T lymphocytes in blood, we used a stain, lyse, no-wash method based on counting beads. For further characterization of T cell subsets, we used a lyse, stain, and wash technique. CSF cells were concentrated by centrifugation and resuspended in 400 μL PBS. Of this suspension, 100 μL was stained for each panel, washed, and resuspended in 100 μL PBS. For absolute cell counts, 100 μL of counting beads were added 5. Individuals were excluded from the study if their CSF sample contained less than 25 leukocytes per staining 11. Since absolute cell numbers in CSF of patients that underwent spinal anesthesia were not significantly different from those of patients that underwent a diagnostic lumbar puncture but were not diagnosed with a neuroinflammatory disease (e.g., tension headache, data not shown), these patient groups were pooled for analysis. The absolute cell numbers of each subset were stratified according to CMV serostatus, and cell numbers in CMV seropositive versus CMV seronegative individuals were compared with the Mann–Whitney test using SPSS version 20 (IBM, Chicago, IL). Figure 2 was made with Prism version 5 (GraphPad, San Diego, CA).
| CMV− | CMV+ | Pa | |
|---|---|---|---|
| N | 12 | 13 | |
| Gender (M/F) | 6/6 | 4/9 | 0.43 |
| Age (years)b | 61 (32–88) | 57 (19–76) | 0.32 |
| Spinal anesthesia/diagnosticc | 9/3 | 6/7 | 0.23 |
| CSF leukocyte counts (×106/L)b | 3 (1–7) | 2 (1–5) | 0.71 |
| CSF protein concentration (g/L)b | 0.34 (0.18–0.49) | 0.33 (0.20–0.44) | 0.37 |
- a P-values were calculated with the Mann–Whitney or Fisher exact test.
- b Median, range.
- c Included were patients without neurological disease who underwent lumbar puncture for spinal anesthesia (usually for orthopaedic surgery) and patients who had a diagnostic lumbar puncture for suspected neurological disease (none of these patients turned out to suffer from a neuro-inflammatory disorder).
- Abbreviations: CMV, cytomegalovirus; N, number of patients; M, male; F, female; CNS, central nervous system; CSF, cerebrospinal fluid.
RESULTS
Patient characteristics are shown in Table 1. Because all CSF samples contained a statistically sufficient number of leukocytes (we measured a median of 498 leukocytes per panel, while more than 25 leukocytes per panel are required), no patients were excluded from analysis. Both groups (CMV seronegative patients and CMV seropositive patients) did not differ significantly with respect to gender, age, and reason for lumbar puncture. Also, total CSF leukocyte counts and CSF protein concentrations were similar in both groups. Figure 1 shows a representative example of the expression of CD45RA and CD27/28 by CD4+ and CD8+ T cells in CSF of a CMV seropositive individual. Figure 2 shows absolute numbers of naïve, central memory, effector memory, and late memory CD4+ and CD8+ T cells in CSF (panel A and B) and blood (panel C and D) of CMV seronegative and CMV seropositive individuals. In the blood, a trend was seen toward higher numbers of CD4+ effector memory and CD4+ late memory T cells in CMV seropositive individuals as compared to CMV seronegative individuals, but these differences did not reach statistical significance (P = 0.17 and P = 0.19, respectively). The numbers of CD4+ effector memory and CD4+ late memory T cells in CSF were uniformly low in CMV carriers and non-carriers.
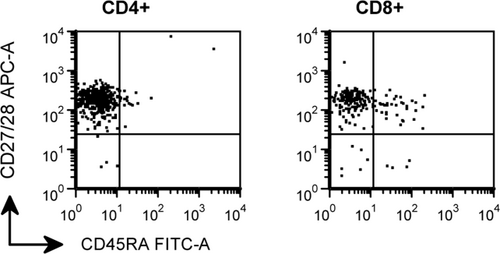
Representative example of the expression of CD45RA and CD27/28 by CD4+ and CD8+ T cells in CSF of a CMV seropositive patient. The vast majority of CD4+ and CD8+ T cells are CD45RA−CD27/28+ central memory T cells. CD45RA−CD27/28− effector memory T cells and CD45RA+CD27/28− late memory T cells were only sporadically detected, similar to CMV seronegative patients (data not shown).
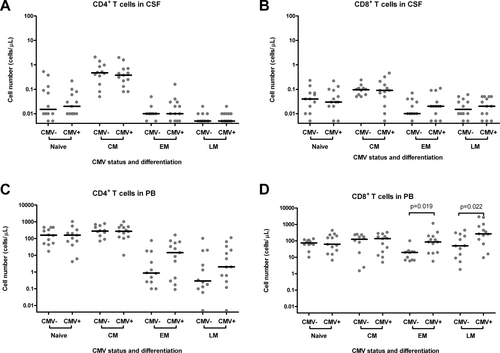
Absolute numbers of naïve, central memory, effector memory, and late memory CD4+ and CD8+ T cells in CSF (panel A and B) and blood (panel C and D) of CMV seronegative and CMV seropositive individuals. Data are shown on logarithmic scales in order to compress the figure. Horizontal lines represent medians. For graphic representation, cell numbers <0.01 cells/μL (corresponding to < 5 measured events) were assigned a value of 0.005 cells/μL. P-values represent the results of the Mann–Whitney test. Abbreviations: CSF, cerebrospinal fluid; PB, peripheral blood; CMV, cytomegalovirus; CMV+, seropositive for CMV; CMV−, seronegative for CMV; CM, central memory; EM, effector memory; LM, late memory.
The numbers of CD8+ effector memory and late memory T cells in the blood were significantly higher in CMV seropositive individuals. Within the CD8+ T lymphocytes, effector memory and late memory cells were only rarely seen in CSF (median, 0.02; range, 0.00–0.11 cells/μL and 0.02; 0.00–0.06 cells/μL, respectively) and did not differ significantly between CMV seronegative and CMV seropositive individuals.
DISCUSSION
In this study, we found no demonstrable difference in the numbers of effector memory and late memory T cells between CSF of CMV seronegative patients and CSF of CMV seropositive individuals, despite significant differences in the numbers of these subsets in the blood. These changes in the blood were most prominent and significant within the CD8+ T cells, and to a lesser extent seen in CD4+ T cells (not reaching significance). The numbers of circulating effector memory and late memory T cells in the blood were shown to increase with age, and are strongly correlated with latent CMV infection 12. Their presence is thought to at least partly result from antigenic stimulation of T cells by latently CMV-infected endothelial and myeloid cells. CMV-specific T cells are thought to prevent viral reactivation by cytolysis of infected cells 3, 12. Interestingly, the lymph nodes of CMV-carrying individuals contain only few effector memory and late memory T cells, and the few CMV-specific T cells present have a central memory phenotype 3. Our findings in CSF support the hypothesis that CD8+ effector memory and late memory T cells under normal circumstances do not enter the CNS. This situation may be due to a low level of expression of chemokine receptors such as CCR5 and CCR6 that are needed for T cell homing to the CNS 8, 10. In addition, lack of expression of molecules such as CD162 (selectin P ligand) and CD49D (α 4 subunit of very late antigen 4 receptor) by T lymphocytes or selectin P and very late antigen 4 by endothelial cells may prevent migration of T lymphocytes across vascular endothelium 4. In this study, we did neither assess the expression of these molecules nor the specificity of the CSF T cells. For this moment, we hypothesize that effector memory and late memory T cells only enter the CNS under exceptional circumstances (for example in case of CMV encephalitis), while under normal conditions, central memory T cells enter and leave the CNS in the context of CNS immunosurveillance.