Levels of circulating endothelial cells in normotensive and severe preeclamptic pregnancies†
How to cite this article: Strijbos MH, Snijder CA, Kraan J, Lamers CHJ, Gratama JW, Duvekot JJ. Levels of circulating endothelial cells in normotensive and severe preeclamptic pregnancies. Cytometry Part B 2010;78B:382–386.
Abstract
Background:
Preeclampsia is a disease hypothesized to originate from widespread endothelial dysfunction or damage. This study investigated whether circulating endothelial cells (CEC) can serve as a surrogate marker for disease severity in patients with preeclampsia, and if their number correlates to serum endothelial biomarkers for activation, dysfunction, or damage of those cells.
Methods:
Blood was drawn consecutively from 30 patients admitted with a diagnosis of severe preeclampsia. Thirty healthy, normotensive, patients matched for age, body mass index, and gestational age served as a control group. We determined the number of CEC and serum concentrations of biomarkers indicative of endothelial damage (thrombomodulin) and activation (E-selectin), and the antiangiogenic protein (endoglin), which reflects endothelial dysfunction.
Results:
Median CEC counts did not differ significantly between preeclamptic patients and the control group (median 5.3 vs. 3.5 CEC/mL, respectively) and were mostly within the normal range (i.e., <20 CEC/mL). However, serum concentrations of thrombomodulin (median 3.6 vs. 5.2 ng/mL; P = 0.006), E-selectin (median 32.0 vs. 42.9 ng/mL; P = 0.02), and especially endoglin (median 5.0 vs. 76.2 ng/mL; P < 0.0001) were significantly increased in severe preeclamptic patients. CEC counts did not correlate with any of the clinical parameters or routinely determined laboratory indices.
Conclusion:
Preeclampsia is characterized by endothelial dysfunction and activation rather than actual endothelial damage as characterized by increased CEC counts. © 2010 International Clinical Cytometry Society
INTRODUCTION
Preeclampsia, defined as the occurrence of hypertension and proteinuria after 20 weeks of gestation, affects up to five percent of all pregnancies worldwide, and is one of the leading causes of maternal and fetal morbidity and mortality (1, 2). Although the exact etiology of preeclampsia is still unknown, there is sufficient clinical and biochemical evidence that the endothelium plays an important role in its pathogenesis (3).
Endothelial dysfunction in preeclampsia is thought to be the result of several mechanisms triggered in response to placental hypoxia caused by abnormal placentation. These mechanisms include the release of soluble antiangiogenic factors, such as soluble fms-like tyrosine kinase 1 receptor (sFlt-1, a soluble receptor for the vascular endothelial growth factor [VEGF]), and soluble endoglin, (a coreceptor for transforming growth factor β-1). Also, release of proinflammatory mediators, such as tumor necrosis factor alpha, interferon gamma, and interleukin 1 and 6 have been reported (3). Prolonged exposure to these mediators is known to cause endothelial activation and dysfunction in pregnant women (4). Indeed, increased serum concentrations of E-selectin and von Willebrand factor, putative markers of endothelial dysfunction, have been observed in preeclamptic women (5).
In addition to endothelial dysfunction, actual damage to the maternal vascular endothelium contributes to the clinical syndrome. For example, it has been shown that sFlt-1 leads to the production of endothelin-1, which subsequently causes glomerular endothelial injury and proteinuria (6). Blood based evidence for endothelial damage in preeclampsia has recently been demonstrated in two studies. These studies reported increased numbers of circulating endothelial cells (CEC) in preeclamptic women (7, 8). Increments in CEC counts are considered to reflect the extent of endothelial injury (9, 10). However, these studies rely on manual enumeration of CEC, which is known to be susceptible to cell loss and therefore to underestimation of counts (11).
In this cross-sectional study, we hypothesize that CEC numbers, determined by a well-validated semiautomatic CEC detection assay with excellent recovery of these cells are elevated in preeclamptic pregnancies, and would reflect endothelial injury. Furthermore, we expected that such increment would not correlate to increments in serum markers of endothelial dysfunction (soluble E-selectin and soluble endoglin) and endothelial injury (soluble thrombomodulin).
