Central memory CD4+ T cells dominate the normal cerebrospinal fluid†
How to cite this article: de Graaf MT, Smitt PAES, Luitwieler RL, van Velzen C, van den Broek PDM, Kraan J, Gratama JW. Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry Part B 2011; 80B: 43–50.
Abstract
Background:
To use cerebrospinal fluid (CSF) immune phenotyping as a diagnostic and research tool, we have set out to establish reference values of white blood cell (WBC) subsets in CSF.
Methods:
We assessed the absolute numbers and percentages of WBC subsets by 6-color flow cytometry in paired CSF and blood samples of 84 individuals without neurological disease who underwent spinal anaesthesia for surgery. Leukocyte (i.e., lymphocytes, granulocytes, and monocytes), lymphocyte (i.e., T [CD4+ and CD8+], NK, NKT and B cells), T cell (i.e., naïve, central memory, effector memory, and regulatory) and dendritic cell subsets (i.e., myeloid and plasmacytoid) were studied.
Results:
CSF showed a predominance of T cells, while granulocytes, B and NK cells were relatively rare compared to blood. The majority of T cells in CSF consisted of CD4+ T cells (∼70%), most of them (∼90%) with a central memory phenotype, while B cells were almost absent (<1%). Among the small population of dendritic cells in CSF, those of the myeloid subtype were more frequent than plasmacytoid dendritic cells (medians: 1.7% and 0.4% of leukocytes, respectively), whilst both subsets made up 0.2% of leukocytes in blood.
Conclusions:
This study reports reference values of absolute numbers and percentages of WBC subsets in CSF, which are essential for further investigation of the immunopathogenesis of neuro-inflammatory diseases. Furthermore, the relative abundance of CD4+ T cells, mainly with a central memory phenotype, and the presence of dendritic cells in CSF suggests an active adaptive immune response under normal conditions in the central nervous system (CNS). © 2010 International Clinical Cytometry Society
Patients with inflammatory disorders of the central nervous system (CNS) typically have increased white blood cell (WBC) counts in their CSF. Little information is yet available on the composition of these cells in normal CSF, as lumbar puncture without a clinical indication is generally considered unethical and flowcytometric studies in CSF mainly focus on detection of CSF involvement in hematologic malignancies (1). Furthermore, the presence of relatively low numbers of cells with rapidly decreasing viability after sampling makes reliable identification and enumeration of leukocyte and lymphocyte subsets in CSF difficult (2). Earlier studies have described the distribution of specific lymphocyte subsets in CSF of patients with neuro-inflammatory diseases such as multiple sclerosis, neuroborreliosis and paraneoplastic neurological syndromes (PNS) in order to investigate the immunopathogenesis of these diseases (3-6). In those studies, patients with other inflammatory or noninflammatory neurological disorders were frequently included as controls instead of individuals without neurological disease. Svenningsson et al. (7, 8) studied normal CSF by assessing the percentages of lymphocyte subsets, but not absolute counts, in CSF of 34 healthy individuals with 2- or 3-color flow cytometry. Here, a predominant presence of T cells, mostly with a central memory phenotype, and low frequencies of B and NK cells, were reported.
In this study, we aimed to also study absolute numbers of WBC subsets in normal CSF, because percentages and absolute numbers can differ widely depending on the clinical setting (9). In specific patients, enumeration of absolute levels of circulating cells and their subsets is of primary importance. F.e., in acquired immune deficiency syndrome (AIDS) absolute counting of CD4+ T cells in peripheral blood is the major laboratory tool for staging human immunodeficiency virus (HIV) infected patients (10). In candidates for autologous or allogeneic hematopoietic stem cell transplantation, enumeration of absolute levels of circulating CD34+ hematopoietic progenitor cells is used as a marker for graft adequacy (11). In our earlier work, PNS patients stood out by highly increased absolute counts of the major lymphocyte subsets in CSF, but above all, by B-cell counts that had increased more than 20-fold as compared with controls without neurological disease (5). This indicates that assessment of absolute counts is also important in CSF and therefore knowledge of normal values is essential.