MATERIALS AND METHODS
Patients and Blood Sampling
Peripheral blood (PB) was obtained from 30 women consecutively admitted to the Department of Obstetrics of the Erasmus MC with the diagnosis severe preeclampsia. As a control group served 30 healthy, normotensive, pregnant controls, visiting the outpatient clinic. Both groups were matched for age, gestational age, and body mass index (BMI). In preeclamptic patients blood sampling was done immediately on admission. Preeclampsia was diagnosed according to the definition of the International Society for the Study of Hypertension (ISSHP). Severe preeclampsia was diagnosed if one or more of the following criteria was present: a blood pressure of 160 mmHg systolic (SBP) or higher or 110 mmHg diastolic (DBP) or higher on two occasions at least 6 h apart; proteinuria of 5 g or more in a 24 h urine specimen or 3+ or greater on two random urine samples collected at least 4 h apart; oliguria of less than 500 mL in 24 h; cerebral or visual disturbances; pulmonary edema or cyanosis; epigastric or right upper-quadrant pain; impaired liver function; thrombocytopenia; fetal growth restriction (12). Patients with previous pregnancies complicated by preeclampsia, gestational diabetes or growth retardation were excluded from this study. The protocol and informed consent forms were approved by the institutional Medical Ethical Review Board, and all patients provided written consent prior to participation. The study is in agreement with the Helsinki declaration of 2000.
Collected Clinical and Laboratory Parameters
For each patient, the following demographic data and clinical parameters were noted: maternal age, length, weight, BMI, systolic, and diastolic blood pressure at admission and the following laboratory parameters: hemoglobin concentration, hematocrit, reticulocytes, thrombocytes, ureum, creatinin, uric acid, total bilirubin, sodium, potassium, calcium, magnesium, total protein, albumin, haptoglobin, aspartate transaminase (ASAT), alanine transaminase (ALAT), lactate dehydrogenase (LDH), and gamma glutamyl transpeptidase (GGT).
Sample Collection
In total, 20 mL of blood was collected: 10 mL was drawn into a serum separator tube and 10 mL in CellSave preservative tubes, which contain EDTA as anticoagulant and a proprietary preservative (Veridex, Raritan, NJ). To avoid contamination with traumatically detached CEC, the CellSave tube was drawn last. All samples were processed within 24 h. Serum was prepared within 1 h after sampling by centrifugation at 1700 g for 10 min. Aliquots were immediately frozen at −80°C until analysis.
CEC Enumeration
The CellTracks® AutoPrep® System and the CellSpotter® Analyzer II System (Veridex) were used to enumerate CEC, and have been described elsewhere (13). Briefly, four milliliters (mL) of blood were used for immunomagnetic enrichment using ferrofluids coupled to an anti-CD146 antibody. This marker is present on endothelial cells, a subset of activated T-lymphocytes, and melanoma cells. Subsequently, the following staining reagents were added: the nuclear dye 4,6-diamidino-2-phenylindole (DAPI), and fluorochrome-conjugated monoclonal antibodies (mAb): phycoerythrin-conjugated CD105, which is present on endothelial cells, and allophycocyanin-conjugated CD45, which is a pan-leukocyte antigen included to exclude hematopoietic cells during analysis. Analysis was done using image cytometry, where CEC were defined as being at least 4 μm in diameter, intact (with the nucleus ≥50% of the surface of the cytoplasm), DAPI+, CD146+, CD105+, and CD45 negative.
Assessment of Soluble Endothelial Markers by ELISA
Serum concentrations of thrombomodulin, E-selectin, and endoglin were determined using ELISA according to the manufacturers' instructions. The thrombomodulin-specific ELISA was purchased from American Diagnostica (Stanford, CT), the other ELISAs were from R& D Systems (Minneapolis, MN). Absorbance was read at 450 nm using a Titertek 212 MS microplate reader (Titertek, Huntsville, AL). Samples were tested in duplicate and related to the relevant standard curves. The lower detection limits of the assays were 2.0 ng/mL for E-selectin; 1.2 ng/mL for thrombomodulin, and 0.07 ng/mL for endoglin.