By using 6-color flow cytometry, we were able to study multiple functionally different WBC subsets in paucicellular CSF samples, such as lymphocytes (CD4+ and CD8+ T cells, NK, NKT, and B cells), granulocytes, monocytes, and dendritic cells (myeloid and plasmacytoid). These subsets were assessed in CSF and blood samples of 84 individuals without neurological disease who underwent spinal anaesthesia for surgery. We considered these study persons normal with respect to the numbers and phenotypes of WBC in their CSF. In addition to description of normal values, we confirmed the predominant presence of central memory CD4+ T cells and the paucity of B, NK, and NKT cells in normal CSF. Additionally, we showed that dendritic cells, both myeloid as well as plasmacytoid are present in normal CSF. Comparison of our results with those in patients with neuro-inflammatory diseases may allow further studies of the immunopathogenesis of such diseases.
METHODS
Study Persons
A total of 84 individuals without neurological disease undergoing spinal anaesthesia for surgery were included between May and August 2008 at the Department of Anaesthesiology, Sint Franciscus Gasthuis, Rotterdam. Individuals were not included when they had a history of (i) neurological disease; (ii) cancer; or (iii) treatment with corticosteroids or cytostatic drugs. The local ethical committee approved the study and written informed consent was obtained from all individuals.
Monoclonal Antibodies
Properly titrated fluorochrome-conjugated monoclonal antibodies (mAb) were used for cell surface labeling. For enumeration of leukocyte and lymphocyte subsets in CSF, a single 6-color mixture was used: CD3 conjugated with fluorescein isothiocyanate (FITC; clone SK7), CD56 conjugated with phycoerythrin (PE; clone C5.9), CD45 conjugated with peridinyl cholorophyllin (PerCP; clone 2D1), CD4 conjugated with PE-Cy7 (clone SK3), CD19 conjugated with allophycocyanin (APC; clone HIB19) and CD8 conjugated with APC-Cy7 (clone SK1). For blood two 4-color mixtures were used: CD3-FITC, CD56-PE, CD45-PerCP and CD19-APC and CD4-FITC (clone HP2/6), CD8-PE (clone SK1), CD45-PerCP and CD3-APC (clone SK7). T cell subsets were assessed with a 6-color panel: CD45RA-FITC (clone L48), CD127-PE (clone hIL-7R-M21), CD4-PerCP (clone SK3), CD25-PE-Cy7 (clone 2A3), CD27/28-APC (clone L128/CD28.2) and CD3-APC-eFluor780 (clone UCHT1). For dendritic cell subset immune phenotyping a 6-color staining protocol described by Della Bella et al. (12) was used: LIN-1-FITC (Anti-Lineage 1 cocktail composed of CD3, CD14, CD16, CD19, CD20, and CD56 monoclonal antibodies), CD45-PerCP, CD123-PE-Cy7 (clone 6H6), CD11c-APC (clone S-HCL-3) and HLA-DR-APC-Cy7 (clone L243). All mAb were obtained from BD Biosciences (San Jose, CA) with the exception of CD56-PE (Dako, Glostrup, Denmark), and CD3-APC-eFluor780, CD19-APC, and CD123-PE-Cy7 (eBioscience, San Diego, CA).
Cerebrospinal Fluid
CSF was obtained during lumbar puncture performed by anaesthesiologists before administration of the anaesthetic drug. Lumbar puncture was done with a 27G atraumatic pencil-point Sprotte needle between the 2nd and 3rd or 3rd and 4th dorsal processes of the lumbar vertebrae in a sitting position. Approximately 5 ml CSF was carefully aspirated. Automated erythrocyte and leukocyte counts were obtained on a Sysmex XE-5000 (Sysmex, Hamburg, Germany). For immune phenotyping, 3-5 ml CSF was collected in tubes containing stabilization medium (RPMI-1640 with 25 mM HEPES, 1 mM glutamin, 2% Pen/Strep, 5% heat-inactivated BCS and 2500 IU heparin) to prevent direct cell lysis (2). Within 6 h after collection, cells were concentrated by centrifugation (8 min, 450g) and resuspended in phosphate-buffered saline (PBS). Next, 100 μl of the sample was incubated for 15 min (15′) at room temperature (RT) in the dark with 10 μl of each of the above-mentioned mAb and after incubation the cells were washed in PBS. For enumeration of absolute numbers of leukocyte and lymphocyte subsets 100 μl PBS and 100 μl Cyto-Cal Count Control counting beads (Duke Scientific Corporation, Palo Alto, CA) were added (2), while for determination of T and dendritic cell subsets 250 μl PBS with 1% paraformaldehyde (PFA) was added. Immediately upon staining, list mode data were acquired on a 6-color FACSCanto flow cytometer (BD Biosciences). Analysis was performed using FCS Express software (De Novo Software, Los Angeles, CA). Our gating strategy for leukocyte and lymphocyte subsets in CSF is presented in Figure 1. Absolute numbers were calculated as described previously (2). First, the number of events for each of the lymphocyte and leukocyte subsets and the number of acquired counting bead events were defined. Next, the absolute number of each cell subset (x106 cells/l) was calculated by using the following formula: (events in cell subset gate/events in counting bead gate) × (counting beads added per tube/volume of CSF sample) (2). This method was validated by comparing flow cytometric absolute cell numbers with microscopic cell numbers in 45 normocellular CSF samples of patients with haematological or solid malignancies (Supporting Information Table). Percentages were calculated out of total leukocytes for the leukocyte and dendritic cell subsets, out of total lymphocytes for the lymphocyte subsets or out of total T cells for the T cell subsets. When less than 25 leukocytes per sample were counted (2), individuals were excluded from the study.