Statistical Analysis
The Shapiro-Wilk W test demonstrated that CEC counts and serum concentrations of thrombomodulin, E-selectin, and endoglin were not normally distributed (CEC distribution is shown in Figure 1). Therefore, comparison between groups was done using the Mann-Whitney U test. Correlations between parameters were calculated using Spearman's correlation coefficient (rs). Statistical differences were considered significant if P < 0.05. Analyses were performed using STATA 10 (StataCorp College Station, TX).
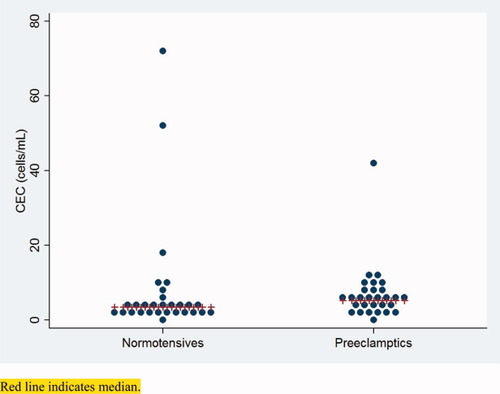
Distribution of CEC between normotensives and preeclamptics. Red line indicates median. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
RESULTS
The demographical, obstetrical, and clinical characteristics of patients and controls are shown in Table 1. Of note, there were clearly more nulliparae among the preeclamptic patients than among the control patients (Table 1). None of the control patients developed signs of hypertension or preeclampsia later in pregnancy.
| Control group (n = 30) | Patients with severe preeclampsia (n = 30) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 31 (28–34) | 31 (28–34) | NS |
| BMI (kg/m2) | 26.5 (22.6–29.8) | 27.4 (22.3–32.7) | NS |
| SBP (mm Hg) | 120 (110–124) | 148 (140–160) | P < 0.0001 |
| DBP (mm Hg) | 75 (65–80) | 90 (90–110) | P < 0.0001 |
| Smoking | |||
| Yes | 1 | 2 | |
| No | 29 | 28 | |
| Obstetrics | |||
| Gestational age (days) | 203 (189–217) | 207 (196–210) | NS |
| Gravidity | |||
| 1 | 2 | 19 | |
| 2 | 14 | 4 | |
| ≥3 | 14 | 14 | |
| Parity | |||
| 0 | 4 | 21 | |
| 1 | 4 | 4 | |
| 2 | 3 | 3 | |
| ≥3 | 2 | 2 | |
| Medication | |||
| Methyldopa | 0 | 19 | |
| Ca2+ reentry blocker | 0 | 15 | |
| Magnesium sulphate | 0 | 14 | |
- Data are shown as medians and interquartile ranges.
- NS = not significant.
Upon analysis of CEC numbers, no significant difference could be detected between the normotensive control group and the patients with severe preeclampsia (median 3.5 CEC [IQR 2–4.8] vs. 5.3 CEC [IQR 3.3–7.5], respectively, P = 0.21) (Table 2). The distributions of CEC counts deviated from normal in both preecclamptic patients and normotensives due to the occurrence of 1 and 3 outliers, respectively. The outliers couldn't be explained by individual clinical features. We did not find any correlation between CEC numbers and demographic and clinical parameters (data not shown). Seven of the 30 preeclamptic patients had not been stabilized during blood sampling. No differences in CEC numbers were found between stabilized and nonstabilized preeclamptic patients (data not shown).
| Parameter | Normotensives | Preeclamptics | |
|---|---|---|---|
| CEC (cells/mL) | 3.5 (2–4.8; n = 29) | 5.3 (3.3–7.5; n = 29) | NS |
| E-selectin (ng/mL) | 32.0 (17.0–42.8; n = 25) | 42.9 (27.4–57.0, n = 28) | P = 0.02 |
| Endoglin (ng/mL) | 5.0 (4.2–7.7; n = 26) | 76.2 (46.6–106.0, n = 28) | P < 0.0001 |
| Thrombomodulin (ng/mL) | 3.6 (2.6–5.2; n = 26) | 5.2 (3.9–7.1, n = 28) | P = 0.006 |
- Data are shown as medians and interquartile ranges. NS = not significant.