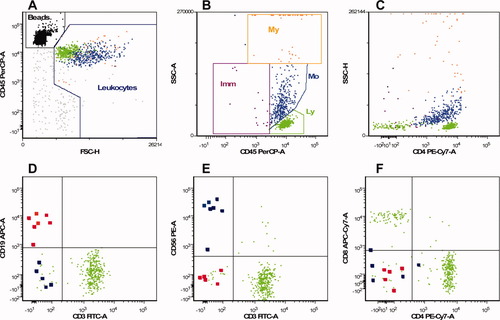
Gating strategy for leukocyte and lymphocyte subsets in CSF Example of a 6-color flow cytometric analysis of a normal CSF sample. Each dot represents a single cell. The units on the x- and y-axes are relative fluorescense intensity. For absolute counting, microspheres are used and defined as FSClo, FL+++ [black dots in panel A]. Debris, erythroytes, and nonleukocyte events (gray) were excluded by defining leukocytes CD45bright, FSCim-hi [panel A]. The leukocyte subsets (My, myeloid lineage; Imm, immature lineage; Mo, monocytes; Ly, lymphocytes) were defined with CD45 expression and side scatter [panel B and Table 2] and shows two major subsets: lymphocytes (CD45+, SSClo, FSCim; green dots) and monocytes (CD45+, SSCim, FSChi, CD4dim; cyan dots). In order to optimize resolution, side scatter area (SSC-A) is used. To visualize all granulocytes separately, a plot with side scatter height (SSC-H) is included [panel C]. Next, within the lymphocyte gate, T cells (CD3+), and sporadic B cells (CD19+; highlighted red dots [panel D]), sporadic NK (CD3−,CD56+; highlighted blue dots) and NKT cells (CD3+,CD56+ [panel E]), CD4+, and CD8+ T cells [panel F] were identified.
Blood
From all included individuals, ∼14 ml blood was drawn in EDTA tubes. For the enumeration of absolute numbers of leukocyte and lymphocyte subsets, a single platform, whole blood, stain, lyse, no-wash method based on counting beads was used. Details of this method have been described elsewhere (13). Briefly, 100 μl of EDTA anti-coagulated whole blood was stained with mixtures of the above-mentioned mAb. After incubation for 15′ at RT, 2 ml NH4Cl lysing buffer was added and 100 μl of Flowcount counting beads (Beckman Coulter, Miami, FL). After another 15′ of incubation at RT, samples were acquired on a FACSCalibur flow cytometer (BD Biosciences). Analysis of list mode data was performed using CellQuestPro software (BD Biosciences). For quantification of T and dendritic cell subsets we used a lyse, stain and wash technique which required an additional washing step after red cell lysis in order to reduce background fluorescence due to unbound mAb. These samples were acquired on a 6-color FACSCanto flow cytometer and analyzed using FCS Express software.
Statistical Analysis
Results were analyzed with SPSS version 15.0 (SPSS Inc., Chicago, IL) and presented graphically in box-and-whisker plots. The boxes represent median and interquartile ranges; the whiskers extend to the adjacent values, i.e., 1.5× the interquartile range rolled back to where there is data. Observed values more extreme than the adjacent values were considered outliers and have been plotted individually. Because the absolute numbers and percentages of subsets in CSF were in general not normally distributed, nonparametric tests were used. Percentages of subsets in CSF and blood were compared with the Wilcoxon test. Earlier studies have shown a significant impact of age and gender on the numbers and phenotypes of lymphocyte subsets in blood (14-16). Therefore, influences of these factors on the subsets in blood as well as CSF were analyzed with the Spearman correlation coefficient (age) or the Wilcoxon test (gender). Differences between groups with a P-value <0.05 were considered significant.
RESULTS
Study Persons
We studied 84 paired CSF and blood samples from 84 individuals without neurological disease. Because all CSF samples contained a statistically sufficient number of leukocytes for the flowcytometric analysis (i.e., more than 25), no specimens were excluded from analysis. Clinical characteristics of the individuals are presented in Table 1. All individuals had normal CSF glucose and protein levels and a normal WBC count. No blood contamination was observed in any of the samples. After the first staining, enough CSF was left to perform additional staining for T cell subsets in 18 paired samples and dendritic cell subsets in 35 paired samples. There were no significant differences in the numbers of leukocyte and lymphocyte subsets between these 18 respectively 35 samples and the other samples. Table 2 outlines the different WBC subsets we studied, their immunological definition, function and absolute number in CSF.
| Individuals without neurological disease | |
|---|---|
| N | 84 |
| Age: median (range) | 53 (17–82) |
| Gender | |
| Male | 39 |
| Female | 45 |
| Cerebrospinal fluid: median (range) | |
| Volume (ml)a | 2.86 (0.80–4.65) |
| Glucose (mmol/l) | 3.3 (0.3–4.9) |
| Protein (g/l) | 0.36 (0.19–0.71) |
| WBC (x106/l) | 1.12 (0.40–3.17) |
| RBC (x106/l) | 0 (0–0) |
| Reason for surgery | |
| Orthopaedicb | 46 |
| Gynaecologicalc | 15 |
| Urologicald | 9 |
| Othere | 14 |
- a Volume of CSF used for determination of absolute numbers of leukocyte and lymphocyte subsets.
- b Arthroscopy of hip or knee or hallux valgus correction.
- c Hysteroscopy, endocervical currettage, abortion or labiaplasty.
- d Orchidectomy, lithotripsy or spermatocele excision.
- e Inguinal or abdominal wall hernia repair, dermatological or vascular surgery.
- WBC, white blood cell; RBC, red blood cell.
| Subset | Immunological definition | Function | Absolute numbera |
|---|---|---|---|
| Leukocytes | CD45+ | White blood cells | 1.12 (0.40–3.17) |
| Granulocytes | CD45+, SSChi | Involved in innate immunity | 0.08 (0.02–0.43) |
| Monocytes | CD45+, SSCim, FSChi, CD4dim | Precursors of macrophages | 0.23 (0.08–1.11) |
| Lymphocytes | CD45+, SSClo, FSCim | 0.66 (0.16–1.88) | |
| T cells | CD3+ | Involved in adaptive immunity | 0.62 (0.15–1.83) |
| CD4+ T cells | CD4+ | Helper T cells | 0.44 (0.08–1.43) |
| Naïve | CD45RA+, 27/28+ | Have not yet encountered an antigen | 0.02 (0.00–0.38) |
| Central memory | CD45RA−, 27/28+ | Have seen or been primed with an antigen | 0.43 (0.05–1.60) |
| Effector memory | CD45RA−, 27/28− | Present in settings of active antigenic stimulation | 0.00 (0.00–0.02) |
| Late memory | CD45RA+, 27/28− | Control of viral reactivation and cancers | 0.00 (0.00–0.02) |
| Regulatory | CD25++, 127+ | Suppression of T cell responses | 0.02 (0.00–0.12) |
| CD8+ T cells | CD8+ | Cytotoxic T cells | 0.13 (0.04–0.40) |
| Naïve | CD45RA+, 27/28+ | Have not yet encountered an antigen | 0.04 (0.01–0.22) |
| Central memory | CD45RA−, 27/28+ | Have seen or been primed with an antigen | 0.10 (0.05–0.46) |
| Effector memory | CD45RA−, 27/28− | Present in settings of active antigenic stimulation | 0.02 (0.00–0.06) |
| Late memory | CD45RA+, 27/28− | Control of viral reactivation and cancers | 0.02 (0.00–0.05) |
| NKT cells | CD56+ | Secretion of cytokines and regulatory function | 0.01 (0.00–0.06) |
| B cells | CD3−, 19+ | Differentiate into plasma cells and secrete antibodies | 0.00 (0.00–0.03) |
| NK cells | CD3−, 56+ | Release lytic granules that kill infected or tumor cells | 0.01 (0.00–0.05) |
| Dendritic cells | CD45+, LIN-1−, HLA-DR+ | Specialized antigen-presenting cells | 0.04 (0.01–0.18) |
| Myeloid | CD11c+, 123− | Derived from myeloid lineage | 0.02 (0.00–0.13) |
| Plasmacytoid | CD11c−, 123+ | Derived from lymphoid lineage | 0.01 (0.00–0.03) |
- SSC, side scatter; FSC, forward scatter; LIN-1, Anti-Lineage 1 cocktail composed of CD3, CD14, CD16, CD19, CD20, and CD56 monoclonal antibodies.