Serum concentrations of thrombomodulin, E-selectin, and endoglin were all markedly elevated in preeclamptic patients when compared to controls (Table 2). Whilst median thrombomodulin and E-selectin levels increased only modestly in preeclampsia (3.6 vs. 5.2 ng/mL and 32.0 vs. 42.9 ng/mL, respectively), endoglin levels increased 15-fold from 5.0 to 76.2 ng/mL.
DISCUSSION
In this study, we compared serum biomarkers of endothelial dysfunction (E-selectin and endoglin) and endothelial injury (thrombomodulin) to CEC counts in two groups of pregnant women: one group with severe preeclampsia, and one group of normotensive women. The groups were matched for gestational age and BMI, but not for parity status. CEC counts in both groups of women were within normal ranges [i.e., <20 CEC/mL; ref (13).]; with the exception of 2 normotensives and 1 preeclamptic patient. However, the concentrations of the soluble endothelial markers E-selectin, thrombomodulin and particularly endoglin were significantly increased in patients with severe preeclampsia. The effect of parity status on CEC counts and markers for endothelial dysfunction or injury is unknown, and cannot be addressed in this patient cohort due to its limited size.
The absence of increased CEC counts in preeclampsia was unexpected, as previous studies reported increased CEC numbers in these patients (7, 8). We believe that this discrepancy can be attributed to several factors. First, the power of our study was insufficient to be able to detect significant differences between two groups with test results that both still fall within the reference ranges of normal. The standard deviation (SD) of CEC counts in healthy, nonpreeclamptic pregnant women in our study was substantially larger (9.3) than the reported SD in the study by Canbakan (SDhealthy = 1.3), possibly as a result of a different immunophenotypical definition of CEC. Based on an SD of 9.3, we calculated that we would need at least 174 patients to detect a difference of 2.8 CEC, i.e., the difference between the medians of both groups in this study, with a 80% power and an alpha of 0.05. The studies by Grundmann (7) and Canbakan (8) included only 10 and 20 preeclamptic patients, respectively.
Secondly, methodological issues seem to play a role. CEC detected with the CellSearch assay are indeed endothelial cells, originate from the vascular lining (14) and express typical markers as demonstrated by global gene expression profiling (15). CEC counts were significantly higher in healthy nonpregnant and pregnant women in the study by Grundmann (7). Theoretically, the inclusion of CD105 in the immunophenotypic definition of CEC in our study could lead to an underestimation of CEC, as CD105 negative CEC (if they exist) are excluded from our analyses. However, the CEC numbers in nonpregnant and normotensive pregnant women reported by Canbakan (8) are comparable to those in our study, despite the fact that they use a similar technique as Grundmann, namely manual CD146-driven immunomagnetic isolation, followed by a confirmatory staining (Canbakan: acridine orange, Grundmann: Ulex Europaeus Lectin-1). Thus, methodological differences (i.e., Canbakan vs. Grundmann) may have contributed to the reported differences in CEC numbers in preeclampsia.
Finally, the duration of the clinical syndrome may have contributed to the different results. In the other two studies blood was sampled at a median gestational age of 35 weeks, in our study at an earlier median gestational age of 29 weeks. However, a correlation between gestational age and CEC counts could not be found in our data.
In previous cardiovascular research, CEC have been increased and associated with arterial stiffness in patients with hypertension, indicating endothelial damage/dysfunction (16). Various hypotheses are considered to elucidate the pathogenesis of vascular damage in women with preeclampsia. The key event in the pathogenesis of preeclampsia is thought to be abnormal placentation and failing vascularization of the placenta (17). The resulting placental hypoperfusion and hypoxia trigger the subsequent release of soluble factors. Three soluble factors related to endothelial damage (thrombomodulin), activation (E-selectin), and especially the antiangiogenic protein endoglin were increased in our study but CEC levels were not. This observation is remarkable, as patients with clinically the most severe form of preeclampsia were studied. Our results suggest that endothelial dysfunction and activation rather than actual damage occurs in preeclamptic patients. Additional prospective studies, which should be sufficiently powered will be needed to elucidate the exact role of the endothelium in preeclampsia and the role of endoglin as a potential early surrogate marker.