- a Medians (5th–95th percentiles) of absolute numbers ×106/l are given.
Leukocyte Subsets
In CSF, the absolute number of leukocytes was ∼5,000× lower than in blood (CSF: median 1.12 × 106/l, blood: median 6048 × 106/l). Lymphocyte (CSF: median 0.66 × 106/l, blood: median 1716 × 106/l) and monocyte counts (CSF: median 0.23 × 106/l, blood: median 414 x106/l) were ∼2,500× and 2,000× lower in CSF than in blood, while granulocyte counts were even ∼45,000× lower (CSF: median 0.08 × 106/l, blood: median 3,568 × 106/l) (Figs. 2A and 2B). This was also reflected in the distribution of the leukocyte subsets which showed major differences between both compartments: CSF contained relatively more lymphocytes and monocytes, but less granulocytes than blood (Figs. 2C and 2D).
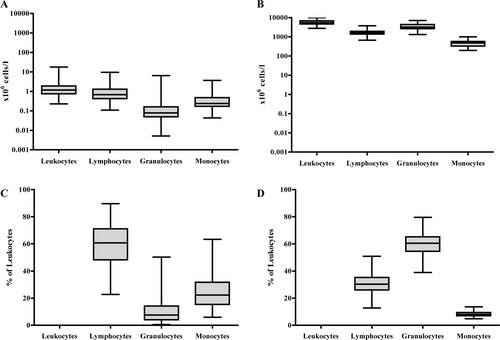
Absolute numbers and percentages of leukocytes and leukocyte subsets in CSF and blood Absolute numbers (i.e., ×106 cells/l) of leukocytes, lymphocytes, granulocytes, and monocytes in CSF (A) and blood (B). Percentages (expressed as percentage of leukocytes) of lymphocytes, granulocytes, and monocytes in CSF (C) and blood (D). In paired analyses, all leukocyte subsets showed significant differences between the percentage in CSF and in blood (P < 1.0 × 10−15).
Lymphocyte Subsets
As in blood, T cells were the most abundant lymphocyte subset in CSF with a predominance of CD4+ over CD8+ T cells, whilst absolute counts of NK, NKT, and B cells were 10- to 100-fold lower than those of T cells (Figs. 3A and 3B). When looking at the percentages of the various lymphocyte subsets in CSF and blood (data not shown), the following differences were apparent: (i) the ratio between CD4+ and CD8+ T cells was shifted significantly in favor of CD4+ T cells in CSF compared to blood (median: 3.0 [5th and 95th percentile: 1.1 and 6.5] versus 2.1 [0.9 and 6.0], respectively; P < 1.0 × 10−6); (ii) NK and NKT cells were <5% in most CSF samples; and (iii) B cells were by far the smallest subset in CSF, whilst NKT cells were the smallest subset in blood.
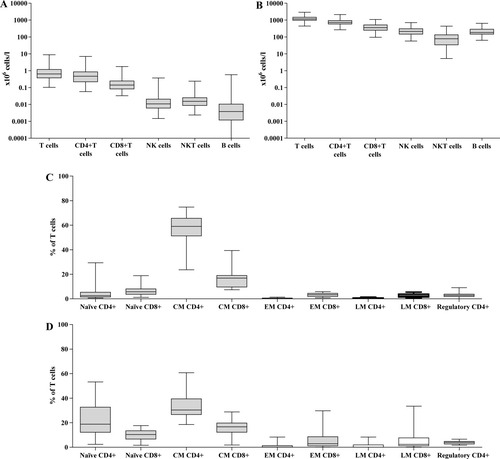
Absolute numbers of lymphocyte subsets and percentages of T cell subsets in CSF and blood Absolute numbers (i.e., ×106 cells/l) of T cells, CD4+ T cells, CD8+ T cells, NK cells, NKT cells, and B cells in CSF (A) and blood (B). Percentages (expressed as percentage of T cells) of naïve, central memory, effector memory, late memory, and regulatory CD4+ and CD8+ T cells in CSF (C) and blood (D). CM, central memory; EM, effector memory; LM, late memory. For absolute B-cell counts, a minimum of five events was required to calculate an absolute count. In 13 of the 84 cases, this threshold was not reached. An indication of these missing data was given in the figure by placing the lower whisker of “B cells” just above the 100 cells/l level.
T Cell Differentiation
We then studied markers reflecting lymphocyte differentiation on T cells in paired CSF and blood samples of a subgroup of 18/84 individuals and determined the percentages of naïve, central memory, effector memory, late memory, and regulatory phenotypes (Figs. 3C and 3D). The T cell subsets we describe here, are by and large similar to the subsets Kim et al. (17) described recently: the naïve phenotype corresponds with Kim's “N compartment,” the central memory phenotype with the “M1 compartment,” the effector memory phenotype with the “M2 compartment” and the late memory phenotype with the “M3 compartment”. We observed that the vast majority of T cells in CSF consisted of central memory CD4+ T cells (∼60% of total T cells and ∼90% of CD4+ T cells). Also, within the CD8+ T cell subset the largest proportion was composed of cells with the central memory phenotype (∼17% of total T cells and ∼60% of CD8+ T cells). This corresponds with earlier studies which showed that the majority of T cells in CSF had a memory phenotype (7, 8, 18, 19), while the proportion of naïve T cells was low (8, 19). Compared to blood, we observed significantly higher percentages of central memory CD4+ T cells (P < 5.0 × 10−4), while the percentages of the naïve phenotypes, both CD4+ (P < 5.0 × 10−4) and CD8+ (P < 5.0 × 10−2), and regulatory CD4+ T cells (P < 5.0 × 10−2), as estimated by the coexpression of CD25 (bright fluorescence) and CD127 on CD4+ T cells, were significantly lower in CSF.
Dendritic Cells
The amount of CSF obtained allowed the additional characterization of dendritic cells in 35/84 samples. We detected a median of 0.04 × 106 dendritic cells/l (5th and 95th percentiles: 0.01 and 0.18 × 106 cells/l) in CSF, and a median of 31 × 106 dendritic cells/l (14 and 52 × 106 cells/l) in blood. Relatively, dendritic cells were 2.2% of leukocytes in CSF versus only 0.4% in blood (P < 5.0 × 10−7). We found a clear predominance of the myeloid subset over the plasmacytoid subset in CSF (medians: 1.7% and 0.4% of leukocytes, respectively), whilst both subsets had similar percentages in blood (median: 0.2%; Fig. 4).
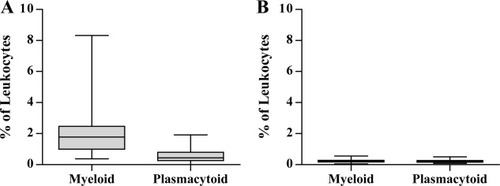
Percentages of dendritic cell subsets in CSF and blood Percentages (expressed as percentage of leukocytes) of myeloid and plasmacytoid dendritic cells in CSF (A) and blood (B).
Impact of Age and Gender
Age and gender had no significant impact on numbers and proportions of leukocytes and their major subsets in CSF and blood (data not shown). With regard to the lymphocyte subsets, absolute counts of CD4+ T cells in CSF, but not in blood, were negatively correlated with age (r = −0.23, P < 0.05), while absolute counts of CD8+ T cells in blood, but not in CSF, were negatively correlated with age (r = −0.27, P < 0.05). Additionally, absolute counts of B cells were negatively correlated with age both in CSF and blood (r = −0.23 and −0.25, respectively; P < 0.05). However, multivariate analysis showed no confounding influence of age and gender on the CSF and blood results of this study (data not shown), which indicates that correction of these WBC reference values for age and gender is not necessary.
DISCUSSION
By using 6-color flow cytometry, we studied absolute numbers and percentages of WBC subsets in paired CSF and blood samples of 84 individuals undergoing spinal anaesthesia before surgery. None of the patients had a history of neurological disease, cancer or treatment with immunosuppressive or cytostatic drugs, factors which may have influenced the numbers of WBC subsets. Furthermore, all CSF samples had normal levels of glucose and protein and normal WBC counts, without any blood contamination. Therefore, we propose that these CSF samples are useful as reference source for WBC subsets in that compartment.
Second, we confirmed the predominant presence of CD4+ T cells with relatively low numbers of granulocytes, B, NK and NKT cells shown in earlier CSF studies (7, 8). The predominance in CSF of cells of the adaptive immune system over those of the innate immune system may be related to the fact that, under normal circumstances, the CNS is much less exposed to pathogens (and auto-antigens) than peripheral organs.
Third, within the CD4+ T cell subset in CSF, most cells have the central memory phenotype (∼90%). This proportion is significantly higher than in blood (∼58%), while the naïve and regulatory phenotypes were less abundant in CSF than in blood. This indicates a selective recruitment of central memory CD4+ T cells into normal CSF (7, 8, 19-21). Hypothetically, central memory T cells search for their specific antigen after migration into the CNS. If they encounter it, they accumulate, initiate inflammation and cause infectious or autoimmune reactions (19, 20, 22); if not they will leave the CNS. This is considered immunological surveillance of the CNS as is also observed in other organs (23). An alternative explanation for the high proportion of memory T cells in CSF, is the absence of migration of naïve T cells into normal CSF. Several studies have shown that activated lymphocytes more readily migrate into the CNS than resting lymphocytes (22-25). It is hypothesized that in case of CNS inflammation, naïve T cells first have to be activated by antigen in perivascular spaces or lymph nodes draining the CNS, before they can enter the CSF (23).
Fourth, the number of B cells is hardly above detection limit (median, 0.004 × 106 cells/l) in normal CSF. This result corresponds with our earlier study in which we examined CSF lymphocyte subsets in healthy controls and PNS patients. Whilst CSF B-cell counts in the healthy controls were very low (median, 0.005 × 106 cells/l), PNS patients showed significantly elevated counts (median: 0.133 × 106 cells/l) (5). This suggests that B cells are recruited to CSF in certain pathological conditions, rather than that they participate in immunosurveillance of the CNS, like the (central memory) T cells do.
Fifth, dendritic cells are relatively more abundant in CSF whilst being “rare events” in blood. The absolute numbers we report here are comparable with the numbers Pashenkov et al. (26) observed in CSF of patients with noninflammatory neurological diseases. In addition to the relatively high frequency of dendritic cells in CSF, we found a predominance of myeloid over plasmacytoid dendritic cells which may be explained by the origin of dendritic cells in CSF. Most myeloid dendritic cells arise in the CNS from blood monocytes (26), while plasmacytoid dendritic cells are all blood-borne (27). Dendritic cells are the most potent antigen presenting cells (28). In CSF they take up antigens that invade the CNS and present them to T cells in secondary lymphoid organs, such as deep cervical lymph nodes (27). In this way, dendritic cells have an important role in the adaptive immune response in CSF. This role is also supported by the finding that in CSF of patients with neuroinflammatory diseases, such as multiple sclerosis, optic neuritis, neuroborreliosis and aseptic meningoencephalitis, numbers of both myeloid and plasmacytoid dendritic cells are significantly elevated (26).
In addition to serving as benchmark for WBC subsets in CSF, this study confirms the predominant presence of central memory CD4+ T cells, which may have selectively been recruited to the CNS to function as immune surveillants. The presence of these cells in CSF would facilitate quick responses to antigens and induction of an adaptive immune response, aided by dendritic cells.
Acknowledgements
The authors are grateful to the anaesthesiologists of the Sint Franciscus Gasthuis for inclusion of individuals and Eric Brouwer for technical assistance.